Abstract
The CD70-CD27 interaction is known to positively regulate T cell expansion and effector function by providing costimulatory signals. In this issue of Immunity, Coquet et al. (2013) show an unexpected T-helper-17-cell-specific negative regulation mediated by CD70-CD27 interaction.
It is well established that the functional differentiation of CD4+ T helper (Th) cells requires three kinds of signals: those of costimulation, the T cell receptor (TCR), and cytokines. Costimulation determines the activation, proliferation, and survival of T cells, thereby generally supporting the differentiation and effector function of Th cells (Nurieva et al., 2011). On the other hand, the fate decision of Th-cell-lineage differentiation is largely determined by the third signal—the local cytokine milieu provided by antigen-presenting cells (APCs). For example, interleukin-12 (IL-12) induces Th1 cells and IL-4 induces Th2 cells, whereas IL-6, together with transforming growth factor β (TGF-β), potently induces Th17 cell differentiation. Under certain conditions, the strength of TCR signaling and different types of costimulations can also modulate Th cell polarization through T-cell-intrinsic mechanisms. For example, the inducible costimulator ICOS positively regulates the differentiation or expansion of Th2, Th17, and T follicular helper (Tfh) cells (Nurieva et al., 2011). In this issue of Immunity, Coquet et al. (2013) show that the so-called CD70-CD27 costimulatory pathway can also exert an inhibitory effect by selectively suppressing the migration and effector gene expression of Th17 cells.
CD27 belongs to the tumor-necrosis-factor-receptor superfamily of costimulators and is constitutively expressed on CD4+ and CD8+ T cells, which can be further upregulated after antigenic stimulation. CD70, the ligand for CD27, is transiently expressed on activated dendritic cells (DCs), B cells, and T cells. CD70-CD27 signaling facilitates the proliferation and survival of activated T and B cells and thereby positively regulates their effector function and memory responses and prevents tolerance induction (Nolte et al., 2009). Previous studies have suggested that CD70-CD27 interaction might also regulate the polarization of CD4+ T cells. For example, anti-CD70 treatment was able to suppress Th17-cell-mediated inflammatory diseases, including experimental autoimmune encephalomyelitis (EAE), and CD70-transgenic mice showed increased interferon-γ-producing CD4+ and CD8+ T cells (Nolte et al., 2009). These data suggest a positive role of CD70-CD27 interaction in regulating Th1 and Th17 cells. However, the negative correlation between CD27 and IL-17 production in γδ T cells indicates that CD27 signaling might actually inhibit IL-17 expression (Ribot et al., 2009), which was further demonstrated in a Th17-cell-differentiation culture (Libregts et al., 2011). Moreover, the effect of CD70-CD27 signaling on Th1 cell differentiation might depend on genetic background or experimental conditions (Libregts et al., 2011). Considering the discrepancy among these studies and the complicated role of CD70-CD27 signaling in the immune system, whether CD70-CD27 costimulation can directly instruct Th cell polarization other than the general costimulatory effect remains unclear.
To clarify the function of CD70-CD27 costimulation in Th cell polarization, especially in Th17 cells, the authors took advantages of several gain-of-function or loss-of-function approaches, including the use of Cd27−/−, Cd70cre/cre (i.e., Cd70−/−), and Cd70 transgenic (tg) mice (CD70 is constitutively expressed on conventional DCs under the control of the Cd11c promoter). In the EAE model, the authors found that, compared with wild-type (WT) mice, both CD27- and CD70-deficient mice showed significantly exacerbated disease, as well as increased mortality. In contrast, Cd70tg mice showed alleviated disease symptoms. Accordingly, the authors observed increased myelin-oligodendrocyte-glyocoprotein-specific Th17 cells in Cd27−/− mice and a decrease in Cd70tg mice in the EAE model. The similar phenotypes between Cd27−/− and Cd70−/− mice and the opposite phenotype in Cd70tg mice provide strong evidence that CD70 indeed signals through CD27 to control Th17 cell responses in vivo, either directly or indirectly. For determining whether this is due to a T-cell-intrinsic effect via CD70-CD27 signaling, an adoptive T-cell-transfer EAE model using Cd27−/− CD4+ T cells and Cd70−/− recipient mice will be useful.
To further characterize the role of CD70-CD27 signaling in Th17 cell differentiation in vitro, the authors adopted a coculture system by using purified WT and Cd27−/− naive CD4+ T cells in the presence of irradiated WT splenocytes and polarizing cytokines. Th17 cell differentiation was normal in both WT and Cd27−/− cells. However, the addition of a CD70 mimicry protein (FcCD70) greatly reduced the frequency of IL-17-producing cells in WT, but not Cd27−/−, cultures. The findings were also confirmed in Th17 cell differentiation performed with an APC-free culture system. It was shown that CD70-CD27 signaling can increase IL-2 expression, thereby inhibiting IL-17 through the antagonistic function between the transcription factors STAT3 and STAT5 (Nolte et al., 2009). To exclude this possibility, the authors blocked IL-2R in their culture, yet FcCD70 still inhibited IL-17 production. Furthermore, FcCD70 impeded Th17 cell differentiation even when it was added 48 hr later, after T cell activation. These results, together with EAE experiments, demonstrate a direct inhibitory role of CD70-CD27 signaling in Th17 cell differentiation independently of the general effect of costimulation on T cell proliferation and survival.
To ascertain the underlying mechanism of how CD70-CD27 signaling controls Th17 cell differentiation, the authors extensively examined the expression of a number of genes associated with Th17 cells and other Th cell lineages. Surprisingly, CD27 signaling only reduced the amount of IL-17, IL-17F, and the chemokine receptor CCR6, but not any other proteins crucial for Th17 cell development, including RORs, Batf, and IL-23R. Inhibition of the chemokine receptor CCR6 by CD27 costimulation was also confirmed in their Cd70tg EAE model. These data showed that CD70-CD27 signaling selectively regulates Th17 cells by controlling both their migration and their effector function, but not the general or early stage of Th17-cell-polarization programming. This is in contrast to the function of ICOSL-ICOS costimulation in Th17 cell and Tfh cell differentiation. Further investigation showed that CD27 signaling inhibited Th17 cells by enhancing the level of phospho-Jun amino-terminal kinase (JNK) given that a JNK inhibitor was able to rescue the inhibitory effect of CD27 costimulation on Th17 cells. Additionally, CD27 costimulation increased the amount of the nonpermissive histone marker H3K27me3 at the IL-17 gene locus, and treatment with DNA-demethylating agent 5-azacytidine significantly restored IL-17 production in CD27-stimulated Th17 cells, suggesting that CD70-CD27 signaling controls IL-17 expression in Th17 cells both transcriptionally and epigenetically.
It is noted that previous studies showed that CD70-blocking antibody prevented EAE in SJL/J mice (Nakajima et al., 2000) and that overexpression of CD70 in B cells enhanced EAE in 2D2 mice (Francosalinas et al., 2012), which is in contrast to Coquet et al. (2013) finding that overexpression of CD70 in DCs reduced EAE but that deficiency of CD70 and CD27 led to enhanced EAE. Moreover, Coquet et al. (2013) showed that CD70-CD27 signaling did not affect Th1 differentiation on the basis of their EAE experiments using Cd70-transgenic and Cd27−/− mice and in vitro differentiation assay using Cd27−/− T cells, suggesting a Th17-specific role of CD70-CD27 interaction. However, this finding is also opposite to the result obtained with transgenic mice with CD70 overexpression in B cells (Arens et al., 2001). These inconsistencies might have been caused by the general effect of CD70-CD27 interaction on T and B cell activation given that constitutive overexpression of CD70 in B cells disrupts the homeostasis of the normal immune system and thus leads to T cell hyper-activation and gradual B cell depletion (Tesselaar et al., 2003). For distinguishing the general costimulatory function and its inhibitory effect on Th17 cells, a careful examination of T cell transfer with the use of Th17- and Th1-biased disease models would be useful. CD70-CD27 costimulation can activate many different signaling pathways, including those of canonical-alternative NF-κB, mitogen-activated protein kinase, and JNK (Nolte et al., 2009). Coquet et al. (2013) showed that CD70-CD27 signaling inhibited Th17 cells by selectively enhancing JNK activation. It is therefore worth identifying the downstream JNK target(s) that directly control(s) the expression of IL-17 and CCR6 in Th17 cells, as well as examining whether CD70-CD27 interaction uses a distinct mechanism in negative regulation of Th17 cells and its other immune-related functions. Interestingly, a subset of APCs has been shown to express CD70 constitutively in the lamina propria, where plenty of Th17 cells are generated at steady phase (Atarashi et al., 2008). However, these cells were shown to be able to induce Th17 differentiation through the bystander effect of secreting IL-6 and IL-23 in the presence of ATP or microbiota (Atarashi et al., 2008). It would still be interesting to know whether these intestinal CD70+ APCs can also restrain excessive Th17 gene expression via directly signaling through CD27 and thereby maintain the balance of gut-associated mucous immune homeostasis. Importantly, considering the varied results achieved in different mouse models and experimental conditions (Nolte et al., 2009), the findings of CD70-CD27 signaling obtained in the mouse system, including its effect on Th17 cell differentiation, need to be reexamined and validated in the human immune system. These additional studies would be important for completely clarifying the specificity of the CD70-CD27 axis in regulating Th17 cells and laying basis for developing therapeutics against Th17-related inflammatory and autoimmune diseases. Despite those remaining issues, the study by Coquet et al. (2013) in this issue of Immunity provides compelling in vitro and in vivo experimental evidence to support a T-cell-intrinsic role of CD70-CD27 interaction in inhibiting Th17 cell differentiation and migration, adding a new brake to prevent a train wreck of Th17-cell-mediated immunity and possibly to secure the homeostasis of the gut-associated immune system (Figure 1).
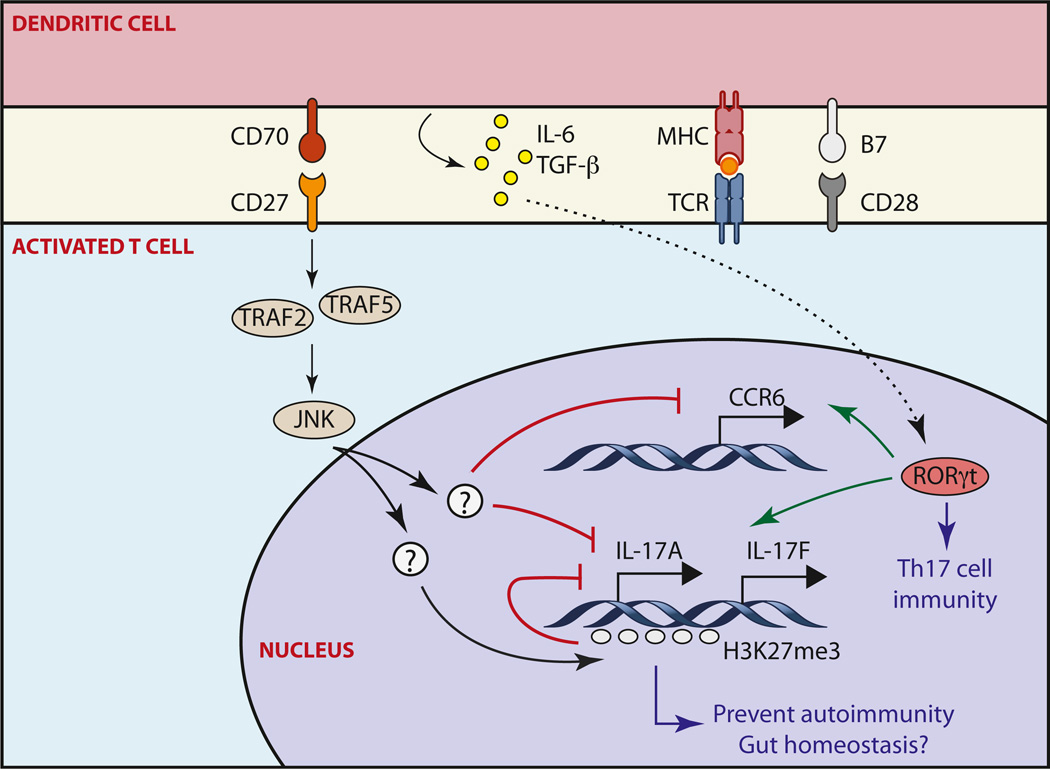
Figure 1. Role of CD70-CD27 Signaling in the Control of Th17 Cell Differentiation.
CD70 is induced in activated DCs and signals through CD27 on activated CD4+ T cells, leading to enhanced phosphorylation of JNK via TRAF2 or TRAF5 signaling. Activation of JNK antagonizes RORγt-induced IL-17, IL-17F, and CCR6 expression via transcriptional suppression or through epigenetically silencing the Il17a locus; this then limits overactivation of Th17-cell-mediated responses and associated autoimmunity and might help to maintain homeostasis of the gut-associated immune system (Nolte et al., 2009).
REFERENCES
- Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Middendorp S, van der Horst G, Kind J, Veraar EAM, Xiao Y, Jacobs H, Borst J. Immunity. 2013;38:53–65. doi: 10.1016/j.immuni.2012.09.009. this issue. [DOI] [PubMed] [Google Scholar]
- Francosalinas G, Cantaert T, Nolte MA, Tak PP, van Lier RA, Baeten DL. J. Neuroimmunol. 2012 doi: 10.1016/j.jneuroim.2012.10.010. Published online November 5 2012. http://dx.doi.org/10.1016/j.jneuroim.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Libregts S, van Olffen RW, van der Sluijs KF, van Lier RA, Nolte MA. Immunol. Lett. 2011;136:177–186. doi: 10.1016/j.imlet.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Oshima H, Nohara C, Morimoto S, Yoshino S, Kobata T, Yagita H, Okumura K. J. Neuroimmunol. 2000;109:188–196. doi: 10.1016/s0165-5728(00)00324-6. [DOI] [PubMed] [Google Scholar]
- Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Immunol. Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Liu X, Dong C. Immunol. Rev. 2011;241:133–144. doi: 10.1111/j.1600-065X.2011.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, van Oers MH, van Lier RA. Nat. Immunol. 2003;4:49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]