Abstract
Infectious etiologies have been hypothesized for Hodgkin and non-Hodgkin lymphoma (HL and NHL) in early life, but findings to date for specific lymphomas and periods of susceptibility are conflicting. We conducted the first national cohort study to examine whether season of birth, a proxy for infectious exposures in the first few months of life, is associated with HL or NHL in childhood through young adulthood. A total of 3,571,574 persons born in Sweden in 1973–2008 were followed up through 2009 to examine the association between season of birth and incidence of HL (943 cases) or NHL (936 cases). We found a sinusoidal pattern in NHL risk by season of birth (P=0.04), with peak risk occurring among birthdates in April. Relative to persons born in fall (September-November), odds ratios for NHL by season of birth were 1.25 (95% CI, 1.04–1.50; P=0.02) for spring (March-May), 1.22 (95% CI, 1.01–1.48; P=0.04) for summer (June-August), and 1.11 (95% CI, 0.91–1.35; P=0.29) for winter (December-February). These findings did not vary by sex, age at diagnosis, or major subtypes. In contrast, there was no seasonal association between birthdate and risk of HL (P=0.78). In this large cohort study, birth in spring or summer was associated with increased risk of NHL (but not HL) in childhood through young adulthood, possibly related to immunologic effects of delayed infectious exposures compared with fall or winter birth. These findings suggest that immunologic responses in early infancy may play an important role in the development of NHL.
Keywords: Hodgkin lymphoma, non-Hodgkin lymphoma, risk factors, seasons
INTRODUCTION
The incidence of Hodgkin and non-Hodgkin lymphoma (HL and NHL) has increased in childhood through young adulthood, but not older adulthood, in the U.S. and Europe in recent decades.1–6 This increase among the young has sparked a growing interest in identifying risk factors that occur in early life. Infectious exposures including Epstein-Barr virus (EBV) infection7–10 are hypothesized to play an important role, but critical periods of susceptibility and differential effects for specific lymphomas are still unclear. Because of seasonal variation of EBV and other common respiratory infections,11–13 season of birth is a reasonable proxy for infectious exposures in the first few months of life. If infectious exposures in early infancy play a role in lymphomagenesis, normal seasonal variation in such exposures would result in a seasonal pattern in the risk of HL or NHL by date of birth. The few studies that have examined this hypothesis have reported discrepant results, but have varied widely in design, analytic methods, age ranges, and populations.14–16 No studies have been conducted using a population-based cohort design with the ability to estimate relative risks of HL or NHL by season of birth. We conducted the first national cohort study to examine whether season of birth is associated with the risk of HL or NHL in childhood through young adulthood.
MATERIAL AND METHODS
Using the Swedish Birth Registry, we identified the month and day of birth for 3,571,574 persons who were born in Sweden in 1973–2008. This cohort was followed up for HL and NHL incidence from birth through December 31, 2009 (maximum attained age was 37 years). All HL and NHL cases were identified from the Swedish Cancer Registry and were classified according to the International Classification of Diseases, 7th revision (code 201 for HL, and codes 200 and 202 for NHL). This registry includes all primary incident cancers in Sweden since 1958 with compulsory reporting nationwide. Histologic subtypes were classified according to Systemized Nomenclature of Medicine (SNOMED) codes since 1993 and synonymous definitions provided by the World Health Organization prior to this period.17 NHL subtypes were categorized as diffuse large B-cell subtype, other or unspecified B-cell subtypes, and T-cell subtypes; and HL subtypes included nodular sclerosis, mixed cellularity, lymphocyte-rich, lymphocyte-depleted, classic HL “not otherwise specified”, and nodular lymphocyte predominant.
Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between season of birth and HL or NHL. Season of birth was modeled using standard methods for seasonal data. First, date of birth (DOB, coded as an integer from 1 to 365) was modeled as a sinusoidal function in the logistic regression model, using an iterative method to identify the date with peak HL or NHL risk and to test for an overall seasonal association, as previously described.18 In the case of a leap year, February 29 was recoded as calendar day 59 so that the respective year had 365 days. Specifically, the trigonometric term entered into the logistic model was
where tmax (the peak birth date for either HL or NHL risk) was determined iteratively by finding the value from 1 to 365 that maximized the model coefficient.18 After identifying the peak date in this manner, the 3-month period of maximum risk was identified by centering on this date.
Unadjusted risk estimates were reported because there was no evidence of confounding by other sociodemographic or familial variables (e.g., sex, birth year, birth order, parental age, parental country of birth, parental education level). We examined first-order interactions between these variables and season of birth with respect to HL or NHL risk using a likelihood ratio test and by visually examining risk estimates across different strata for each variable. In addition, multinomial logistic regression was used to test for heterogeneity in the association between season of birth and earlier-onset (age <15 years) vs. later-onset (age ≥15 years) HL or NHL. We also repeated the same models described above to explore associations between season of birth and the most common HL or NHL subtypes. All statistical tests were 2-sided and used an α-level of 0.05. Goodness of fit was evaluated using the Pearson chi-squared test, which indicated a good fit in all models. All analyses were conducted using Stata version 13.0.19 This study was approved by the Regional Ethics Committee of Lund University in Sweden.
RESULTS
Among the 3,571,574 persons in this cohort, 936 NHL and 943 HL cases were identified in 66.3 million person-years of follow-up. The overall incidence rates (per 100,000 person-years) were 1.41 for NHL and 1.42 for HL. The mean duration of follow-up was 18.6 years (SD 10.4, median 18.6), and the mean ages at diagnosis were 13.8 years (SD 10.0, median 11.8) for NHL and 20.2 years (SD 6.4, median 20.1) for HL.
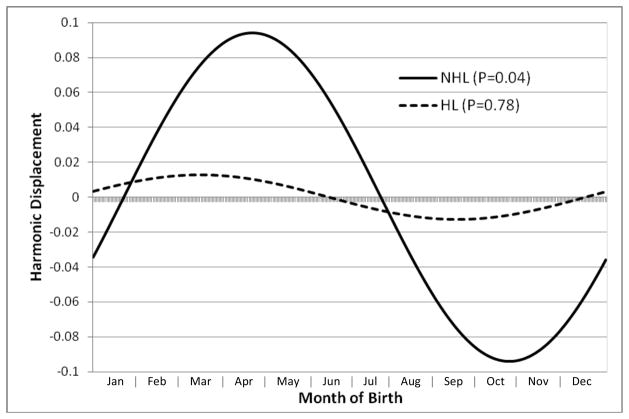
NHL incidence (per 100,000 person-years) was highest among persons born in spring (1.49) or summer (1.52), and lowest among those born in fall (1.26); whereas HL incidence varied little across different seasons of birth (range 1.38 to 1.46; Table 1). In the sinusoidal logistic model, we found a significant association between season of birth and NHL (P=0.04), with peak risk corresponding to a birth date of April 24. Relative to persons born in fall, those born in spring or summer had significantly increased odds of NHL in childhood through young adulthood (see Table 2). The odds ratio for NHL among persons born in the 3-month peak (March 10-June 8, identified by centering around the peak birth date of April 24) relative to the 3-month nadir (September 8-December 7) was 1.27 (95% CI, 1.05–1.53; P=0.01). In contrast, there was no significant association between season of birth and HL (P=0.78 in the sinusoidal model). Figure 1 shows the seasonal pattern in relative risks of NHL and HL from the sinusoidal model. Further adjustment for other variables (e.g., sex, birth year, birth order, maternal age, maternal and paternal country of birth, maternal and paternal education) either individually or in any combination had a negligible effect on the risk estimates (see fully adjusted model in Supplementary Table 1).
Table 1.
Incidence of non-Hodgkin and Hodgkin lymphoma in 1973–2009 by season or month of birth
| Births N (%) | NHL cases | HL cases | |||
|---|---|---|---|---|---|
|
| |||||
| n (%) | Ratea | n (%) | Ratea | ||
| Overall | 3,571,574 (100.0) | 936 (100.0) | 1.41 | 943 (100.0) | 1.42 |
| Spring | 990,773 (27.7) | 281 (30.0) | 1.49 | 259 (27.5) | 1.38 |
| March | 334,787 (9.4) | 103 (11.0) | 1.60 | 90 (9.5) | 1.40 |
| April | 331,520 (9.3) | 94 (10.0) | 1.49 | 92 (9.8) | 1.46 |
| May | 324,466 (9.1) | 84 (9.0) | 1.38 | 77 (8.2) | 1.26 |
| Summer | 915,888 (25.6) | 255 (27.2) | 1.52 | 242 (25.7) | 1.44 |
| June | 305,267 (8.6) | 92 (9.8) | 1.63 | 85 (9.0) | 1.50 |
| July | 310,043 (8.7) | 84 (9.0) | 1.49 | 77 (8.2) | 1.36 |
| August | 300,578 (8.4) | 79 (8.4) | 1.45 | 80 (8.5) | 1.47 |
| Fall | 822,327 (23.0) | 187 (20.0) | 1.26 | 218 (23.1) | 1.46 |
| September | 291,196 (8.2) | 60 (6.4) | 1.13 | 80 (8.5) | 1.50 |
| October | 278,533 (7.8) | 65 (6.9) | 1.29 | 78 (8.3) | 1.55 |
| November | 252,598 (7.1) | 62 (6.6) | 1.36 | 60 (6.4) | 1.32 |
| Winter | 842,586 (23.6) | 213 (22.8) | 1.35 | 224 (23.8) | 1.42 |
| December | 253,596 (7.1) | 66 (7.1) | 1.44 | 64 (6.8) | 1.40 |
| January | 297,241 (8.3) | 81 (8.7) | 1.43 | 81 (8.6) | 1.43 |
| February | 291,749 (8.2) | 66 (7.1) | 1.19 | 79 (8.4) | 1.42 |
Incidence rate per 100,000 person years.
Abbreviations: HL = Hodgkin lymphoma, NHL = non-Hodgkin lymphoma.
Table 2.
Odds ratios for associations between season of birth and non-Hodgkin or Hodgkin lymphoma in 1973–2009
| NHL | HL | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Spring (Mar-May) | 1.25 | 1.04, 1.50 | 0.02 | 0.99 | 0.82, 1.18 | 0.88 |
| Summer (Jun-Aug) | 1.22 | 1.01, 1.48 | 0.04 | 1.00 | 0.83, 1.20 | 0.97 |
| Fall (Sep-Nov) | Ref. | Ref. | ||||
| Winter (Dec-Feb) | 1.11 | 0.91, 1.35 | 0.29 | 1.00 | 0.83, 1.21 | 0.98 |
| 3-month peaka | 1.27 | 1.05, 1.53 | 0.01 | 1.01 | 0.84, 1.20 | 0.94 |
| 3-month nadirb | Ref. | Ref. | ||||
In sinusoidal models, the birthdate with peak NHL risk was Apr 24 (3-month peak: Mar 10-Jun 8) and with peak HL risk was Mar 18 (3-month peak: Feb 1-May 2).
In sinusoidal models, the birthdate with lowest NHL risk was Oct 23 (3-month nadir: Sep 8-Dec 7) and with lowest HL risk was Sep 16 (3-month nadir: Aug 2-Oct 31).
Abbreviations: CI = confidence interval, HL = Hodgkin lymphoma, NHL = non-Hodgkin lymphoma, OR = odds ratio.
Figure 1.
Sinusoidal logistic regression results for association between birthdate and risk of non- Hodgkin or Hodgkin lymphoma (NHL or HL) in childhood through young adulthood. Harmonic displacement represents the log odds ratio for a given time point relative to the mean for the entire calendar year.
We found no interactions between season of birth and other variables with respect to NHL or HL risk. In particular, there was no evidence that the observed association between season of birth and NHL varied by sex (Pinteraction=0.88), birth year (Pinteraction=0.56, modeled as a categorical variable by decade), birth order (Pinteraction=0.63, modeled as 1, 2, 3, ≥4), or sibship size (Pinteraction=0.29, modeled as 1, 2, 3, ≥4). There also was no heterogeneity by age at NHL diagnosis, comparing cases diagnosed at ages <15 years (n=554) with those diagnosed at ≥15 years (n=382) (P=0.57). HL results also did not vary by age at diagnosis, comparing ages <15 years (n=179) vs. ≥15 years (n=764) (P=0.31).
The results for NHL subtypes were consistent with those for NHL overall. In the sinusoidal logistic model, the birthdate with peak risk for diffuse large B-cell subtype was April 11 (P=0.02, based on 320 cases), for other or unspecified B-cell subtypes was April 6 (P=0.54, based on 170 cases), for T-cell subtypes was April 21 (P=0.48, based on 114 cases), and subtype was unavailable for 332 cases. Among HL subtypes, the birthdate with peak risk of nodular sclerosis subtype was May 11 (P=0.11, based on 572 cases) and for mixed cellularity was January 11 (P=0.07, based on 76 cases). Other HL subtypes were too infrequent for analysis, including nodular lymphocyte predominant (n=16), lymphocyte-rich (n=8), and lymphocyte-depleted (n=6), or were inadequately specific (classic HL “not otherwise specified”, n=123) or unavailable (n=142).
DISCUSSION
In this large national cohort study, persons who were born in spring or summer had a significantly increased risk of NHL in childhood through young adulthood. These findings may be related to immunologic effects of delayed infectious exposures in the first few months of life compared with persons born in fall or winter. In contrast, season of birth was not associated with risk of HL in childhood through young adulthood.
The few previous studies of season of birth and HL or NHL have yielded conflicting results, but were based on different methods and populations. A study of 2,079 HL and 991 NHL cases diagnosed at ages 15–24 years in England reported a seasonal association for NHL among males only (P=0.04), with a bimodal peak among birthdates in January and July; whereas HL had no seasonal association with birthdate (P=0.46).15 A smaller study of children aged <15 years in northern England reported null results for both HL (128 cases) and NHL (134 cases).16 A Danish study of 299 HL and 253 NHL cases diagnosed at ages <20 years reported a weak seasonal association for HL with a peak among birthdates in July, and no association with NHL.14 Our study was the first with a population-based cohort design for estimating relative risks of HL and NHL by season of birth. We found a significant seasonal pattern for NHL, irrespective of gender, with peak risk among birthdates in April. All major NHL subtype groups appeared to have a similar pattern, although statistical power was limited for subtype-specific analyses.
These findings are consistent with an association we previously reported between low birth order and NHL in this cohort (Ptrend=0.02),20 which may be related to infectious etiologies. The “delayed exposure hypothesis” postulates that delayed exposure to EBV or other infectious agents (as may occur among first-born because of the lack of older siblings) impairs normal maturation of the immune system from a T helper cell type 2 (Th2) to a T helper cell type 1 (Th1) preponderance. This results in an abnormal immune response that may predispose to the development of NHL.21 In contrast, birth order was not associated with HL in this cohort (Ptrend=0.28).22 The link between spring or summer birth and NHL (but not HL) in the current study is consistent with these prior findings for birth order. It suggests that immunologic effects from delayed infectious exposures may play a role in the development of NHL, and that early infancy may be an important window of susceptibility to these effects.
The main strength of this study was its large population-based cohort design with nearly complete nationwide ascertainment of HL and NHL. Although season of birth is a reasonable proxy for infectious exposures in early infancy, our study was limited by the inability to directly assess EBV status, other specific infections, and infectious exposures later in life that may also influence disease risk. Additional studies with detailed information on infectious exposures will be needed to further elucidate the etiologic pathways and critical windows of susceptibility. Longer-term follow-up of this and other large cohorts will also be needed to examine these associations in older adulthood.
In summary, this large national cohort study found that spring or summer birth was associated with increased risk of NHL (but not HL) in childhood though young adulthood among persons born in Sweden during 1973–2008. These findings may be related to immunologic effects from delayed infectious exposures in early infancy, which may play a role in the development of NHL. Clinical studies with more detailed information on infectious exposures and immune responses are warranted to further delineate the underlying mechanisms.
Supplementary Material
Novelty and impact.
We conducted the first national cohort study to examine whether season of birth, a proxy for infectious exposures in the first few months of life, is associated with non-Hodgkin or Hodgkin lymphoma (NHL or HL). Persons born in spring or summer had significantly increased risk of NHL (but not HL) in childhood through young adulthood, suggesting that immunologic effects of delayed infectious exposures in early infancy may play an important role in the development of NHL.
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R03 CA171017); the Swedish Research Council; and ALF project grant, Lund, Sweden. The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations
- CI
confidence interval
- DOB
date of birth
- EBV
Epstein-Barr virus
- HL
Hodgkin lymphoma
- NHL
non-Hodgkin lymphoma
- OR
odds ratio
- SD
standard deviation
- Th1
T helper cell type 1
- Th2
T helper cell type 2
Footnotes
There were no conflicts of interest.
Author Contributions: Dr. Jan Sundquist had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Crump, K. Sundquist, Sieh, Winkleby, J. Sundquist.
Acquisition of data: K. Sundquist, J. Sundquist.
Analysis and interpretation of data: Crump, K. Sundquist, Sieh, Winkleby, J. Sundquist.
Drafting of the manuscript: Crump.
Critical revision of the manuscript for important intellectual content: Crump, K. Sundquist, Sieh, Winkleby, J. Sundquist.
Statistical analysis: Crump, J. Sundquist.
Obtained funding: Crump, J. Sundquist.
References
- 1.Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW, Lacour B, Parkin M. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004;364:2097–105. doi: 10.1016/S0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Eisner MP, Kosary CL, et al. E. SEER Cancer Statistics Review, 1975–2000. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 3.Hjalgrim H, Askling J, Pukkala E, Hansen S, Munksgaard L, Frisch M. Incidence of Hodgkin’s disease in Nordic countries. Lancet. 2001;358:297–8. doi: 10.1016/S0140-6736(01)05498-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen YT, Zheng T, Chou MC, Boyle P, Holford TR. The increase of Hodgkin’s disease incidence among young adults. Experience in Connecticut, 1935–1992. Cancer. 1997;79:2209–18. [PubMed] [Google Scholar]
- 5.Clavel J, Steliarova-Foucher E, Berger C, Danon S, Valerianova Z. Hodgkin’s disease incidence and survival in European children and adolescents (1978–1997): report from the Automated Cancer Information System project. Eur J Cancer. 2006;42:2037–49. doi: 10.1016/j.ejca.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Curado MP, Edwards B, Shin HR, Ferlay J, Heanue M, Boyle P, Storm H. Cancer Incidence in Five Continents. 160. IX. IARC Scientific Publication; 2009. [Google Scholar]
- 7.Mueller NE, Lennette ET, Dupnik K, Birmann BM. Antibody titers against EBNA1 and EBNA2 in relation to Hodgkin lymphoma and history of infectious mononucleosis. Int J Cancer. 2011 doi: 10.1002/ijc.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedobitek G, Young LS, Herbst H. Epstein-Barr virus infection and the pathogenesis of malignant lymphomas. Cancer Surv. 1997;30:143–62. [PubMed] [Google Scholar]
- 9.Hjalgrim H, Askling J, Sorensen P, Madsen M, Rosdahl N, Storm HH, Hamilton-Dutoit S, Eriksen LS, Frisch M, Ekbom A, Melbye M. Risk of Hodgkin’s disease and other cancers after infectious mononucleosis. J Natl Cancer Inst. 2000;92:1522–8. doi: 10.1093/jnci/92.18.1522. [DOI] [PubMed] [Google Scholar]
- 10.Diepstra A, Niens M, Vellenga E, van Imhoff GW, Nolte IM, Schaapveld M, van der Steege G, van den Berg A, Kibbelaar RE, te Meerman GJ, Poppema S. Association with HLA class I in Epstein-Barr-virus-positive and with HLA class III in Epstein-Barr-virus-negative Hodgkin’s lymphoma. Lancet. 2005;365:2216–24. doi: 10.1016/S0140-6736(05)66780-3. [DOI] [PubMed] [Google Scholar]
- 11.Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, Viboud C. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8:e54445. doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas AS, Brown T, Reid D. Infectious mononucleosis and Hodgkin’s disease--a similar seasonality. Leuk Lymphoma. 1996;23:323–31. doi: 10.3109/10428199609054835. [DOI] [PubMed] [Google Scholar]
- 13.Olofsson S, Brittain-Long R, Andersson LM, Westin J, Lindh M. PCR for detection of respiratory viruses: seasonal variations of virus infections. Expert Rev Anti Infect Ther. 2011;9:615–26. doi: 10.1586/eri.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langagergaard V, Norgard B, Mellemkjaer L, Pedersen L, Rothman KJ, Sorensen HT. Seasonal variation in month of birth and diagnosis in children and adolescents with Hodgkin disease and non-Hodgkin lymphoma. J Pediatr Hematol Oncol. 2003;25:534–8. doi: 10.1097/00043426-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 15.van Laar M, Kinsey SE, Picton SV, Feltbower RG. First description of seasonality of birth and diagnosis amongst teenagers and young adults with cancer aged 15–24 years in England, 1996–2005. BMC Cancer. 2013;13:365. doi: 10.1186/1471-2407-13-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basta NO, James PW, Craft AW, McNally RJ. Season of birth and diagnosis for childhood cancer in Northern England, 1968–2005. Paediatr Perinat Epidemiol. 2010;24:309–18. doi: 10.1111/j.1365-3016.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe ESHN, Stein H, Vardiman JW. Pathology and genetics of tumours of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 18.Efird JT, Nielsen SS. A method to model season of birth as a surrogate environmental risk factor for disease. Int J Environ Res Public Health. 2008;5:49–53. doi: 10.3390/ijerph5010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp. Stata Statistical Software: Release. 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 20.Crump C, Sundquist K, Sieh W, Winkleby MA, Sundquist J. Perinatal and family risk factors for non-Hodgkin lymphoma in early life: a Swedish national cohort study. J Natl Cancer Inst. 2012;104:923–30. doi: 10.1093/jnci/djs225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vineis P, Miligi L, Crosignani P, Fontana A, Masala G, Nanni O, Ramazzotti V, Rodella S, Stagnaro E, Tumino R, Vigano C, Vindigni C, et al. Delayed infection, family size and malignant lymphomas. J Epidemiol Community Health. 2000;54:907–11. doi: 10.1136/jech.54.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crump C, Sundquist K, Sieh W, Winkleby MA, Sundquist J. Perinatal and family risk factors for Hodgkin lymphoma in childhood through young adulthood. Am J Epidemiol. 2012;176:1147–58. doi: 10.1093/aje/kws212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.