Abstract
Background
The impact of palmoplantar psoriasis on health-related quality of life (QoL) is largely unknown.
Objective
To compare clinical characteristics and patient-reported outcomes between patients with palmoplantar psoriasis and moderate-to-severe plaque psoriasis.
Methods
We conducted a cross-sectional study of patients with plaque psoriasis (N=1,153) and palmoplantar psoriasis (N=66) currently receiving systemic or light treatment for psoriasis.
Results
Patients with palmoplantar psoriasis were more likely to report Dermatology Life Quality Index scores that correspond to at least a moderate impact on QoL (odds ratio [OR] 2.08; 95% confidence interval [CI], 1.20-3.61); problems with mobility (OR 1.98; 95% CI, 1.10-3.58), self-care (OR 3.12; 95% CI, 1.24-7.86), and usual activities (OR 2.47; 95% CI, 1.44-4.22) on the European Quality of Life-5 Dimensions questionnaire; and heavy topical prescription use of at least twice daily in the preceding week (OR 2.81; 95% CI, 1.63-4.85) than those with plaque psoriasis.
Limitations
Our assessment tools may not account for all dimensions of health-related QoL affected by palmoplantar disease, and these results may not be generalizable to patients with milder forms of psoriasis.
Conclusion
Patients with palmoplantar psoriasis suffer from greater health-related QoL impairment and are more likely to report heavy use of topical prescriptions than those with moderate-to-severe plaque psoriasis.
Keywords: Psoriasis, palmoplantar psoriasis, plaque psoriasis, health-related quality of life, patient-reported outcomes, epidemiology
Introduction
Psoriasis is a chronic inflammatory disease that affects 2-4% of the population worldwide.1, 2 It is associated with a higher risk of cardiovascular,3-6 metabolic,7 and renal disease,8 and patients may experience significant impairment of health-related quality of life (HRQoL) even with localized disease.9-20 Palmoplantar psoriasis (psoriasis localized to the palms and/or soles) is reported to affect approximately 5% of all psoriasis patients, and although it is a disabling and difficult-to-treat variant of psoriasis, its epidemiology is poorly defined and few studies have evaluated its impact on patient-reported outcomes.21-36 A study that surveyed 579 psoriasis patients found that palmoplantar psoriasis (n=124, 39%) causes greater physical disability than psoriasis without palm and sole involvement; however, no differences were observed in psychological distress, HRQoL, and global quality of life (QoL).21 Data on potential confounders such as treatment information and co-morbidities were not available. Hence, there still exists a substantial need to augment our understanding of the impact of palmoplantar psoriasis on patients’ subjective well-being.
The purpose of this study was to compare patient-reported outcomes and clinical characteristics between patients with plaque and palmoplantar psoriasis who were evaluated during routine follow-up and were receiving systemic or light therapy for their psoriasis at the time of data collection. We hypothesized that patients with palmoplantar psoriasis would have a lower HRQoL and report a greater negative impact of their skin disease on their lives than patients with plaque psoriasis.
Methods
Study design
We conducted a descriptive, cross-sectional study to determine the impact of plaque or palmoplantar psoriasis on patients’ HRQoL and their use of prescription topical medications. Consecutive patients being seen by their dermatology providers for routine follow-up care were enrolled, and data were collected using dermatologist assessments and patient questionnaires.37 The study was approved by the Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients.
Setting
Data were collected by 10 dermatologists and 2 physician assistants from 1755 patients seen at 10 dermatology sites across the United States participating in the Dermatology Clinical Effectiveness Research Network (DCERN). Data were collected prospectively at a single regularly scheduled clinic appointment per patient, from February 2010 through June 2011, at 2 academic centers (University of Pennsylvania and University of Utah, each with a hospital-based site and a community-based site) and 6 private practices in Georgia, Pennsylvania, New York, and Colorado.37
Participants
Patients were enrolled consecutively under broad inclusion criteria, as previously described.37 Participants were eligible if they were currently receiving or previously received systemic or light therapy for psoriasis, or had a history of at least 5% body surface area (BSA) involvement.37 New patients became eligible at their next regular visit.37 In the analyses presented herein, we included patients who were currently receiving systemic or light therapy for a primary indication of plaque or palmoplantar psoriasis as defined by the treating clinician. We excluded patients whose indication for treatment was another variant of psoriasis.
Variables
Data were collected by study coordinators using standardized forms. Detailed information was collected on medical and social history, psoriasis treatments, socio-demographic factors, and psoriasis characteristics.37 Patient-reported data were confirmed using the dermatology clinic record and clinical assessments. Estimated total BSA involvement (%) and the Physician Global Assessment (PGA) scale (scored 0-5 for erythema, induration, and scaling, then averaged) were used by clinicians as measures of severity.38 Primary psoriasis treatment indication served as the main exposure while other variables served as potential confounders or effect modifiers. The patient-reported outcomes were the Dermatology Life Quality Index (DLQI),39 the European Quality of Life-5 Dimensions (EQ-5D),40 and the frequency of concurrent prescription topical medication use. The DLQI is a 10-question validated questionnaire that measures the degree to which a patient’s life is affected by his/her skin condition (i.e., psoriasis), and the score ranges from 0 to 30 with higher scores indicating greater QoL impairment. The DLQI score was dichotomized into two categories: 0-5 indicating no to small impact and 6-30 indicating moderate to extremely large impact on QoL. The EQ-5D provides a generic measure of HRQoL using a descriptive profile consisting of five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) with three levels per dimension (no problems, some/moderate problems, and extreme problems). We grouped the responses into two categories: no problems and any problems. We also calculated the U.S. population-based EQ-5D index score, which was dichotomized to below and above the sample population median.41, 42 The EQ-5D also includes a single value for self-perceived health state on a scale of 0 to 100 (the visual analog scale, or VAS), with 100 being the best imaginable state. Lastly, patients were asked how often they had used topical prescription medications in the week prior to data collection, and heavy use (daily use with ≥ 2 applications per day) was assessed as an outcome.
Statistical analysis
Descriptive statistics were used to summarize demographics and clinical characteristics. Univariate analyses were conducted using Student’s t-tests or Wilcoxon rank-sum tests for continuous variables, and χ2 or Fisher’s exact tests for categorical variables. Subjects with missing values (1.4%) were omitted from the analysis.
For multivariable analyses, logistic regression models were fitted for the dichotomized outcomes DLQI, EQ-5D index score, EQ-5D dimensions, and topical prescription use. A quantile regression model was fitted for the EQ-5D VAS outcome. Potential confounders were selected using a purposeful selection approach based on significant associations with both exposure and outcome (p<0.25).43 We reached our final model using a backwards elimination approach, retaining predetermined confounders (age and sex) and significant covariates (p<0.05 on the Wald test or changing the effect of interest by >10%). Model fit was assessed using the Hosmer-Lemeshow goodness-of-fit test, and adjustments were made for multiple comparisons using the Holm-Bonferroni procedure.44 The secondary analyses involving the EQ-5D domains were not adjusted for multiplicity because they were primarily exploratory. We also performed multiple sensitivity analyses: first, we included all 1600 plaque and palmoplantar psoriasis patients enrolled in DCERN regardless of current treatment; second, we excluded patients with overlapping disease (23 plaque psoriasis patients with palmoplantar involvement [2.0%] and 20 palmoplantar psoriasis patients with features of generalized plaque disease [30%]); and third, we adjusted all outcomes for type of current treatment (biologic, oral systemic, or phototherapy).
Results
We included 1153 plaque and 66 palmoplantar psoriasis patients currently receiving systemic or light therapy in our study (Figure 1). Their demographic information and clinical characteristics are shown in Table I. Patients with palmoplantar psoriasis were older than patients with plaque psoriasis with mean ages (standard deviation) of 53.8 years (12.6) vs. 48.7 years (15.2) (p=0.007) respectively, and were more likely to be female (75.8% vs. 48%; p<0.001) and to be current or past smokers (74.2% vs. 51.4%; p=0.001) than patients with plaque psoriasis.
Figure 1.
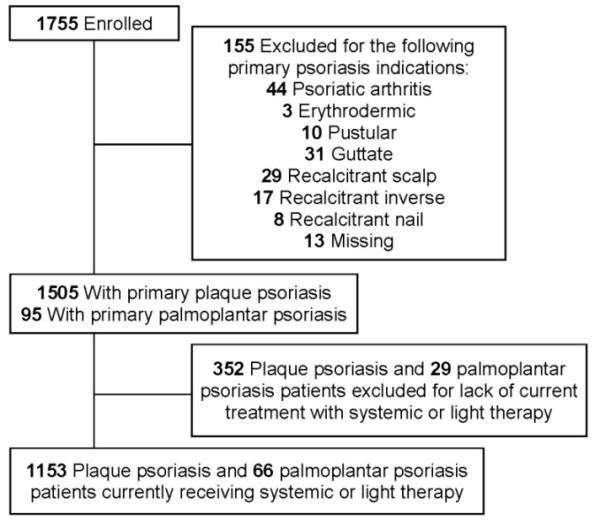
Flow diagram of patient inclusion.
Table I.
Baseline and psoriasis characteristics of patients with plaque versus palmoplantar psoriasis.
| Characteristic | Plaque Psoriasis (N=1153) |
Palmoplantar Psoriasis (N=66) |
P-value |
|---|---|---|---|
|
| |||
| Age, year | |||
| Mean (SD) | 48.7 (15.2) | 53.8 (12.6) | 0.007 a |
| Median (IQR) | 49 (38, 59) | 54.5 (46, 62) | 0.005 b |
|
| |||
| Female sex, N (%) | 554 (48) | 50 (75.8) | <0.001 c |
|
| |||
| Smoking status, N (%) | 0.001 c | ||
| Current smoker | 198 (17.2) | 16 (24.2) | |
| Past smoker | 394 (34.2) | 33 (50) | |
| Never smoked | 561 (48.7) | 17 (25.8) | |
|
| |||
| Current drinking status, N (%) | 0.69 c | ||
| Heavy d | 53 (4.6) | 4 (6.1) | |
| Moderate | 741 (64.3) | 40 (60.6) | |
| None in last year | 359 (31.1) | 22 (33.3) | |
|
| |||
| BMI, median (IQR) | 28.9 (25.2, 33.3) | 30.1 (26.2, 34.3) | 0.35 b |
|
| |||
| Total number of co-morbidities, median (IQR) |
2 (1, 4) | 3 (1, 4) | 0.024 b |
|
| |||
| Practice setting of dermatologist, N (%) |
0.009 c | ||
| Academic | 688 (59.7) | 50 (75.8) | |
| Private | 465 (40.3) | 16 (24.2) | |
|
| |||
| Age of psoriasis onset, median (IQR) | 25 (16, 40) | 44 (34, 53) | <0.001 b |
|
| |||
| Psoriasis severity at its worst, N (%) | <0.001 c | ||
| Mild, <3 palms | 109 (9.5) | 19 (29.2) | |
| Moderate, 3-10 palms | 362 (31.4) | 34 (52.3) | |
| Severe, >10 palms | 682 (59.2) | 12 (18.5) | |
|
| |||
| Family history, N (%) | 0.34 c | ||
| Yes | 481 (45.8) | 22 (38.6) | |
| No | 570 (54.2) | 35 (61.4) | |
|
| |||
| Median duration of psoriasis, y (IQR) | 19 (8, 30) | 6.5 (2, 19) | <0.001 b |
|
| |||
| Psoriatic arthritis diagnosed by a physician, N (%) |
298 (25.9) | 14 (21.2) | 0.47 c |
|
| |||
| Total body surface area involved (BSA), % |
|||
| Median (IQR) | 2.5 (0.8, 6) | 1 (0.3, 2.1) | <0.001 b |
|
| |||
| Physician Global Assessment (PGA) | |||
| Median (IQR) | 1.67 (1, 2) | 1.33 (1, 2) | 0.68 b |
|
| |||
| Type of current treatment e | |||
|
| |||
| Biologic, N (%) | 643 (55.8) | 29 (43.9) | 0.074 c,f |
| Adalimumab | 224 (19.4) | 6 (9.1) | |
| Etanercept | 245 (21.3) | 17 (25.8) | |
| Infliximab | 86 (7.5) | 1 (15) | |
| Alefacept | 1 (0.1) | 0 (0) | |
| Ustekinumab | 87 (7.6) | 5 (7.6) | |
|
| |||
| Oral systemic, N (%) | 429 (37.2) | 36 (54.6) | 0.006 c,f |
| Methotrexate | 316 (27.4) | 14 (21.2) | |
| Cyclosporine | 30 (2.6) | 5 (7.6) | |
| Acitretin | 73 (6.3) | 19 (28.8) | |
| Intramuscular steroids | 3 (0.3) | 0 (0) | |
| 6-thioguanine/Other | 21 (1.8) | 3 (4.5) | |
|
| |||
| Phototherapy, N (%) | 335 (29.1) | 13 (19.7) | 0.12 c,f |
| Non-prescribed light g | 153 (13.3) | 5 (7.6) | |
| UVB h | 182 (15.8) | 1 (15) | |
| PUVA (oral and topical) | 5 (0.7) | 6 (9.1) | |
| Excimer laser | 24 (2.1) | 1 (1.5) | |
|
| |||
| Number of current treatments, N (%) | 0.45 c | ||
| 1 | 875 (75.9) | 51 (77.3) | |
| 2 | 260 (22.6) | 13 (19.7) | |
| >2 | 18 (1.5) | 2 (3.0) | |
Significant values with P < 0.05 are shown in bold.
Student’s t-test
Wilcoxon rank-sum test
Fisher’s exact
>2 Drinks per day for men and >1 drink per day for women.
Proportions add up to >100 because of patients who are on multiple therapies.
The total proportions of patients in each category of treatment (biologic, oral systemic or phototherapy) were used for comparison.
Includes natural sunlight and tanning beds.
Includes UVB, broadband UVB, narrowband UVB, and home UVB.
Regarding psoriasis characteristics, patients with palmoplantar disease had a later median (interquartile range [IQR]) age of psoriasis onset (44 years [34, 53] vs. 25 years [16, 40]; p<0.001) and lower median (IQR) BSA involvement at the time of data collection (1% [0.3%, 2.1%] vs. 2.5% [0.8%, 6%]; p<0.001) than patients with plaque psoriasis. With respect to current treatments, a higher percentage of palmoplantar vs. plaque psoriasis patients were on oral systemic therapy (55% vs. 37%; p=0.006), with no significant differences in biologics or phototherapy. Of those receiving oral systemic therapy, 28.8% of palmoplantar psoriasis patients vs. 6.4% of plaque psoriasis patients were on acitretin, which is a widely used therapy for the pustular variant of palmoplantar psoriasis (i.e., palmoplantar pustulosis).27, 45, 46 There were no significant differences in family history of psoriasis, prevalence of psoriatic arthritis, and PGA scores between the two groups.
The unadjusted outcome analyses are shown in Table II. There were no significant differences in the levels of pain/discomfort, anxiety/depression, and self-perceived health state between the two groups. The significantly different outcomes (DLQI, EQ-5D, and heavy topical prescription use) were further analyzed using adjusted regression models (Table III). Of note, the adjusted odds of reporting at least a moderate impact on skin-specific HRQoL (DLQI>5) were 2.08 (95% confidence interval [CI], 1.20-3.61) times higher for patients with palmoplantar psoriasis compared to those with plaque psoriasis. Patients with palmoplantar and plaque psoriasis were equally likely to report general HRQoL greater than the 50th percentile (EQ-5D index score > median) (odds ratio [OR] 0.88; 95% CI, 0.51-1.51). The overall trend of palmoplantar psoriasis patients having lower EQ-5D scores than plaque psoriasis patients in unadjusted analyses was preserved in adjusted analyses (median [IQR] scores of 0.83 [0.75, 1.0] vs. 0.84 [0.80, 1.0], respectively; p=0.02), but this may not be clinically significant given that the minimally important difference in EQ-5D has been reported as 0.05.47
Table II.
Patient-reported outcomes in patients with plaque psoriasis versus palmoplantar psoriasis.
| Plaque psoriasis (N=1153) |
Palmoplantar Psoriasis (N=66) |
P-value | |
|---|---|---|---|
|
| |||
| DLQI | |||
|
| |||
| DLQI, median (IQR) | 3 (1, 6) | 4 (1, 9) | 0.037 a |
|
| |||
| DLQI category, N (%) | 0.004 b | ||
| 0-5 No-small effect | 839 (73.5) | 37 (56.1) | |
| 6-30 Moderate-extremely large effect | 302 (26.5) | 29 (43.9) | |
|
| |||
| EQ-5D | |||
|
| |||
| EQ-5D index (U.S.) median (IQR) |
0.84 (0.80, 1.0) | 0.83 (0.75, 1.0) | 0.022 a |
|
| |||
| EQ-5D Mobility c, N (%) | 0.027 b | ||
| No problems | 918 (79.8) | 44 (67.7) | |
| Problems | 232 (20.2) | 21 (32.3) | |
|
| |||
| EQ-5D Self-care c, N (%) | 0.019 b | ||
| No problems | 1094 (95.0) | 57 (87.7) | |
| Problems | 57 (5.0) | 8 (12.3) | |
|
| |||
| EQ-5D Usual Activities c, N (%) | <0.001 b | ||
| No problems | 949 (82.5) | 40 (61.5) | |
| Problems | 201 (17.5) | 25 (38.5) | |
|
| |||
| EQ-5D Pain/Discomfort c, N (%) | 0.053 b | ||
| No problems | 659 (57.3) | 29 (44.6) | |
| Problems | 492 (42.7) | 36 (55.4) | |
|
| |||
| EQ-5D Anxiety/Depression c, N (%) | 0.21 b | ||
| No problems | 817 (71) | 41 (63.1) | |
| Problems | 334 (29) | 24 (36.9) | |
|
| |||
| EQ-5D VAS score | |||
| Mean (SD) | 78.1 (18.3) | 74.3 (19.3) | 0.098 d |
| Median (IQR) | 80 (70, 90) | 80 (70, 90) | 0.072 a |
|
| |||
| Topical prescription use in the last week | |||
|
| |||
| Median number of days used (IQR) | 3 (0, 7) | 7 (1, 7) | 0.001 a |
|
| |||
| Number of patients who reported no use, N (%) |
416 (36.1) | 15 (22.7) | 0.033 b |
|
| |||
| Number of patients who reported heavy use (daily use with ≥ 2 applications per day), N (%) |
158 (13.7) | 23 (34.9) | <0.001 b |
Significant values with P < 0.05 are shown in bold.
Wilcoxon rank-sum test
Fisher’s exact
Of the three possible responses for each EQ-5D dimension (no problems/some or moderate problems/severe problems), we combined responses to the moderate and severe categories to form two levels: no problems and problems.
Student’s t-test
Table III.
Patient-reported outcomes for palmoplantar psoriasis patients compared to plaque psoriasis patients.
| Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
Adjusted P-value |
Adjusted P- value for Multiple Comparisons a |
|
|---|---|---|---|---|
|
DLQI score 6-30
(Moderate-Extremely Large impact on HRQoL) |
2.18 (1.32-3.60) | 2.08 (1.20-3.61) b | 0.009 | 0.036 |
|
EQ-5D index score
> median |
0.68 (0.41-1.14) | 0.88 (0.51-1.51) c | 0.63 | 0.63 |
|
Problems with
Mobility (EQ-5D) |
1.89 (1.10-3.24) | 1.98 (1.10-3.58) d | 0.023 | |
|
Problems with Self-
Care (EQ-5D) |
2.69 (1.23-5.92) | 3.12 (1.24-7.86) e | 0.016 | |
|
Problems with Usual
Activities (EQ-5D) |
2.95 (1.75-4.98) | 2.47 (1.44-4.22) f | 0.001 | |
|
Heavy prescription topical use in the last week g |
3.37 (1.98-5.74) | 2.81 (1.63-4.85) h | 0.0002 | 0.001 |
Significant values with P < 0.05 are shown in bold.
Adjusted for multiple comparisons using the Holm-Bonferroni procedure.
Adjusted for age, sex, ethnicity, site, number of co-morbidities, psoriasis duration, and psoriasis severity at its worst.
Adjusted for age, sex, number of co-morbidities, smoking status, and marital status.
Adjusted for age, sex, marital status, number of co-morbidities, smoking status, and psoriasis severity at its worst.
Adjusted for age, sex, number of co-morbidities, psoriasis severity at its worst, and age of psoriasis onset.
Adjusted for age, sex, smoking status, number of co-morbidities.
Daily use with at least two applications per day.
Adjusted for age, sex, and type of therapy.
Exploratory multivariable regression analyses revealed that patients with palmoplantar psoriasis were significantly more likely to report problems with mobility (OR 1.98; 95% CI, 1.10-3.58), self-care (OR 3.12; 95% CI, 1.24-7.86), and usual activities (OR 2.47; 95% CI, 1.44-4.22). Finally, the odds of heavy topical prescription use were 2.81 (95% CI, 1.63-4.85) times higher for patients with palmoplantar psoriasis than those with plaque psoriasis. The findings were robust to multiple sensitivity analyses (Table IV).
Table IV.
Adjusted odds ratios and 95% confidence intervals for sensitivity analyses.
| Primary analysis |
Sensitivity Analyses | |||
|---|---|---|---|---|
| Excluding patients with overlapping features |
Including all patients regardless of current treatment |
Adjusted for type of therapy a |
||
| N | 1219 | 1176 | 1600 | 1219 |
|
DLQI score 6-30
(Moderate- Extremely Large impact on HRQoL) |
2.08 (1.20-3.61) |
2.78 (1.45-5.33) |
1.65 (1.03-2.63) |
2.19 (1.25- 3.83) |
|
EQ-5D index score
> median |
0.88 (0.51-1.51) |
0.88 (0.46-1.69) |
0.81 (0.50- 1.30) |
0.87 (0.51-1.51) |
|
Problems with
Mobility (EQ-5D) |
1.98 (1.10-3.58) |
2.40 (1.20- 4.82) |
1.90 (1.13- 3.20) |
1.87 (1.04- 3.39) |
|
Problems with Self-
Care (EQ-5D) |
3.12 (1.24-7.86) |
5.38 (1.95- 14.8) |
2.86 (1.17- 6.98) |
1.87 (1.23- 7.65) |
|
Problems with Usual
Activities (EQ-5D) |
2.47 (1.44-4.22) |
3.27 (1.76- 6.06) |
2.17 (1.35- 3.48) |
2.42 (1.39- 4.23) |
|
Heavy prescription
topical use in the last week |
2.81 (1.63-4.85) |
3.54 (1.89-6.66) |
2.50 (1.58-3.94) |
N/A b |
Biologic, oral systemic or light therapy.
Not applicable; primary analysis was adjusted for type of therapy.
Discussion
We found that palmoplantar psoriasis is associated with substantial impairment of HRQoL. Specifically, compared to moderate-to-severe plaque psoriasis, palmoplantar psoriasis is independently associated with a greater impact on skin-related QoL; a greater impairment of mobility, self-care, and usual activities; and a greater dependency on topical medications. Notably, palmoplantar psoriasis patients reported more difficulty with activities of daily living while no differences were observed for general pain/discomfort and anxiety/depression. This may suggest that the discomfort experienced by the palmoplantar group is related to psoriasis affecting locations that are crucial for function, whereas patients with plaque psoriasis and more extensive disease may experience similar levels of overall discomfort because of factors not captured by the EQ-5D. In terms of dermatologist assessments, the fact that there was no difference in PGA scores suggests that while palmoplantar patients typically have lower BSA involvement (usually <5%),48 there is no significant difference in the composite of erythema, scaling, and induration.
Our findings are consistent with the few epidemiological studies on palmoplantar psoriasis,49-54 and build upon the study of Pettey et al, which found that palmoplantar involvement causes more physical disability as measured by the Psoriasis Disability Scale, but found no differences in generic HRQoL using the Short Form-36 Health Survey.21 Like Pettey et al,21 we also observed a significant difference between plaque and palmoplantar psoriasis patients using a dermatology-specific measure (DLQI), without differences in generic HRQoL (EQ-5D index). However, our participation rate was considerably higher (95% vs. 54.7%), and the DLQI and EQ-5D have better correlations with clinical endpoints than the SF-36 and are used more frequently in the literature than the PDS.55
Our study has several strengths. To our knowledge, this is the first multi-center study to assess differences in patient-reported outcomes between plaque and palmoplantar psoriasis patients. It includes patients who were seen by general dermatologists and psoriasis specialists, and we minimized selection bias by consecutively including routine patients at a high participation rate. We had clinicians determine the primary exposure to reduce misclassification bias, and we adjusted for many potential confounders in our primary and sensitivity analyses, including age, sex, number of co-morbidities, psoriatic arthritis, education, employment, smoking/drinking history, marital status, psoriasis severity, age of psoriasis onset, and type of therapy. We minimized type I errors by adjusting for multiple comparisons for all outcomes other than exploratory analyses.
Important limitations to consider include the possibility of remaining unmeasured or unknown confounders. Moreover, because only patients currently receiving systemic or light therapy were included, our results may not be generalizable to patients with milder forms of psoriasis. Misclassification of the exposure may have also occurred, given the possibility of clinical overlap between plaque and palmoplantar psoriasis. However, when we performed sensitivity analyses excluding patients with overlapping features to reduce potential misclassification, we observed stronger associations, suggesting that such misclassification is more likely to bias our results towards the null. Additionally, since there is no universal method for evaluating HRQoL in palmoplantar psoriasis, it is possible that the tools we used (DLQI and EQ-5D) do not account for all dimensions of HRQoL affected by palmoplantar disease. The DLQI is used frequently in psoriasis studies, but has not been validated in palmoplantar psoriasis. Similarly, the EQ-5D was developed for general medical conditions and may not capture all relevant aspects of dermatologic disease.10 Other QoL assessment tools that were suggested for use in palmoplantar psoriasis have not been validated.22
There is also a lack of effective tools to compare disease severity across different variants of psoriasis. Existing severity measures such as BSA involvement and the PGA were developed for plaque psoriasis, and may be inadequate for assessing palmoplantar psoriasis severity by failing to capture features that are specific to palmoplantar disease, such as the presence of pustules, fissures, or edema. Moreover, since this is a descriptive study with a relatively small sample size, it may not have been powered to detect differences that may exist in the EQ-5D dimensions of pain/discomfort and anxiety/depression.
Finally, we evaluated palmoplantar psoriasis as a single disease and did not make distinctions between its two phenotypes, palmoplantar plaque psoriasis and palmoplantar pustulosis. While these two types overlap clinically, some evidence suggests that palmoplantar pustulosis may be a genetically distinct form of psoriasis.51, 56, 57 In this study we focused on investigating the effects that anatomical involvement of the palms and soles can have on QoL, but future endeavors to compare patient-reported outcomes between the two subtypes of palmoplantar psoriasis may provide further insight.
Conclusions
In palmoplantar psoriasis, there appears to be a clear disconnect between severity measured using traditional psoriasis assessment tools and impact of the disease on patients’ HRQoL. Our study shows that even with less BSA involvement and similar PGA scores, patients with palmoplantar disease are more likely to suffer from a significant impact on skin-related QoL, have problems with activities of daily living, and rely on topical prescription medications than patients with moderate-to-severe plaque psoriasis. Clinicians should pay particular attention to level of functional impairment rather than relying on traditional instruments to evaluate severity, and be aware of a greater tendency for heavy topical use in this patient group, even in those who are on other treatments. Further research is warranted to confirm our findings and examine the validity of applying existing HRQoL measures to palmoplantar psoriasis.
Capsule summary.
Palmoplantar psoriasis is a disabling variant of psoriasis that primarily affects the palms and soles.
Patients with palmoplantar psoriasis suffer from greater health-related quality of life impairment than those with moderate-to-severe plaque psoriasis.
Clinicians should pay particular attention to functional impairment when treating palmoplantar psoriasis.
Acknowledgments
We would like to acknowledge Dermatology Clinical Effectiveness Research Network (DCERN) investigators including Drs. Jamie Weisman, Stephen M. Schleicher, Robert E. Kalb, Brian R. Sperber, Michael B. Stierstorfer, and Bruce A. Brod for their contribution to data collection.
Funding/Support: This study was supported by grant RC1-AR058204 and K24-AR064310 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Dr. Gelfand), Dermatology Foundation Career Development Award (Takeshita), T32-AR07465 (Shin) from the National Institutes of Health, and an unrestricted grant from Eli Lilly.
Abbreviations and acronyms
- BMI
Body mass index
- BSA
Body surface area
- CI
Confidence interval
- DCERN
Dermatology Clinical Effectiveness Research Network
- DLQI
Dermatology Life Quality Index
- EQ-5D
European Quality of Life-5 Dimensions
- HRQoL
Health-related quality of life
- IQR
Interquartile range
- OR
Odds ratio
- PASI
Psoriasis Area and Severity Index
- PGA
Physician Global Assessment
- QoL
Quality of life
- SD
Standard deviation
- VAS
Visual analog scale (part of EQ-5D)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Jina Chung had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Chung, Gelfand, Callis Duffin, and Krueger. Acquisition of data: Gelfand, Callis Duffin, Krueger, Shin, and Van Voorhees. Analysis and interpretation of data: Chung, Gelfand, Shin and Edson-Heredia. Drafting of the manuscript: Chung, Gelfand and Callis Duffin. Critical revision of the manuscript for important intellectual content: Chung, Takeshita, Callis Duffin, Krueger, Robertson, Shin, Troxel, Van Voorhees, Edson-Heredia and Gelfand. Statistical analysis: Chung, Shin, and Troxel. Obtained funding: Gelfand and Callis Duffin. Administrative, technical, or material support: Robertson and Shin. Study supervision: Gelfand, Krueger.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study, in the collection and management of the data, or in the preparation of the manuscript. An author from Eli Lilly participated in data analysis and interpretation, manuscript review, and decision to submit the manuscript. None of the other sponsors participated in data analysis/interpretation, review, or decision to submit the manuscript.
Financial Disclosure: Dr. Callis Duffin was an investigator, consultant, and/or speaker for AbbVie, Amgen, ApoPharma, Bristol-Myers Squibb, Celgene, Eli Lilly, Genzyme, Incyte, Janssen Biotech, Novo Nordisk, Pfizer, and Wyeth, receiving honoraria and/or salary; served on the advisory board of Amgen; and received residency/fellowship program funding from AbbVie and Amgen. Dr. Krueger served as a consultant for AbbVie, Amgen, and Janssen Biotech; had grants or has pending grants from AbbVie and Amgen; and received payment for lectures and travel-related expenses from AbbVie, Amgen, and Janssen Biotech. Dr. Robertson is employed by the National Psoriasis Foundation, which receives unrestricted financial support from companies that make products used to treat psoriasis and psoriatic arthritis, including AbbVie, Amgen, Celgene, Eli Lilly, Galderma Laboratories, L.P., Janssen Biotech, Leo Pharma, Novartis, Pfizer, and Stiefel, a GSK company. Dr. Robertson has also served as an uncompensated member of advisory boards at AbbVie and Merck. Dr. Van Voorhees served on advisory boards for Amgen, AbbVie, Genentech, Warner Chilcott, Leo, and Janssen Biotech; served as an investigator for Amgen, AbbVie, receiving grants; served as a consultant for Amgen. E. Edson-Heredia is a full time employee and stockholder of Eli Lilly. Dr. Gelfand served as a consultant for AbbVie, Amgen, Eli Lilly, Merck, Janssen Biotech, Novartis, and Pfizer, receiving honoraria; had grants or has pending grants from AbbVie, Amgen, Genentech, Novartis, Eli Lilly, and Pfizer; and received payment for continuing medical education work related to psoriasis.
References
- 1.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60(2):218–24. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–85. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 3.Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–9. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006;296(14):1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 5.Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–8. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–6. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 8.Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. Bmj. 2013;15(347) doi: 10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr., Reboussin DM. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology. 1999;41(3 Pt 1):401–7. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–4. [PubMed] [Google Scholar]
- 12.Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PLoS One. 2012;7(12):28. doi: 10.1371/journal.pone.0052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35. doi: 10.1186/1477-7525-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23(4):681–94. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Fortune DG, Main CJ, O’Sullivan TM, Griffiths CE. Quality of life in patients with psoriasis: the contribution of clinical variables and psoriasis-specific stress. Br J Dermatol. 1997;137(5):755–60. [PubMed] [Google Scholar]
- 16.Finlay AY, Coles EC. The effect of severe psoriasis on the quality of life of 369 patients. Br J Dermatol. 1995;132(2):236–44. doi: 10.1111/j.1365-2133.1995.tb05019.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiss SC, Kimball AB, Liewehr DJ, Blauvelt A, Turner ML, Emanuel EJ. Quantifying the harmful effect of psoriasis on health-related quality of life. J Am Acad Dermatol. 2002;47(4):512–8. doi: 10.1067/mjd.2002.122755. [DOI] [PubMed] [Google Scholar]
- 18.Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin. 1996;14(3):485–96. doi: 10.1016/s0733-8635(05)70376-4. [DOI] [PubMed] [Google Scholar]
- 19.Ginsburg IH, Link BG. Feelings of stigmatization in patients with psoriasis. J Am Acad Dermatol. 1989;20(1):53–63. doi: 10.1016/s0190-9622(89)70007-4. [DOI] [PubMed] [Google Scholar]
- 20.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–9. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 21.Pettey AA, Balkrishnan R, Rapp SR, Fleischer AB, Feldman SR. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49(2):271–5. doi: 10.1067/s0190-9622(03)01479-8. [DOI] [PubMed] [Google Scholar]
- 22.Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60(6):1024–31. doi: 10.1016/j.jaad.2008.11.910. [DOI] [PubMed] [Google Scholar]
- 23.Elahmed HH. Rapid improvement of palmoplantar psoriasis after cessation of smoking. Sultan Qaboos Univ Med J. 2013;13(1):188–9. doi: 10.12816/0003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adisen E, Tekin O, Gulekon A, Gurer MA. A retrospective analysis of treatment responses of palmoplantar psoriasis in 114 patients. J Eur Acad Dermatol Venereol. 2009;23(7):814–9. doi: 10.1111/j.1468-3083.2009.03197.x. [DOI] [PubMed] [Google Scholar]
- 25.Guenther LC. Alefacept is safe and efficacious in the treatment of palmar plantar pustulosis. J Cutan Med Surg. 2007;11(6):202–5. doi: 10.2310/7750.2007.00036. [DOI] [PubMed] [Google Scholar]
- 26.Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007 Jan;156(1):178–80. doi: 10.1111/j.1365-2133.2006.07571.x. [DOI] [PubMed] [Google Scholar]
- 27.Schroder K, Zaun H, Holzmann H, Altmeyer P, el-Gammal S. Pustulosis palmo-plantaris. Clinical and histological changes during etretin (acitretin) therapy. Acta Derm Venereol Suppl. 1989;146:111–6. [PubMed] [Google Scholar]
- 28.Al-Mutairi N, Joshi A, Nour-Eldin O. Punctate palmoplantar keratoderma (Buschke-Fischer-Brauer disease) with psoriasis: a rare association showing excellent response to acitretin. J Drugs Dermatol. 2005;4(5):627–34. [PubMed] [Google Scholar]
- 29.Ettler K, Richards B. Acitretin therapy for palmoplantar pustulosis combined with UVA and topical 8-MOP. Int J Dermatol. 2001 Aug;40(8):541–2. doi: 10.1046/j.1365-4362.2001.01094-3.x. [DOI] [PubMed] [Google Scholar]
- 30.Rivard J, Janiga J, Lim HW. Tacrolimus ointment 0.1% alone and in combination with medium-dose UVA1 in the treatment of palmar or plantar psoriasis. J Drugs Dermatol. 2006;5(6):505–10. [PubMed] [Google Scholar]
- 31.Cohen DJ, Scherschun L. Case reports: practical experience with efalizumab in hand and foot psoriasis. J Drugs Dermatol. 2007;6(12):1224–30. [PubMed] [Google Scholar]
- 32.Kircik L. Treatment of hand and foot psoriasis with emphasis on efalizumab. Skin Therapy Lett. 2007;12(9):4–7. [PubMed] [Google Scholar]
- 33.Ravi Kumar BC, Kaur I, Kumar B. Topical methotrexate therapy in palmoplantar psoriasis. Indian J Dermatol Venereol Leprol. 1999;65(6):270–2. [PubMed] [Google Scholar]
- 34.Coleman WR, Lowe NJ, David M, Halder RM. Palmoplantar psoriasis: experience with 8-methoxypsoralen soaks plus ultraviolet A with the use of a high-output metal halide device. J Am Acad Dermatol. 1989;20(6):1078–82. doi: 10.1016/s0190-9622(89)70136-5. [DOI] [PubMed] [Google Scholar]
- 35.Zeichner JA. Use of Topical Coal Tar Foam for the Treatment of Psoriasis in Difficult-to-treat Areas. J Clin Aesthet Dermatol. 2010;3(9):37–40. [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert J, Dowlatshahi EA, de la Brassinne M, Nijsten T. A descriptive study of psoriasis characteristics, severity and impact among 3,269 patients: results of a Belgian cross sectional study (BELPSO) Eur J Dermatol. 2012;22(2):231–7. doi: 10.1684/ejd.2011.1623. [DOI] [PubMed] [Google Scholar]
- 37.Gelfand JM, Wan J, Callis Duffin K, Krueger GG, Kalb RE, Weisman JD, et al. Comparative effectiveness of commonly used systemic treatments or phototherapy for moderate to severe plaque psoriasis in the clinical practice setting. Arch Dermatol. 2012;148(4):487–94. doi: 10.1001/archdermatol.2012.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2012;66(3):369–75. doi: 10.1016/j.jaad.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 40.EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 41.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 42.van der Zanden BP, Dijkgraaf MG, Blanken P, de Borgie CA, van Ree JM, van den Brink W. Validity of the EQ-5D as a generic health outcome instrument in a heroin-dependent population. Drug Alcohol Depend. 2006;82(2):111–8. doi: 10.1016/j.drugalcdep.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(17):1751–0473. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16(22):2529–42. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Lassus A, Geiger JM. Acitretin and etretinate in the treatment of palmoplantar pustulosis: a double-blind comparative trial. Br J Dermatol. 1988;119(6):755–9. doi: 10.1111/j.1365-2133.1988.tb03499.x. [DOI] [PubMed] [Google Scholar]
- 46.Ormerod AD, Campalani E, Goodfield MJ. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162(5):952–63. doi: 10.1111/j.1365-2133.2010.09755.x. [DOI] [PubMed] [Google Scholar]
- 47.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Kumar B, Saraswat A, Kaur I. Palmoplantar lesions in psoriasis: a study of 3065 patients. Acta Derm Venereol. 2002;82(3):192–5. doi: 10.1080/00015550260132488. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson MO, Hagforsen E, Lundin IP, Michaelsson G. Palmoplantar pustulosis: a clinical and immunohistological study. Br J Dermatol. 1998;138(3):390–8. doi: 10.1046/j.1365-2133.1998.02113.x. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and Smoking: A Systematic Review and Meta-Analysis. Br J Dermatol. 2013;11(10):12670. doi: 10.1111/bjd.12670. [DOI] [PubMed] [Google Scholar]
- 51.Brunasso AM, Puntoni M, Aberer W, Delfino C, Fancelli L, Massone C. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol. 2013;168(6):1243–51. doi: 10.1111/bjd.12223. [DOI] [PubMed] [Google Scholar]
- 52.Herron MD, Hinckley M, Hoffman MS, Papenfuss J, Hansen CB, Callis KP, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141(12):1527–34. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 53.Hellgren L, Mobacken H. Pustulosis palmaris et plantaris. Prevalence, clinical observations and prognosis. Acta Derm Venereol. 1971;51(4):284–8. [PubMed] [Google Scholar]
- 54.Enfors W, Molin L. Pustulosis palmaris et plantaris. A follow-up study of a ten-year material. Acta Derm Venereol. 1971;51(4):289–94. [PubMed] [Google Scholar]
- 55.Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health Qual Life Outcomes. 2006;4:71. doi: 10.1186/1477-7525-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ammoury A, El Sayed F, Dhaybi R, Bazex J. Palmoplantar pustulosis should not be considered as a variant of psoriasis. J Eur Acad Dermatol Venereol. 2008 Mar;22(3):392–3. doi: 10.1111/j.1468-3083.2007.02344.x. doi: 10.1111/j.1468-3083.2007.02344.x. [DOI] [PubMed] [Google Scholar]
- 57.Asumalahti K, Ameen M, Suomela S, Hagforsen E, Michaelsson G, Evans J, et al. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J Invest Dermatol. 2003;120(4):627–32. doi: 10.1046/j.1523-1747.2003.12094.x. [DOI] [PubMed] [Google Scholar]


