Abstract
Among primates, humans exhibit the most profound degree of age-related brain volumetric decline in particular regions, such as the hippocampus and the frontal lobe. Recent studies have shown that our closest living relatives, the chimpanzees, experience little to no volumetric decline in gray and white matter over the adult lifespan. However, these previous studies were limited with a small sample of chimpanzees of the most advanced ages. In the present study, we sought to further test for potential age-related decline in cortical organization in chimpanzees by expanding the sample size of aged chimpanzees. We used the BrainVisa software to measure total brain volume, gray and white matter volumes, gray matter thickness, and gyrification index in a cross-sectional sample of 219 captive chimpanzees (8-53 years old), with 38 subjects being 40 or more years of age. Mean depth and cortical fold opening of 11 major sulci of the chimpanzee brains were also measured. We found that chimpanzees showed increased gyrification with age and a cubic relationship between age and white matter volume. For the association between age and sulci depth and width, the results were mostly non-significant with the exception of one negative correlation between age and the fronto-orbital sulcus. In short, results showed that chimpanzees exhibit few age-related changes in global cortical organization, sulci folding and sulci width. These findings support previous studies and the theory that the age-related changes in the human brain is due to an extended lifespan.
Keywords: aging, chimpanzees, gray matter, white matter, cortical thickness, gyrification
Normal aging in humans is a complex process that brings about many structural changes in the brain. Research shows that normal brain aging in humans is characterized by particularly severe volume loss in regions involved in memory and executive functions, such as the hippocampus and frontal lobe (Abe et al., 2008; Peters, 2006; Raz et al., 1997; Rosen et al., 2002; Tisserand et al., 2004). The volumetric decline is associated with shrinkage of gray matter (GM) and white matter (WM) volumes and enlargement of the cerebrospinal fluid (CSF) spaces, resulting in an increase of the opening of cortical sulci (Ge et al., 2002; Lemaitre et al., 2012; Matsumae et al., 1996; Pfefferbaum et al., 1994; Sherwood et al., 2011; Sullivan et al., 1995). Evidence suggests that cerebral gray matter volume decreases at a linear rate with age in adulthood, whereas hippocampal volume is relatively stable until middle age, after which there is an accelerated rate of shrinkage (Ge et al., 2002; Good et al., 2001; Raz et al., 2005; Sherwood et al., 2011; Taki et al., 2011; Walhovd et al., 2005). White matter volume shows a quadratic change over the lifespan, in which it increases until the middle age period and then decreases with increasing age (Ge et al., 2002; Giorgio et al., 2010; Sowell et al., 2003; Walhovd et al., 2005; Westlye et al., 2010).
From a comparative perspective, previous studies have examined age-related changes in the brains of other mammals, and specifically primates. Nonhuman primates are of particular interest for studying the neurobiological correlates of aging because of their close phylogenetic relationship with humans (Finch and Austad, 2012). Nonhuman primates are often used as models to understand the effects of aging independent of the cellular changes that cause age-related neurodegenerative disorders commonly seen in humans such as Alzheimer's disease (Gearing et al., 1996; Kimura et al., 2003; Koo et al., 2012; Lemaitre et al., 2012; Sherwood et al., 2011; Squire et al., 1988). Characteristics of neurodegenerative diseases, such as diffuse plaques and vascular lesions, have been observed in the hippocampus and frontal lobes of aged macaque monkeys, chimpanzees, gorillas and orangutans (Gearing et al., 1997; Kimura, 2001; Poduri et al., 1994). At the present time there are only two reports of the existence of neurofibrillary tangles in great apes including one in a 41 year old chimpanzee (Rosen et al., 2008), and more recently in a sample of lowland gorillas (Perez et al., 2013). Finally, there is also evidence that apes and monkeys show age-related volumetric decline in the striatum and modest reductions in total brain volume (Alexander et al., 2008; Herndon et al., 1999; Matochik et al., 2000; Rapp and Amaral, 1992).
In terms of behavior and cognition, age-related decline in motor and cognitive functions have been described extensively in monkeys (Herndon et al., 1997; Lacreuse et al., 2005; Moss et al., 1988; Rapp and Amaral, 1989). In humans, the age-related volumetric reductions of the frontal lobe and the hippocampus are associated with decline in cognitive processes, including fluid reasoning, mental processing speed, episodic memory, and spatial ability (Park et al., 2001; Salthouse, 1996; Verhaeghen and Salthouse, 1997; Whalley et al., 2004). In aged nonhuman primates, there are impairments in delayed response tasks, delayed matching-to-sample tasks, delayed recognition span tasks, reversal learning tasks, and conceptual set-shifting task (Bartus et al., 1978; Hara et al., 2012; Moss et al., 1988). Far fewer data are available from great apes, including chimpanzees; however, a recent study by Lacreuse et al. (2014) reported a moderate but statistically significant decline in spatial memory in a sample of 4 chimpanzees over the age of 50 years old.
It has been hypothesized that a possible factor explaining the more pronounced pathological and cognitive changes found in humans compared to nonhuman primates is increased longevity (Hawkes, 2003; Herndon, 2009). Compared to other primates, humans have evolved an extended lifespan, particularly post reproductively, which has been proposed to increase the risk for the development of cognitive impairments and associated brain changes in later life (Herndon, 2009). The “grandmother hypothesis” suggests that human longevity resulted from selection for longer post-menopausal survival in women, who could contribute to the cooperative care of dependent offspring in their families (Finch and Sapolsky, 1999; Hawkes, 2003). According to Herndon (2009), chimpanzees' shorter post-menopausal lifespan might allow them to avoid cognitive impairments or neurodegenerative disorders that occur during the very latest stages of life in humans, such as Parkinson's or Alzheimer's disease.
To date, there are two studies on age-related changes in cortical organization in chimpanzee brains based on post-mortem or in vivo magnetic resonance imaging. Sherwood et al. (2011) examined age-related changes in cortical organization in chimpanzees compared to humans. They measured the volumes of brain regions in 69 chimpanzees and found that there was little evidence of marked age-related change. Specifically, chimpanzees did not show statistically significant volumetric age-related decline in gray and white matter volume for either the entire brain or frontal lobe or hippocampus. More recently, Chen et al. (2013) found that chimpanzees do show age-related declines in both gray and white matter, but the declines were much smaller than typically occur in older humans. One limitation of this prior research was the minimal number of very old or “aged” subjects, defined as those chimpanzees greater than 40 years of age. For instance, there were only 7 subjects over the age of 40 in the previous study by Sherwood et al., with only one being a male. Similarly, Chen et al. (2013) had only a small portion of chimpanzees over the age of 40 and the sample consisted entirely of females. Thus, both Chen et al. (2013) and Sherwood et al. (2011) may not have had enough statistical power to detect more robust age-related changes in cortical organization among the most geriatric chimpanzees, and particularly older males.
The aim of the current research was to further test for potential age-related decline in cortical organization in chimpanzees. This study differs from previous reports on age-related changes in the chimpanzee brain in two important ways. First, this study had a larger sample of male and female chimpanzees that included substantially more individuals representing the upper end of their lifespan. Second, we employed a different methodology and approach to the measurement of different dimensions of cortical organization. Here, we used the BrainVisa (BV) software to measure the organization and folding in the cerebral cortex. This software has been previously employed to assess age-related changes in human and baboon brains (Kochunov et al., 2005). Using the BV software, we measured the total brain volume, gray and white matter volumes, gray matter thickness, and gyrification index of 219 captive chimpanzees, with 38 subjects being 40 or more years of age, therefore considerably expanding the sample size in the oldest cohort of individuals. Furthermore, we measured the mean depth and cortical fold opening of 11 major sulci of the chimpanzee brains. We hypothesized that if age-related changes in the chimpanzee brain are reduced compared to humans, then age would account for a small proportion of variance in cortical organization, sulci depth and fold opening in our sample.
Methods
Subjects
There were 219 captive chimpanzees (134 females, 85 males) in this study including 84 chimpanzees housed at the Yerkes National Primate Research Center (YNPRC) and 135 chimpanzees housed at The University of Texas M. D. Anderson Cancer Center (UTMDACC). Ages at the time of their magnetic resonance image scans ranged from 8 to 53 years (Mean = 27.04, SD = 6.74). In addition to analyzing the neuroanatomical variables against chronological age, we also classified the chimpanzee into 4 age groups including adolescent or sub-adult individuals (<=15 years), young adults (16 to 25 years), middle-aged adults (26 to 39 years) and elderly (40+ years). The samples sizes in the sub-adult, young adult, middle-aged, and elderly groups were 33, 86, 62, and 38, respectively.
Magnetic Resonance Image Collection
All chimpanzees were scanned in vivo during one of their annual physical examinations. Magnetic resonance image (MRI) scans followed standard procedures at the YNPRC and UTMDACC and were designed to minimize stress. Thus, the animals were first sedated with ketamine (10 mg/kg) or telazol (3-5mg/kg) and were subsequently anaesthetized with propofol (40–60 mg/kg/h). They were then transported to the MRI scanning facility and placed in a supine position in the scanner with their head in a human-head coil. Upon completion of the MRI, chimpanzees were briefly singly-housed for 2-24 hours to permit close monitoring and safe recovery from the anesthesia prior to return to the home social group. All procedures were approved by the Institutional Animal Care and Use Committees at YNPRC and UTMDACC and also followed the guidelines of the Institute of Medicine on the use of chimpanzees in research. Seventy-six chimpanzees were scanned using a 3.0 Tesla scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA). T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320, with 0.6 × 0.6 × 0.6 resolution). The remaining 143 chimpanzees were scanned using a 1.5T G.E. echo-speed Horizon LX MR scanner (GE Medical Systems, Milwaukee, WI). T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, matrix size = 256 × 256, with 0.7 × 0.7 × 1.2 resolution).
MRI Processing
BrainVISA 4.0.1 (BV) is a freely distributed software (http://brainvisa.info) that can be used for a range of morphometrics, including measurement of cortical folding of the brain (Mangin et al., 2004). Initially, using Analyze 8.1, all MRIs were skull-stripped, cropped, and reformatted at 0.7 cubic isotropic resolution and subsequently imported into BV. Extracting the sulci from the cortex in the brain scans involves a series of steps in a pipeline process within BV (Mangin et al., 2004) (see Figure 1) and these methods are described in detail elsewhere (Bogart et al., 2012). Initially, the anterior and posterior commissures were manually specified on the MRI where they intersect with the mid-sagittal slice to align the brain. The first step was to correct for special inhomogeneities in the signal intensity providing a spatially smooth bias field with a stable distribution of tissue intensities (Figure 1b). Next, the analysis of the signal histogram and mathematical morphology were computed to obtain a binary mask of the brain (Figure 1c). The mask was then split into the left and right hemispheres and the cerebellum (Figure 1d). A negative mould of the white matter was computed from the split-brain mask. The outside boundary of this mould results from a 5 mm morphological closing of the masked hemisphere, filling up the folds. The grey/white interface is the inside boundary preserving deformations assuring the spherical topology of the mould (Figure 1e). Finally the mould was skeletonised to detect cortical folding, while topological constraints guarantee the resulting surfaces have no holes (Figure 1f-g). The deepest part of the fold indicates the buried gyrus given the grey/white interface. The final steps results in creating the cortical fold graph with the extracted sulci (Figure 1h).
Figure 1.
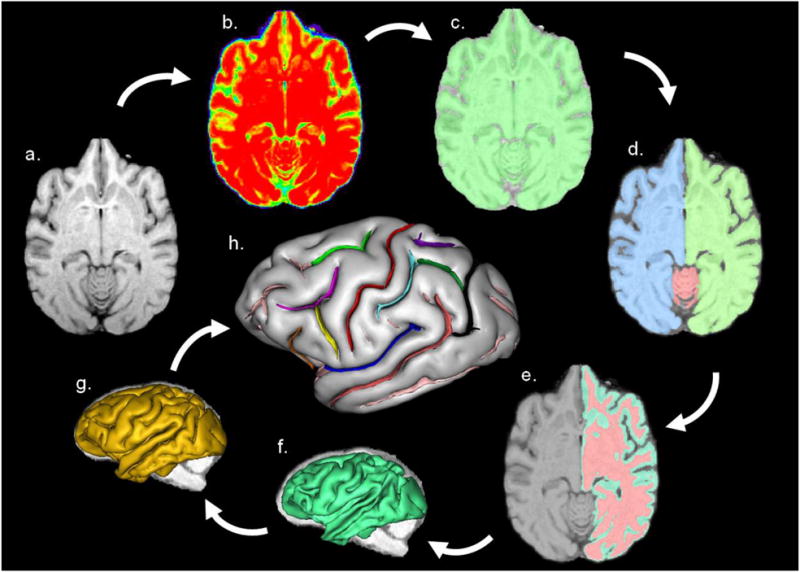
BrainVISA's pipeline processing steps a) MR image of a skull-stripped chimpanzee brain, b) stable tissue intensities creating bias field, c) binary mask of the brain, d) split mask of left and right hemispheres and cerebellum, e) gray and white interface, f) a negative mould of the white matter, g) skeletonised mould of cortical folding, h) cortical fold graph of chimpanzee sulci manually labelled. Sulci: red = central, light green = superior precentral, orange = fronto-orbital, yellow = precentral inferior, light purple = inferior frontal, dark blue = Sylvian fissure, dark pink = superior temporal, light blue = inferior postcentral, dark purple = superior postcentral, dark green = intraparietal, and brown = superior parietal. Image taken from Bogart et al. (2012).
In this study, 11 sulci of the chimpanzee brain were manually labeled following Bailey et al.'s (1950) definitions (Figure 2). The sulci selected in the frontal lobe were the central (CS), superior precentral (SPC), precentral inferior (PCI), inferior frontal (IFS), and fronto-orbital (FO) sulci. The superior precentral sulcus in chimpanzees is triradiate in formation, with one branch extending toward the frontal pole (Bailey et al., 1950), while the posterior end runs medial to lateral. We included all limbs of SPC in this study. PCI often includes the superior limb running parallel to the central sulcus; however, we were interested in including only that portion of PCI that is used to define the inferior frontal gyrus (IFG) in chimpanzees (Keller et al., 2009). Thus, measures were obtained on the inferior limb, which is considered the posterior border of the IFG in the chimpanzee brain. Furthermore, PCI can be bifurcated (Keller et al., 2009; Sherwood et al., 2003), and we included all inferior branches of the PCI in our measurement of this sulcus. FO in the chimpanzee constitutes the anterior border of IFG and corresponds to the human ascending ramus (Keller et al., 2009). The temporal lobe sulci consisted of the Sylvian fissure (SF) and superior temporal sulcus (STS), while the parietal lobe sulci included the superior postcentral (SPCS), inferior postcentral (IPCS), superior parietal sulcus (SPS) and intraparietal (IP) sulci.
Figure 2.
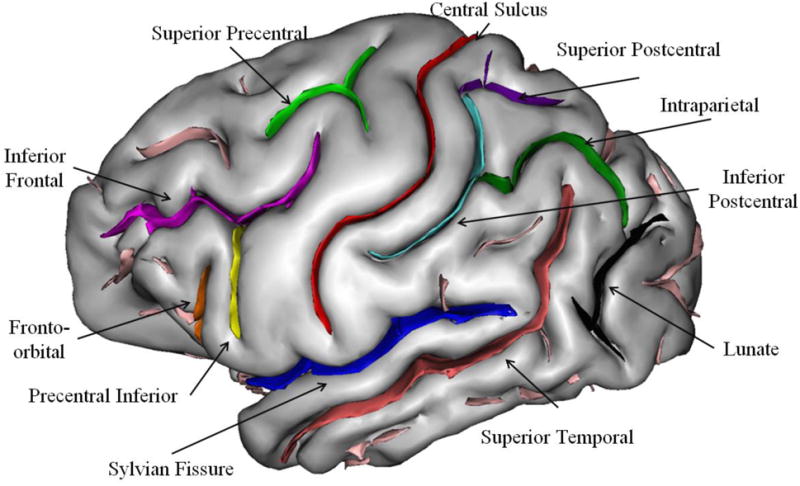
3D-rendering of chimpanzee brain with each of the 11 sulci of interest labeled on the surface. Image taken from Bogart et al. (2012)
Sulci Measures
Two measures were obtained from each sulcus using BrainVISA's (BV) morphometric tools, and these included: 1) mean depth (mm) and 2) sulcus fold opening (mm). Mean depth is the average depth of the sulcus along its principal axis of projection (i.e., dorsal-ventral, anterior-posterior, see Figure 3a). Sulcus fold opening was quantified by measuring the average mean distance between the two sides of the sulcus (see Figure 3b).
Figure 3.
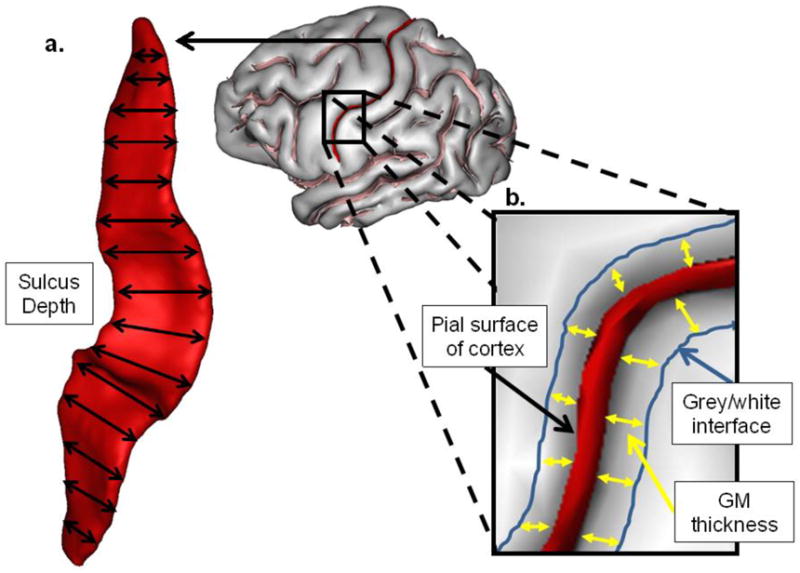
The central sulcus is extracted to demonstrate sulcus measures computed. a) Mean depth is the calculated average of the depth measures along the entire length of the sulcus. b) fold opening is represented as the distance between the two sides of the sulcus.
Global Cortical Measures
Using BV, we further calculated hemispheric gray and white matter volumes (cc), overall gyrification index (GI), and average cerebral gray matter thickness (mm) for each subject. To calculate the gray (GM) and white matter (WM) volumes, the program used the grey/white interface generated in the sulci extraction pipeline process to generate a cerebral spinal fluid (CSF) interface, providing the total volumes in each hemisphere. The gray and white matter volumes included subcortical structures such as the thalamus and basal ganglia; however, the cerebellum and related brain stem structures were excluded from calculations of total GM and WM volume. As has been done in many previous studies, the overall gyrification index (GI) of the cerebral cortex was determined by measuring the surface area sulcal internal contours of the cortex and dividing it by the total surface area for each hemisphere (Armstrong et al., 1993; Rilling and Insel, 1999; Rogers et al., 2010; Zilles et al., 1989). Overall average cortical gray matter thickness (CGM) was computed using a plugin developed for BV by Kochunov and colleagues (2012). This plugin also has a tool that measures the average gray matter thickness within each hemisphere using the pial and gray-white interface created in the pipeline process for each brain. Whole-brain CGM was calculated for the explicit purpose of adjusting individual sulcal gray matter thickness (see Statistical Analyses below).
Statistical Analyses
For all brain measures, the left and right hemisphere values for each subject were averaged to derive a single mean. For the total brain, white and gray matter volumes, cortical thickness and gyrification index, we used multiple analysis of covariance (MANCOVA)with scanner strength (1.5T, 3T) sex and age group serving as the between-group factors while body weight served as the covariate. For the sulci measures, MANOVA was used with scanner type, sex and age group as the between group factors. To be consistent with other studies that have examined age-related changes in different measures of the brain, we also fit linear, quadratic and cubic functions between age and each of the brain measures to assess which, if any, best explain the variation in values. All analyses were performed using Statistical Package for Social Science (SPSS) software. Post-hoc tests, when necessary, were conducted using Tukey's Honestly Significant Different (HSD) tests. For all statistical tests, alpha was set to p < 0.05.
Results
Global Cortical Measures
Prior to conducting the sex and age effect analyses, we performed a Pearson Product Moment correlation between age and body weight and found that older subjects had lower body weights (r = -.223, p = .001). Because age was associated with body weight and body weight can correlate with brain size within and between different primate species, we subsequently used body weight as a covariate in the analyses examining sex and age effects on the global measures of cortical organization.
In the initial analysis, we performed MANCOVA with the total brain volume (TBV), gray and white matter volume (GM_VOL, WM_VOL), cortical thickness (CT) and gyrification (GI) serving as dependent measures while scanner type, sex and age group were the between group factors. Body weight served as a covariate. Significant main effects were found for sex F(5, 198)=3.728, p = .003, age group F(15, 600)=1.785, p = .033 and scanner type F(5, 198)=3.733 p= .003. None of the interaction terms between age, sex, scanner type and the covariate body weight were significant. The mean male and female TBV, GM_VOL, WM_VOL, GM and GI values and their standard errors are shown in Figure 4a and 4b. Because the scanner type variable was significant, we have plotted separate graphs for the data collected at 1.5T and 3.0T scanner strength.
Figure 4.
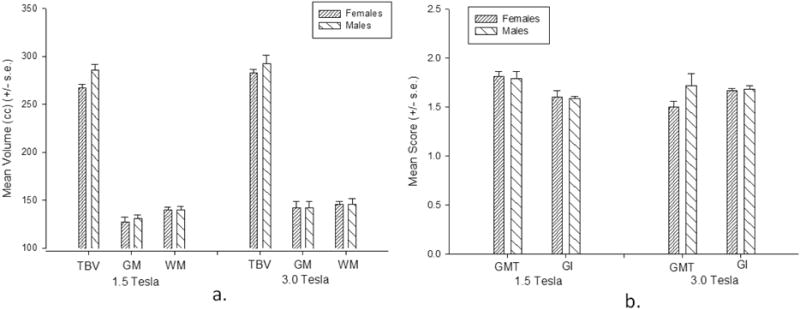
Mean global cortical organization measurements (+/− s.e.) in male and females chimpanzees at each scanner strength. a) TBV = total brain volume, GM = gray matter volume, WM = white matter volume b) GMT = gray matter thickness, GI = gyrification index.
The subsequent univariate F-tests revealed that males had larger TBV F(1, 202)=5.360, p = .022, and WM_VOL F(1, 202)=7.706, p = .006 values than females. No significant sex differences were found in GM_VOL, gray matter thickness, or gyrification. The mean global cortical organization values for each age group and brain measure are shown in Figure 5a and 5b. The univariate F-tests indicated that significant age group differences were found for GI F(3, 202)=2.797, p = .041 and GM_VOL F(3, 202)=3.478, p = .017. For the GI scores, post-hoc analysis indicated that the mean gyrification scores for the sub-adult groups were significantly higher than all other age groups. None of the remaining means differed significantly from each other. For GM_VOL, post-hoc analysis failed to reveal any significant differences between age groups. We also fit linear, quadratic and cubic regressions between age and the values for each of the brain measures, after controlling for the scanner type. The results are shown in Table 1 and Figure 6a to 6e. There was a significant cubic association between age and white matter volume and significant linear, quadratic and cubic associations with gyrification. Separate analyses of the associations between age and the measures obtained for the global cortical measures for males and females revealed nearly identical results for both sexes, with both having significant linear, quadratic and cubic associations between age and gyrification (see Table 1). We have provided the mean global brain organization findings and associated F-test for each scanner type in Table 2. Mean GMT was greater in the 1.5 compared to the 3T scans whereas GI and gray matter volumes were significantly higher in the 3T compared to 1.5T scans. No significant differences were found for white matter and total brain volume.
Figure 5.
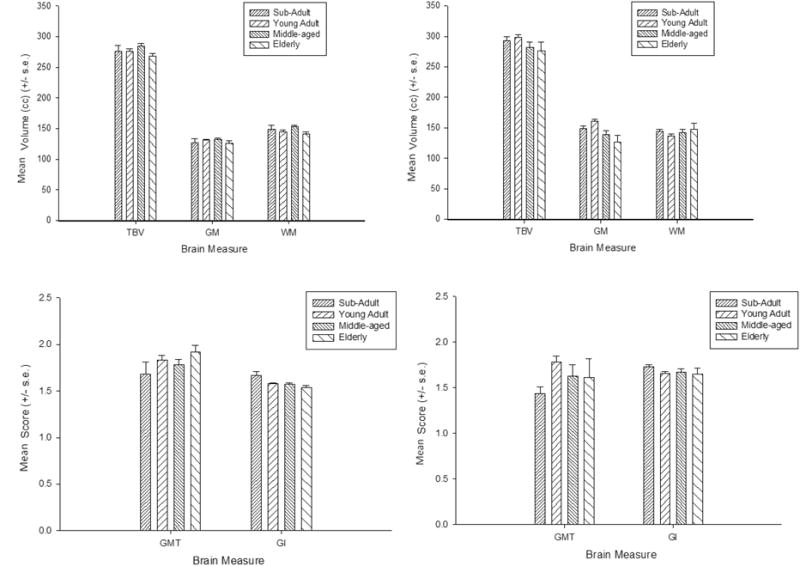
Mean global cortical organization measurements (+/− s.e.) in the different age groups and scanner strength. a,b) TBV = total brain volume, GM = gray matter volume, WM = white matter volume c,d) GMT = gray matter thickness, GI = gyrification index. Left column represent data collected at 1.5T and right column is 3.0Tesla
Table 1. Partial r Values for Linear, Quadratic and Cubic Associations Between Age and Total Brain Volume (TBV), Grey Matter Volume (GM_Vol), White Matter Volume (WM_Vol), Grey Matter Thickness (GMT) and Gyrification (GI).
| Region | Linear | Quadratic | Cubic |
|---|---|---|---|
| Overall | |||
| TBV | −.050 | −.056 | −.122 |
| GM_Vol | −.057 | −.065 | −.066 |
| WM_Vol | −.084 | −.144 | −.138* |
| CMT | .081 | .067 | .060 |
| GI | −.206** | −.199** | −.195** |
| Females | |||
| TBV | −.015 | −.015 | −.016 |
| GM_Vol | .024 | .027 | .029 |
| WM_Vol | −.073 | −.099 | −.121 |
| CMT | .081 | .066 | .060 |
| GI | −.192* | −.192* | −.196** |
| Males | |||
| TBV | −.096 | −.130 | −.160 |
| GM_Vol | −.109 | −.141 | −.066 |
| WM_Vol | .009 | −.024 | −.049 |
| CMT | .157 | .155 | .152 |
| GI | −.280** | −.260* | −.237* |
p < .01
p <.05
p <.10
Figure 6.
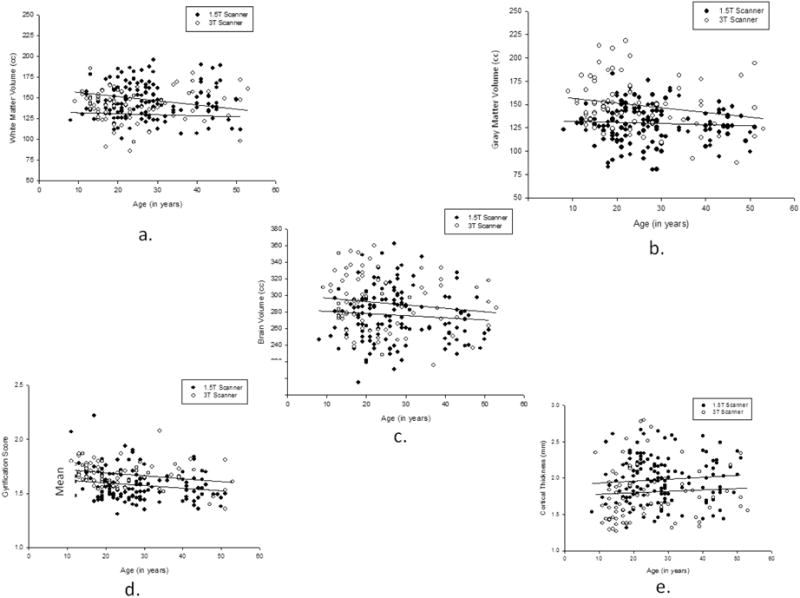
Scatterplots of the relationship between age and a) WM b) GM c) TBV d) GMT and e) GI with separate regression lines drawn for chimpanzee scanned with 1.5T and 3.0T scanners.
Table 2. Descriptive Statistics and Associated F-values for the Effect of Scanner Types on Global Brain Measures.
| Scanner Type | ||||
|---|---|---|---|---|
| Measures | 1.5T | 3.0T | F | p |
| TBV | 276.71 | 287.78 | 3.336 | .069 |
| (3.29) | (5.09) | |||
| Grey Matter | 65.28 | 70.61 | 5.271 | .023 |
| (1.26) | (1.95) | |||
| White Matter | 73.38 | 71.79 | 0.633 | .427 |
| (1.08) | (1.68) | |||
| GMT | 1.81 | 1.61 | 5.826 | .017. |
| (.04) | (.06) | |||
| GI | 1.59 | 1.67 | 10.17 | .002 |
| (.01) | (.02) | |||
TBV, Grey and White Matter values are in cc. For all univariate F-tests, there were 1 and 210 degrees of freedom.
Sulci Mean Depth and Fold Opening
For mean depth and fold opening, separate MANCOVAs were performed. In each MANCOVA, the mean depth or fold opening scores for each sulcus were the dependent measures while sex and age group were the between group factors. Scanner type served as a covariate. For mean depth, the MANCOVA revealed a significant main effect for sex F(11, 193)=1.839, p = .05 and the covariate scanner type F(11, 193)=10.820, p = .001. No significant interactions were found between age group, sex or scanner type. The subsequent univariate F-tests revealed significant male-female differences for the CS F(1, 203)=6.929, p = .01, SPC F(1, 203)=5.247, p = .023, and STS F(1, 203)=4.254, p = .04. The average mean depth for each sex and sulcus can be seen in Figure 7. Females had smaller mean depth scores than males for all the sulci. The linear, quadratic and cubic associations between mean depth and age for each sulcus are shown in Table 3. Significant linear, quadratic and cubic associations were found between age and the mean depth of the CS and FO sulci. A significant quadratic and cubic association was found between age and the mean depth of the SPCS. In terms of covariate scanner type, univariate F-tests revealed significant differences for SF F(1, 203)=38.011, p = .001, STS F(1, 203)=34.233, p = .001, IP F(1, 203)=27.998, p = .001 and SPS F(1, 203)=16.705, p = .001. In all cases, the mean depth for the sulcus was significantly higher for the 1.5 compared to 3.0T images.
Figure 7.
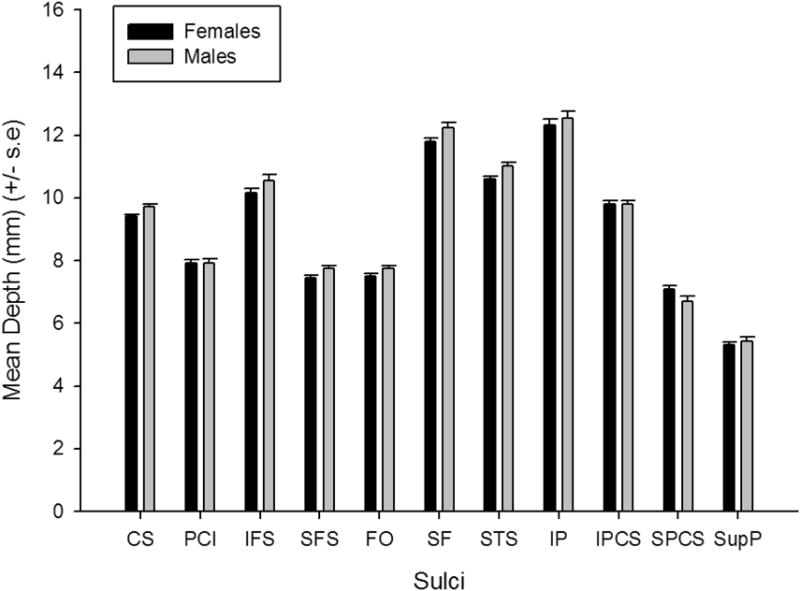
Table 3. Linear, Quadratic and Cubic Correlations Between Age and the Mean Depth and Fold Opening for Each Sulcus.
| Mean Depth | Fold Open | |||||
|---|---|---|---|---|---|---|
| Sulcus | Linear | Quadratic | Cubic | Linear | Quadratic | Cubic |
| CS | −.158* | −.167* | −.168* | .087 | .090 | .088 |
| PCI | −.049 | −.047 | −.046 | .007 | .003 | .000 |
| IFS | −.058 | −.047 | −.037 | .042 | .050 | .052 |
| SFS | −.017 | −.019 | −.021 | .098 | .085 | .074 |
| FO | −.187** | −.176** | −.158* | .171* | .161* | .144* |
| SF | .086 | .073 | .063 | .034 | .036 | .035 |
| STS | −.091 | −.096 | −.102 | .022 | .060 | .011 |
| IP | −.103 | −.111 | −.112 | −.004 | .001 | .003 |
| IPCS | −.053 | −.047 | −.042 | .015 | .017 | .018 |
| SPCS | .119 | .140* | .153* | −.107 | −.116 | -.118 |
| SupPar | −.075 | −.080 | −.079 | .092 | .086 | .077 |
p < .01
p <.05
p <.10
For the fold opening measure, the MANCOVA only revealed a significant main effect for scanner type F(11, 197) = 6.199 p = .001. Significant univariate F-tests were found for FO F(1, 203)=5.769, p = .017 and IP F(1, 203)=9.067, p = .003. FO values were higher in the 1.5 compared to 3T scans for FO whereas the opposite pattern of difference was found for the IP sulcus. Linear, quadratic and cubic associations between age and the old opening scores for each sulcus are shown in Table 3. Significant linear, quadratic and cubic associations were found between age and the fold opening of the FO sulcus.
Discussion
Brain aging in nonhuman primates displays both similarities and differences from humans (Koo et al., 2012; Nagahara et al., 2010; Poduri et al., 1994). Studies have shown that there is little to no changes in cortical organization in the chimpanzee brain (Sherwood et al., 2011; Chen et al., 2013), yet these studies had a small number of chimpanzees over the age of 40. This study is the single largest study ever examining age-related changes in cortical organization in nonhuman primates and specifically chimpanzees. In addition, we found that there was little evidence of age-related decline in cortical organization including 1) total brain and gray matter volume 2) cortical thickness and 3) the mean depth and fold opening of 11 different major cortical sulci. Of all the measures that we analyzed in the current study, aging effects were found only for total cortical gyrification and white matter volume. In short, the macrostructural organization of the chimpanzee brain does not appear to change dramatically with aging.
In general, the results showing a lack of significant or only small age-related changes in cortical organization in chimpanzees are consistent with the findings reported by Sherwood et al. (2011) and others (Chen et al., 2013; Herndon et al., 1999). However, this study differed from other reports in that we had a larger sample size, particularly among individuals in the oldest cohort. Moreover, rather than focus on variation in specific regions of interest, this study included measures of global organization and the depth and degree of fold opening of select sulci.
Sub-adult chimpanzee subjects had higher gyrification index (GI) scores compared to adult, middle-aged and elderly chimpanzees. We also found that there was a quadratic and cubic relationship between age and white matter volume, meaning that white matter volume increases earlier in life, then decreases in later years. This is consistent with data from humans and other primates, namely that cerebral white matter increases until middle adulthood and then decreases with increasing age (Ge et al., 2002; Hopkins and Phillips, 2010; Phillips and Sherwood, 2008; Pierre et al., 2008). A similar pattern for results has been found in humans for the relationship between age and gyrification (Hogstrom et al., in press) (see Table 1 and Figure 5a to 5f). However, the proportion of variance in white matter volume and gyrification that was attributable to age in the chimpanzee brains was less than 5%, which is quite small compared to what has been reported in human brains (see Table 1).
Results also showed there was a sex difference in white matter and total brain volume with males having larger values than females across the lifespan. These results are consistent with some findings in humans (Allen et al., 2003; Groeschel et al., 2010; Luders et al., 2006; Luders et al., 2002) but not all (Gur et al., 2002; Leonard et al., 2008; Luders and Toga, 2010; Xu et al., 2000). Though the sex difference in white matter volume were significant even after adjusting for body weight, when we calculated the ratio in gray to white matter in our sample, we found no significant differences between males and females. Thus, the differences are in absolute size with no obvious functional consequences.
There are at least two potential limitations to this study. First, since the study was cross-sectional and there is the possibility of cohort effects that might have influenced the results. Furthermore, age is confounded with rearing experiences in the chimpanzees. Most of the oldest chimpanzees were wild-caught whereas most, if not all, of the younger individuals were captive born. Thus, it is possible, though unlikely, that variation in the rearing experiences may account for the results rather than age per se. However, given the limited number of significant age effects reported here, we are skeptical that removing the wild-caught individuals from the analyses would make any difference in the outcome of the results. Second, we used two different scanners in the study. Though we statistically controlled for this variable, ultimately scanning all the individuals with the same machine would have been ideal. Nonetheless, given the consistency of our findings with those previously reported in chimpanzees where scanner magnet strength was controlled (Chen et al., 2013), we do not believe that this is a significant limitation nor that this variable masked or influenced the results in any way that would alter the interpretation.
The results highlight the unusual characteristics of human aging and longevity, which seem to require an adaptive account such as the Grandmother Hypothesis (Hawkes, 2003). The Grandmother Hypothesis proposes that the short post-menopausal period of nonhuman primates allows them to avoid neurodegenerative diseases that are often seen in the later stages of life in humans. A key issue in defining post-menopausal life is, of course, determining when menopause occurs. Menopause in human females begins when there is a cessation of ovulation, which is then followed by a post-reproductive period, which has been shown to be about one third of their lifespans (LaCreuse et al., 2008; Atsalis & Videan, 2009). By contrast, though menopause occurs in many nonhuman primate species (Walker and Herndon, 2008), reproductive senescence coincides with the end of the lifespan, occurring at approximately the same time or shortly thereafter (Walker and Herndon, 2008). With specific regard to chimpanzees, though older females show reduction in reproductive fecundity (Roof et al., 2005), the complete cessation of ovulation and menses prior to death is rare (Alberts et al., 2013; Atsalis and Videan, 2009; Herndon and Lacreuse, 2009; Herndon et al., 2012; Lacreuse et al., 2008; Videan et al., 2006).
Beside differences in post-reproductive lifespan, it should also be noted that there are differences between chimpanzees and humans in life style, diet and exercise, factors known to influence age-related changes in cognition and cortical organization. For instance, by human standards, chimpanzee lifestyles and diets are much healthier comprising largely fruits and vegetables (Milton, 1999; Wrangham et al., 1991). Furthermore, chimpanzees are physically active throughout their lifespan and do not develop largely sedentary lives with increasing age, though activity levels can decrease. In humans, healthy lifestyles and physical activity has been shown to have a protective effect against the development of age-related decline in cognitive functions and cortical organization (Ahlskog et al., 2011; Elwood et al., 2013; Vivar et al., 2013). Therefore, these lifestyle factors may also contribute to the relatively healthy aging found in chimpanzees.
In summary, the results of this study show that chimpanzees exhibit few age-related changes in global cortical organization, sulci depth and sulci width. Elderly chimpanzees were found to have less gyrified brains than sub-adult and young-adult individuals but the size of this effect was relatively small with age accounting for only 5.1% of the variance. These results highlight the unusual character of human brain aging and longevity, which seem to require an adaptive account such as the Grandmother Hypothesis (Hawkes, 2003). Additionally, the lengthy period of brain decline characteristic of human aging might contribute to our vulnerability to AD and other age-related neurodegenerative diseases.
Highlights.
Tested tor potential age-related decline in cortical organization in chimpanzees.
Used BrainVisa to measure organization and folding in the cerebral cortex.
This is the single largest study examining age-related changes in chimpanzees.
Chimpanzees exhibit few age-nelated changes in global cortical organization.
Acknowledgments
This research was supported by NIH grants MH-92923, NS-42867, NS-73134,HD-60563 to WDH, NIA P01AG-026423 to JGH Cooperative Agreement RR-15090 to M.D. Anderson Cancer Center, and National Center for Research Resources P51RR000165 to YNPRC, which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132). We would like to thank Yerkes National Primate Research Center and the University of Texas MD Anderson Cancer Center and their respective veterinary and care staffs for assistance in MRI collection. American Psychological Association guidelines for the treatment of animals were followed during all aspects of this study. Inquires regarding this paper may be sent to: William D. Hopkins, Neuroscience Institute, Georgia State University, P.O. Box 5030, Atlanta, Georgia 30302-5030. Email: whopkins4@gsu.edu or whopkin@emory.edu
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Ohtomo K. Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiology of Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinical Proceddings. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts SC, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A, Stoinski TS, Strier KB, Morris WF, Bronikowski AM. Reproductive aging patterns in primates reveal that humans are distinct. Proceedings of the National Academy of Sciences. 2013;110:13440–13445. doi: 10.1073/pnas.1311857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Chen KC, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, Barnes CA. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. Journal of Neuroscience. 2008;28:2710–2718. doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. NeuroImage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Zilles K, Schleicher A. Cortical folding and the evolution of the human brain Journal of Human Evolution. 1993;20:341–348. [Google Scholar]
- Atsalis S, Videan E. Reproductive aging in captive and wild common chimpanzees: factors influencing the rate of follicular depletion. American Journal of Primatology. 2009;71:271–282. doi: 10.1002/ajp.20650. [DOI] [PubMed] [Google Scholar]
- Bailey P, von Bonin G, McCulloch WS. The isocortex of the chimpanzee. University of Illinois Press; Urbana-Champaign: 1950. [Google Scholar]
- Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: Debilitating effects on short-term memory. Journal of Gerontology. 1978;33:858–871. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- Bogart SL, Mangin JF, Schapiro SJ, Reamer L, Bennett AJ, Pierre PJ, Hopkins WD. Cortical sulci asymmetries in chimpanzees and macaques: A new look at an old idea. NeuroImage. 2012;61:533–541. doi: 10.1016/j.neuroimage.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XEB, Li L, Glasser MF, Westyle LT, Fjell AM, Walhovd KB, Hu X, Herndon JG, Preuss TM, Rilling JK. Brain aging in humans, chimpanzees (Pan troglodytes) and hresus macaques (Macaca mulatta): magnetic resonance images of macro- and microstructural changes. Neurobiology of Aging. 2013;34:2248–2260. doi: 10.1016/j.neurobiolaging.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood P, Galante J, Pickering J, Palmer S, Bayer A, Ben-Shlomo Y, Logngley M, Gallacher J. Healthy lifestyles reduce the incidence of chronic diseases and dementia: Evidence from athe Caerphilly cohort study. PLoS One. 2013;8:e81877. doi: 10.1371/journal.pone.0081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Austad SN. Primate aging in the mammalian scheme: the puzzle of extreme variation in brain aging. Age. 2012;34:1075–1091. doi: 10.1007/s11357-011-9355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Sapolsky RM. The evolution of Alzheimer's disease, the reproductive schedule, and apoE isoforms Neurobiology of Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: Volumetric MR imaging analysis. American Journal of Neuroradiology. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra SS. A beta40 is a major form of beta-amyloid in nonhuman primates. Neurobiology of Aging. 1996;17:903–908. doi: 10.1016/s0197-4580(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. NeuroImage. 2010;51:943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of aging in 465 normal adult brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Groeschel S, Vollmer B, King MD, Connelly A. Developmental chnages in cerebral grey and white matter volume form infancy to adulthood. Intenational Journal of Developmental Neuroscience. 2010;28:481–489. doi: 10.1016/j.ijdevneu.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cerebral Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, Morrison JH. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age. 2012;34:1051–1073. doi: 10.1007/s11357-011-9278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K. Grandmothers and the evolution of human longevity. The American Journal of Human Genetics. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- Herndon JG. The grandmother effect: Implications for studies on aging and cognition. Gerontology. 2009;56:73–79. doi: 10.1159/000236045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Lacreuse A. Reproductive aging in captive and wild common chimpanzees: Factors influencing the rate of follicular depletion. In: Atsalis S, Videan E, editors. American Journal of Primatology. Vol. 71. 2009. pp. 271–282. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Paredes J, Wilson ME, Bloomsmith MA, Chennareddi L, Walker ML. Menopause occurs late in life in the captive chimpanzee (Pan troglodytes) Age. 2012;34:1145–1156. doi: 10.1007/s11357-011-9351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. Brain weight throughout the life span of the chimpanzee. The Journal of Comparative Neurology. 1999;409:567–572. [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: Age-related patterns of surface area, thickness and gyrification. Cerebral Cortex; in press. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Phillips KA. Cross-sectional analysis of the association between age and corpus callosum size in chimpanzees (Pan troglodytes) Developmental Psychobiology. 2010;52:133–141. doi: 10.1002/dev.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Hopkins W. A comparative magnetic resonance imaging study of the anatomy, variability, and asymmetry of Broca's area in the human and chimpanzee brain. Journal of Neuroscience. 2009;29:14607–14616. doi: 10.1523/JNEUROSCI.2892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Tanemura K, Nakamura S, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes in Alzheimer's disease-associated proteins in cynomolgus monkey brains. Biochemical and Biophysical Research Communications. 2003;310:303–311. doi: 10.1016/j.bbrc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Rogers W, Mangin JF, Lancaster J. A library of cortical morphology analysis tools to study development, aging and genetics of cerebral cortex. Neuroinformatics. 2012;10:81–96. doi: 10.1007/s12021-011-9127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov PV, Mangin JF, Coyle T, Lancaster JL, Thompson P, Riviere D, Cointepas Y, Regis J, Schlosser A, Royall DR, Zilles K, Mazziotta J, Toga AW, Fox PT. Age-related morphology trends in cortical sulci. Human Brain Mapping. 2005;26:210–220. doi: 10.1002/hbm.20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BB, Schettler SP, Murray DE, Lee JM, Killiany RJ, Rosene DL, Kim DS, Ronen I. Age-related effects on cortical thickness patterns of the Rhesus monkey brain. Neurobiology of Aging. 2012;33:200.e223–200e.231. doi: 10.1016/j.neurobiolaging.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Chennareddi L, Gould KG, Hawkes K, Wijayawardana SR, Chen J, Easley KA, Herndon JG. Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes) Biology of Reproduction. 2008;79:407–412. doi: 10.1095/biolreprod.108.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Diehl MM, Goh MY, Hall MJ, Volk AM, Chhabra RK, Herndon JG. Sex differences in age-related motor slowing in the rhesus monkey: behavioral and neuroimaging data. Neurobiology of Aging. 2005;26:543–551. doi: 10.1016/j.neurobiolaging.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Russell JL, Hopkins WD, Herndon JG. Cognitive and motor aging in female chimpanzees. Neurobiology of Aging. 2014;35:623–632. doi: 10.1016/j.neurobiolaging.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of Aging. 2012;33:671.e671–617.e679. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome SE, Halderman LK, Otto R, Eckert MA, Chiarello C. Size matters: Cerebral volume influences sex differences in neuroanatomy. Cerebral Cortex. 2008;18:2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, DeLuca H, Jancke L, Toga AW. Gender effects on cortial thickness and the influence of scaling Human Brain Mapping. 2006;27:314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Steinmetz H, Jancke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002:2371–2374. doi: 10.1097/01.wnr.0000049603.85580.da. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW. Sex differences in brain anatomy. In: Savic I, editor. Sex differences in the human brain, their underpinnings and implications. Elsevier; Amsterdam: 2010. [Google Scholar]
- Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, Collins DL, Evans AC, Regis J. Object-based morphometry of the cerebral cortex. Medical Imaging. 2004;23:968–982. doi: 10.1109/TMI.2004.831204. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Chefer SI, Lane MA, Woolf RI, Morris ED, Ingram DK, Roth GS, London ED. Age-related decline in striatal volume in monkeys as measured by magnetic resonance imaging. Neurobiology of Aging. 2000;21:591–598. doi: 10.1016/s0197-4580(00)00134-2. [DOI] [PubMed] [Google Scholar]
- Matsumae M, Kikinis R, Morocz IA, Lorenzo AV, Sandor T, Albert MS, Black PM, Jolesz FA. Age-related changes in intracrannial compartment volumes in normal adults assessed by magnetic resonance imaging. Journal of Neurosurgery. 1996;84:982–991. doi: 10.3171/jns.1996.84.6.0982. [DOI] [PubMed] [Google Scholar]
- Milton K. Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us? Nutrition. 1999;15:488–498. doi: 10.1016/s0899-9007(99)00078-7. [DOI] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiology of Aging. 1988;9:495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiology of Aging. 2010;31:1020–1031. doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marchuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues in Clinical Neuroscience. 2001;3 doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Ageing and the brain. Postgraduate Medical Journal. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A qunatitative magnetic resonance imaging stufy of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC. Cortical development in brown capuchin monkeys: A structural MRI study. NeuroImage. 2008;43:657–664. doi: 10.1016/j.neuroimage.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre PJ, Hopkins WD, Taglialatela JP, Lees CJ, Bennett AJ. Age-related neuroanatomical differences from the juvenile period to adulthood in mother-reared macaques (Macaca radiata) Brain Research. 2008;1226:56–60. doi: 10.1016/j.brainres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Poduri A, Gearing M, Rebeck GW, Mirra SS, Tigges J, Hyman BT. Apolipoprotein E$ and beta amyloid in senile plaques and cerebral blood vessels of aged rhesus monkeys. The American Journal of Pathology. 1994;144:1183–1187. [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. The Journal of Neuroscience. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends in Neurosciences. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thorton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kochunov PV, Zilles K, Shelledy W, Lancaster JL, Thompson P, Duggirala R, Blangero J, Fox PT, Glahn DC. On the genetic architecture of cortical folding and brain volume in primates. NeuroImage. 2010;53:1103–1108. doi: 10.1016/j.neuroimage.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof KA, Hopkins WD, Izard MK, Hook M, Schapiro SJ. Maternal age, parity, and reproductive outcome in captive chimpanzees (Pan troglodytes) American Journal of Primatology. 2005;67:199–207. doi: 10.1002/ajp.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JDE. Variable effects of ageing on frontal lobe contributions to memory. Neuroreport. 2002;13:1425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psycholgical Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca's area homologue in great apes: Implication for language evolution. The Anatomical Record. 2003;217A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Gordon AD, Allen JS, Phillips KA, Erwin JM, Hof PR, Hopkins WD. Aging of the cerebral cortex differs between humans and chimpanzees. Proceedings of the National Academy of Sciences USA. 2011;108:13029–13034. doi: 10.1073/pnas.1016709108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S, Chen KS. Memory: Brain systems and behavior. Trends in Neurosciences. 1988;11:170–175. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age related decline in MRI volumes of temporal gray matter but not hippocampus. Neurobiology of Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. Correlations among brain gray matter volumes, age, and hemisphere in healthy individuals. PLoS One. 2011;6:e22734. doi: 10.1371/journal.pone.0022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MPJ, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cerebral Cortex. 2004;14:966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Videan EN, Fritz J, Heward CB, Murphy J. The effects of aging on hormone and reproductive cycles in female chimpanzees (Pan troglodytes) Comparative Medicine. 2006;56 [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. All About Running: Synaptic Plasticity, Growth Factors and Adult Hippocampal Neurogenesis. In: Belzung C, Wigmor P, editors. Neurogenesis and Neural Plasticity. Springer; Berlin: 2013. pp. 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilersten DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Walker ML, Herndon JG. Menopause in nonhuman primates. Biology of Reproduction. 2008;79:398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Whalley LT, Deary IJ, Apleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews. 2004;3:369–381. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wrangham RW, Conklin NL, Chapman CA, Hunt KD. The significance of fibrous foods for Kibale Forest chimpanzees. Philosophical Transactions of the Royal Society B: Biological Sciences. 1991;334:171–178. doi: 10.1098/rstb.1991.0106. [DOI] [PubMed] [Google Scholar]
- Xu J, Kobayashi S, Yamaguchi S, Lijima K, Okada K, Yamashita K. Gender effects on age-related changes in brain structure. American Journal of Neuroradiology. 2000;21:112–118. [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain, Behavior and Evolution. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]


