Abstract
Background
It is valuable to find the potential activity of regulating the excessive mucin secretion by the compounds derived from various medicinal plants. We investigated whether aqueous extract of the root bark of Morus alba L. (AMA), kuwanon E, kuwanon G, mulberrofuran G, and morusin significantly affect the secretion and production of airway mucin using in vivo and in vitro experimental models.
Methods
Effect of AMA was examined on hypersecretion of airway mucin in sulfur dioxide-induced acute bronchitis in rats. Confluent NCI-H292 cells were pretreated with ethanolic extract, kuwanon E, kuwanon G, mulberrofuran G, or morusin for 30 minutes and then stimulated with phorbol 12-myristate 13-acetate (PMA) for 24 hours. The MUC5AC mucin secretion and production were measured by enzyme-linked immunosorbent assay.
Results
AMA stimulated the secretion of airway mucin in sulfur dioxide-induced bronchitis rat model; aqueous extract, ethanolic extract, kuwanon E, kuwanon G, mulberrofuran G and morusin inhibited the production of MUC5AC mucin induced by PMA from NCI-H292 cells, respectively.
Conclusion
These results suggest that extract of the root bark and the natural products derived from Morus alba L. can regulate the secretion and production of airway mucin and, at least in part, explains the folk use of extract of Morus alba L. as mucoregulators in diverse inflammatory pulmonary diseases.
Keywords: Mucins, Biological Products
Introduction
Mucus in the pulmonary system is very important in defensive action against various particles, noxious chemicals and invading pathogenic microbes. This defensive action of pulmonary mucus is attributed to the physicochemical property of mucins, i.e., viscoelasticity. Mucins are high molecular weight glycoproteins present in the airway mucus and produced by goblet cells in the surface epithelium as well as mucous cells in the submucosal gland. However, hypersecretion of airway mucus is one of the major symptoms associated with severe pulmonary diseases including chronic bronchitis, cystic fibrosis, bronchiectasis and asthma1,2. Therefore, we suggest it is valuable to find the potential activity of regulating (inhibiting) the excess mucin secretion (production) by the compounds derived from various medicinal plants. We have tried to investigate the possible activities of some natural products on mucin secretion from cultured airway epithelial cells. As a result of our trial, we previously reported that several natural compounds affected mucin secretion and/or production from airway epithelial cells3,4,5. According to traditional oriental medicine, the root bark of Morus alba L. has been used for controlling pulmonary inflammatory diseases6 and the natural products derived it, kuwanon E, kuwanon G, mulberrofuran G, and morusin have been reported to show antibacterial, anti-inflammatory, antiviral and anticancer effects7,8,9,10. However, to the best of our knowledge, there is no report about the potential effect of extract of the root bark of Morus alba L. and kuwanon E, kuwanon G, mulberrofuran G, and morusin (Figure 1), the natural products derived from it, on production and secretion of airway mucin. Therefore, in this study, we checked whether extract of the root bark of Morus alba L., kuwanon E, kuwanon G, mulberrofuran G, and morusin significantly affect the production and secretion of airway mucin using in vivo and in vitro experimental models reflecting the hypersecretion and/or hyperproduction of mucus observed in inflammatory pulmonary diseases. For in vivo experiment, effect of aqueous extract (AE) of the root bark of Morus alba L. was checked on hypersecretion of pulmonary mucin in sulfur dioxide-induced bronchitis in rats11. For in vitro experiment, effects of AE, ethanolic extract (EE), kuwanon E, kuwanon G, mulberrofuran G, and morusin were checked on production of airway mucin from NCI-H292 cells, a human pulmonary mucoepidermoid cell line, which are frequently used for the purpose of elucidating intracellular signaling pathways involved in airway mucin production12,13,14.
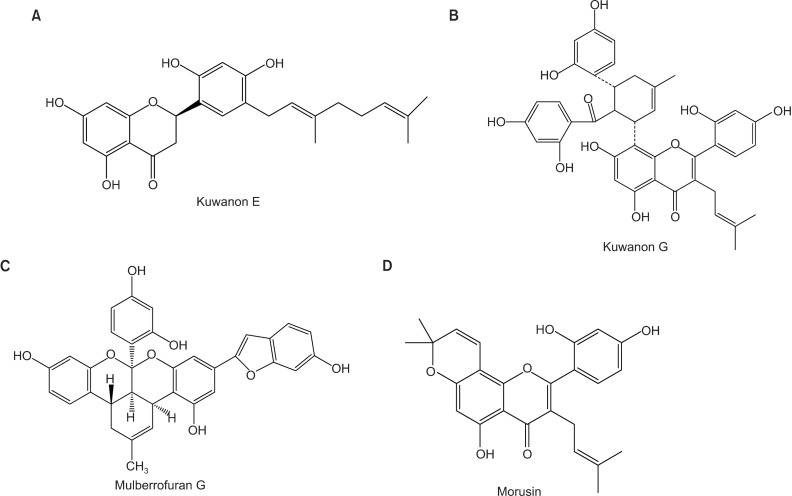
Figure 1.
Chemical structure of natural products derived from the root bark of Morus alba L. including kuwanon E (A), kuwanon G (B), mulberrofuran G (C), and morusin (D).
Materials and Methods
1. Materials
All the chemicals and reagents used in this experiment were purchased from Sigma (St. Louis, MO, USA) unless otherwise specified. Kuwanon E (purity, 98.0%), kuwanon G (purity, 98.0%), mulberrofuran G (purity, 98.0%), and morusin (purity, 98.0%) were isolated, purified and identified by analytical chemists in the Laboratory of Pharmacognosy, Department of Pharmacy, Chosun University (Gwangju, Korea). Briefly, the root barks of Morus alba L. were collected in Gyeongju, Gyeongbuk province, Korea in 2011 and taxonomically identified by Professor Jae Hyun Lee in Department of Herbology, School of Oriental Medicine, Dongguk University (Gyeongju, Korea). The voucher specimen (CSU-1048-17) were deposited in the Herbarium of the College of Pharmacy, Chosun University. The dried root barks of M. alba (100 g) were extracted three times with H2O and 70% ethanol (EtOH) at 95℃. The extracts were filtrated and dried in vacuo to afford H2O ext. (20.1 g) and 70% EtOH ext. (20.4 g), respectively. In order to isolate and purify morusin, kuwanon E, kuwanon G, and mulberrofuran G, the dried root barks of M. alba (12 kg) were extracted three times with methanol (MeOH) under reflux and 1,511.6 g of residue were produced. The MeOH extract was suspended in water and then partitioned sequentially with equal volumes of dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol (n-BuOH). Each fractions were evaporated in vaccuo to yield the residues of CH2Cl2 (318.2 g), EtOAc (192.2 g), n-BuOH (182.4 g), and water (534.3 g) extract. The EtOAc fraction (53 g) was chromatographed over a silica gel column using a gradient solvent system of n-hexane-EtOAc (2:1 to 1:8, EtOAc, MeOH) to give five subfractions (E1-E5). Subfraction E1 was subjected to silica gel column chromatography (CC) eluting with a gradient solvent system of n-hex:EtOAc (10:1 to 4:1, MeOH) to yield seven subfractions (E11-E17). Subfraction E15 was purified by MCI gel CC (MeOH:H2O, 3:1) to give morusin (122.4 mg). Subfraction E2 was subjected to MCI gel CC eluting with a gradient solvent system of MeOH:H2O (1:1 to 3:1) to yield sixteen subfractions (E21-E216). Subfraction E22 was purified by LiChroprep RP 18 CC (MeOH:H2O, 2:3 to 1:1) to give norartocarpanone (3.42 mg). Subfraction E24 was subjected to silica gel CC (CHCl3:MeOH:H2O, 12:1:0.1 to 8:1:0.1) to give six fractions (E241-E246). Subfraction E245 was purified by LiChroprep RP 18 CC (MeOH:H2O, 1:1) to yield kuwanon G (1,011 mg). Subfraction E27 was purified repeated silica gel CC (CHCl3:MeOH:H2O, 25:1:0.1 to 10:1:0.1) and LiChroprep RP 18 CC (MeOH:H2O, 2:3) to yield mulberofuran G (15.9 mg), and kuwanon E (43.7 mg). The physico-chemical data including 1H NMR, 13C NMR, and HSQC of these compounds were identical with those reported in the literature15,16,17,18.
2. Animals
Pathogen-free male Sprague-Dawley rats (Daehan Biolink, Seoul, Korea), 5 weeks of age weighing 200-220 g, were used. The animals were housed five per cage and were provided with the distilled water and food ad libitum. They were kept under a 12 hour light/dark cycle (light on 08:00-20:00) at constant temperature (22.5℃) and humidity (55%). Animals were cared through all of the experimental procedures in accordance with the Guide for the Care and Use of Laboratory Animals regulated by Chungnam National University, Daejeon, Korea.
3. Experimental design
Twenty-five rats were randomly divided into the following five groups: normal control; sulfur dioxide (SO2)-only exposure; SO2 exposure plus aqueous extract of the root bark of Morus alba L. (AMA) 100 mg/kg; SO2 exposure plus AMA 300 mg/kg; SO2 exposure plus dexamethasone 0.5 mg/kg. SO2 was exposed to rats by inhalation and AMA was administered per oral. A positive control, dexamethasone, was administered to rats via intraperitoneal injection. A 15% solution of sodium metabisulfite was aerosolized into a Plexiglas exposure chamber, using an ultrasonic humidifier (Samsung Electronics Inc., Seoul, Korea). The concentration of sulfur dioxide (SO2) gas generated by this apparatus was measured to be 150 ppm. Rats were exposed to SO2 for 3 hours per day, 5 days per week, 3 weeks and AMA was administered during the last 2 weeks out of 3 weeks in total. Normal control group were exposed to fresh air in a similar environment without SO2 exposure.
4. Bronchoalveolar lavage fluid (BALF) collection and quantitation of in vivo mucins in BALF
Rats were euthanized on the last day of experiment and the trachea was cannulated by using sterile polyethylene tube. Bronchoalveolar lavage was performed four times with 5.0 mL of ice-cold phosphate-buffered saline (PBS; PH 7.4) with 80% of recovery rate. Floating cells and cell debris were removed by centrifugation of BALF at 12,000 ×g for 5 minutes. The BALF samples were stored at -70℃ until assayed for their mucin contents. The amount of mucins in each BALF sample was measured by using enzyme-linked immunosorbent assay (ELISA). The BALF samples were prepared with PBS at 1:10 dilution, and 100 µL of each sample was incubated at 42℃ in a 96-well plate, until dry. Plates were washed three times with PBS and blocked with 2% bovine serum albumin (BSA; fraction V) for 1 hour at room temperature. Plates were again washed three times with PBS and then incubated with 100 µL of 45M1 (NeoMarkers, Fremont, CA, USA), a mouse monoclonal MUC5AC antibody (1:200), which was diluted with PBS containing 0.05% Tween 20 and dispensed into each well. After 1 hour, the wells were washed three times with PBS, and 100 µL of horseradish peroxidase-goat anti-mouse IgG conjugate (1:3,000) was dispensed into each well. After 1 hour, plates were washed three times with PBS. Color reaction was developed with 3,3',5,5'-tetramethylbenzidine (TMB) peroxide solution and stopped with 1N H2SO4. Absorbance was read at 450 nm.
5. Histopathologic analysis of tracheal tissues
The combination of the alcian blue and the periodic acid-Schiff (PAS) techniques were used for detecting acidic mucins in tracheal tissues. Formaldehyde-fixed and paraffin-embedded tracheal tissues were cut at 5 µm. Sections were stained with the standard alcian blue (pH 2.5) method followed by the PAS technique. The alcian blue at a pH of 2.5 stained all acidic mucins blue19.
6. Cell culture and treatment of agents
NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured (seeding density: 1×104 cells/well in 24 well plate) in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) in the presence of penicillin (100 units/mL), streptomycin (100 µg/mL), and HEPES (25 mM) at 37℃ in a humidified, 5% CO2/95% air, water-jacketed incubator. For serum deprivation, confluent cells (5×105 cells/well in 24 well plate) were washed twice with PBS and recultured in RPMI 1640 with 0.2% FBS for 24 hours. After 24 hours of serum deprivation, cells were pretreated with AE (1, 10, and 100 µg/mL), 70% EE (1, 10, and 100 µg/mL), kuwanon E (1, 10, and 100 µM), kuwanon G (1, 10, and 100 µM), mulberrofuran G (1, 10, and 100 µM), and morusin (1, 10, and 100 µM) for 30 minutes and treated with phorbol 12-myristate 13-acetate (PMA; 10 ng/mL) for 24 hours in serum-free RPMI 1640, respectively. AE, EE, kuwanon E, kuwanon G, mulberrofuran G, and morusin were dissolved in dimethylsulfoxide, diluted in PBS and treated in culture medium (final concentrations of dimethylsulfoxide were 0.5%), respectively. The final pH values of these solutions were between 7.0 and 7.4. Culture medium and 0.5% dimethylsulfoxide in medium did not affect mucin production from NCI-H292 cells. After 24 hours, cells were lysed with buffer solution containing 20 mM Tris, 0.5% NP-40, 250mM NaCl, 3 mM EDTA, 3 mM EGTA and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) and collected to measure the production of MUC5AC mucin (in 24-well culture plate).
7. MUC5AC mucin analysis using ELISA
MUC5AC mucin was measured by using ELISA. Cell lysates were prepared with PBS at 1:10 dilution, and 100 µL of each sample was incubated at 42℃ in a 96-well plate, until dry. Plates were washed three times with PBS and blocked with 2% BSA for 1 hour at room temperature. Plates were again washed three times with PBS and then incubated with 100 µL of 45M1, a mouse monoclonal MUC5AC antibody (1:200, NeoMarkers), which was diluted with PBS containing 0.05% Tween 20 and dispensed into each well. After 1 hour, the wells were washed three times with PBS, and 100 µL of horseradish peroxidase-goat anti-mouse IgG conjugate (1:3,000) was dispensed into each well. After 1 hour, plates were washed three times with PBS. Color reaction was developed with 3,3',5,5'-tetramethylbenzidine (TMB) peroxide solution and stopped with 1N H2SO4. Absorbance was read at 450 nm.
8. Statistics
Means of individual group were converted to percent control and expressed as mean±SEM. The difference between groups was assessed using one-way ANOVA and Duncan's Multiple Range test as a post-hoc test. p<0.05 was considered as significantly different.
Results
1. Effect of AMA on secretion of in vivo airway mucin and histopathologic changes in tracheal tissue of rats exposed to sulfur dioxide
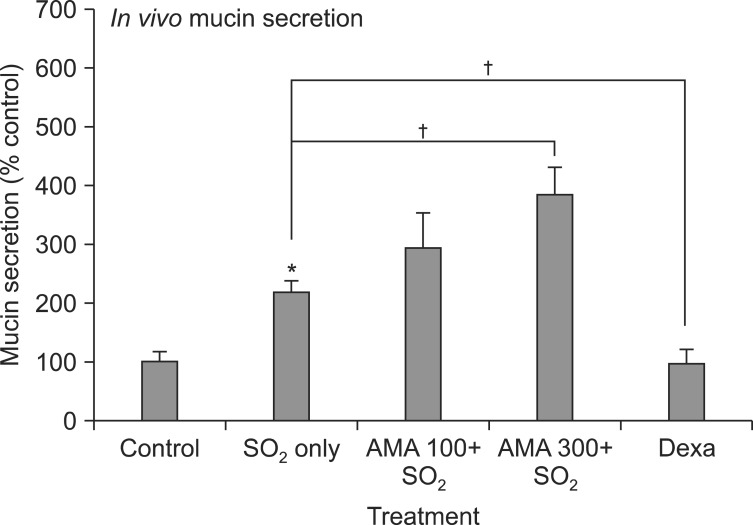
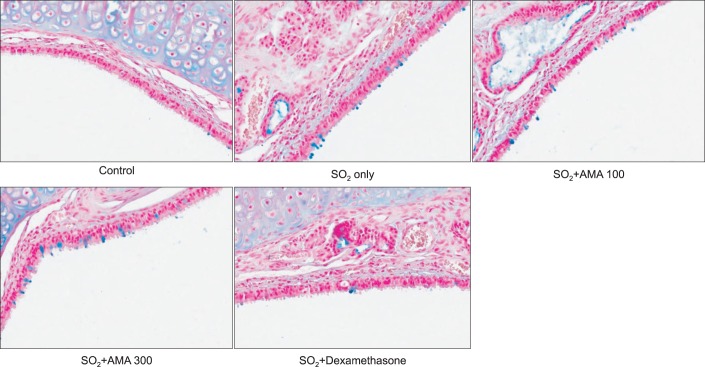
As can be seen in Figures 2 and 3, SO2 exposure to rats for 3 weeks resulted in significant increase in mucin secretion and mucosubstances (acidic mucins) in tracheal tissues, compared with the normal control group. Dexamethasone, a positive control, significantly inhibited mucin secretion and mucosubstances (acidic mucins) in tracheal tissues, due to its prominent antiinflammatory effect. However, AMA stimulated the secretion of mucin and mucosubstances (acidic mucins) in tracheal tissues of rat. The amounts of mucin in the BALF samples were 100±18%, 217±20%, 295±57%, 386±45% and 98±21% for control, SO2 alone, SO2 plus AMA 100 mg/kg, SO2 plus AMA 300 mg/kg, and SO2 plus dexamethasone 0.5 mg/kg, respectively (Figure 2).
Figure 2.
Effect of aqueous extract of the root bark of Morus alba L. (AMA) on secretion of in vivo airway mucin from rats exposed to sulfur dioxide. Rats were exposed to sulfur dioxide and effect of orally-administered AMA on secretion of in vivo airway mucin was investigated. Three independent experiments were performed and the representative data were shown. Each bar represents a mean±SEM from 5 rats. Concentration unit is mg/kg body weight. *Significantly different from control (p<0.05). †Significantly different from SO2 alone (p<0.05). Dexa: dexamethasone; SO2: sulfur dioxide.
Figure 3.
Effect of aqueous extract of the root bark of Morus alba L. (AMA) on epithelial mucosubstances in trachea of rats exposed to sulfur dioxide. Rats were exposed to sulfur dioxide and effect of orally-administered AMA on epithelial mucosubstances (acidic mucins) in trachea was investigated as described in Materials and Methods (×200; hematoxylin and eosin and periodic acid-Schiff-Alcian Blue staining; blue, mucins).
2. Effects of AE and 70% EE of Morus alba L. on PMA-induced MUC5AC production from NCI-H292 cells
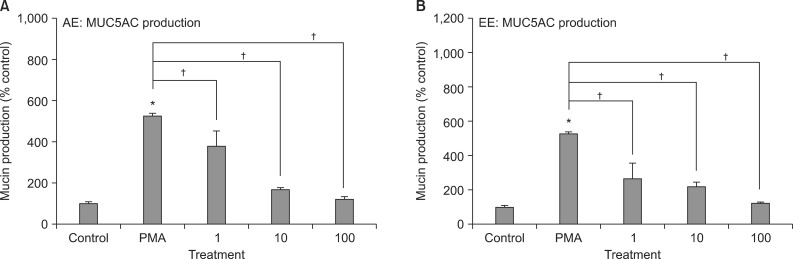
As can be seen in Figure 4, AE and 70% EE of Morus alba L. significantly inhibited PMA-induced MUC5AC production from NCI-H292 cells, respectively. The amounts of mucin in the cells of AE-treated cultures were 100±7%, 528±6%, 379±73%, 165±10%, and 123±9% for control, 10 ng/mL of PMA alone, PMA plus AE 1 µg/mL, PMA plus AE 10 µg/mL and PMA plus AE 100 µg/mL, respectively (Figure 4A). The amounts of mucin in the cells of 70% EE-treated cultures were 100±7%, 528±6%, 262±91%, 216±25%, and 120±5% for control, 10 ng/mL of PMA alone, PMA plus EE 1 µg/mL, PMA plus EE 10 µg/mL, and PMA plus EE 100 µg/mL, respectively (Figure 4B).
Figure 4.
Effects of aqueous extract and 70% ethanolic extract of Morus alba L. on phorbol 12-myristate 13-acetate (PMA)-induced MUC5AC production from NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations of aqueous extract (AE) (A) and 70% ethanolic extract (EE) (B) of Morus alba L. for 30 minutes and then stimulated with PMA (10 ng/mL) for 24 hours. Cell lysates were collected for measurement of MUC5AC mucin production by enzyme-linked immunosorbent assay. Three independent experiments were performed and the representative data were shown. Each bar represents a mean±SEM of 3 culture wells in comparison with that of control set at 100%. Concentration unit is µM. *Significantly different from control (p<0.05). †Significantly different from PMA alone (p<0.05).
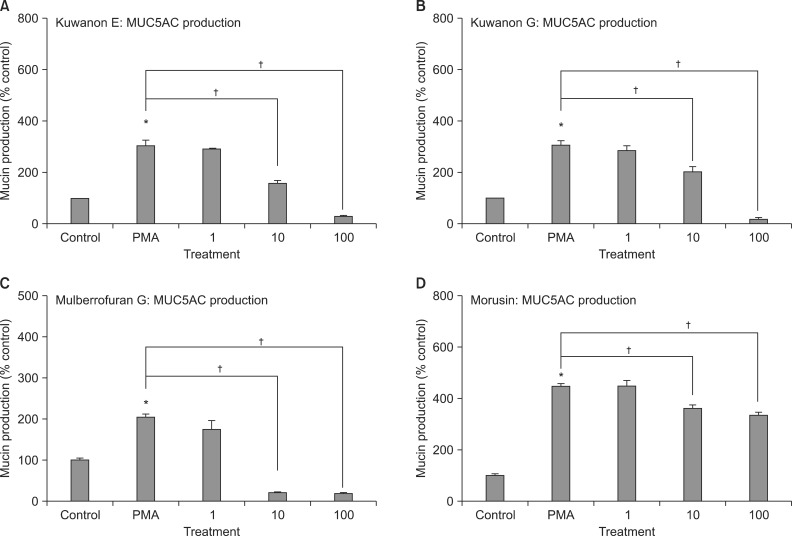
3. Effects of kuwanon E, kuwanon G, mulberrofuran G, and morusin on PMA-induced MUC5AC production from NCI-H292 cells
As can be seen in Figure 5, kuwanon E, kuwanon G, mulberrofuran G, and morusin significantly inhibited PMA-induced MUC5AC production from NCI-H292 cells, respectively. The amounts of mucin in the cells of kuwanon E-treated cultures were 100±1%, 308±17%, 290±5%, 159±9%, and 32±1% for control, 10 ng/mL of PMA alone, PMA plus kuwanon E 10-6 M, PMA plus kuwanon E 10-5 M, and PMA plus kuwanon E 10-4 M, respectively (Figure 5A). The amounts of mucin in the cells of kuwanon G-treated cultures were 100±1%, 308±17%, 286±19%, 204±18%, and 23±1% for control, 10 ng/mL of PMA alone, PMA plus kuwanon G 10-6 M, PMA plus kuwanon G 10-5 M, and PMA plus kuwanon G 10-4 M, respectively (Figure 5B). The amounts of mucin in the cells of mulberrofuran G-treated cultures were 100±5%, 205±5%, 172±24%, 20±1%, and 18±1% for control, 10 ng/mL of PMA alone, PMA plus mulberrofuran G 10-6 M, PMA plus mulberrofuran G 10-5 M, and PMA plus mulberrofuran G 10-4 M, respectively (Figure 5C). The amounts of mucin in the cells of morusin-treated cultures were 100±5%, 446±14%, 451±22%, 364±12%, and 339±8% for control, 10 ng/mL of PMA alone, PMA plus morusin 10-6 M, PMA plus morusin 10-5 M, and PMA plus morusin 10-4 M, respectively (Figure 5D).
Figure 5.
Effects of kuwanon E (A), kuwanon G (B), mulberrofuran G (C), and morusin (D) on phorbol 12-myristate 13-acetate (PMA)-induced MUC5AC production from NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations of each compound for 30 minutes and then stimulated with PMA (10 ng/mL) for 24 hours, respectively. Cell lysates were collected for measurement of MUC5AC mucin production by enzyme-linked immunosorbent assay. Three independent experiments were performed and the representative data were shown. Each bar represents a mean±SEM of 3 culture wells in comparison with that of control set at 100%. Concentration unit is µM. *Significantly different from control (p<0.05). †Significantly different from PMA alone (p<0.05).
Discussion
As aforementioned in introduction, although the root bark of Morus alba L. was used for the regulation of inflammatory pulmonary diseases in folk medicine, there is no report about the pharmacologic action of extract of Morus alba L., kuwanon E, kuwanon G, mulberrofuran G, and morusin on airway mucin secretion and production from inflammatory pulmonary disease model. As can be seen in results, SO2 exposure via aerosolized sodium metabisulfite to rats for 3 weeks resulted in significant increase in mucin secretion and mucosubstances (acidic mucins) in tracheal tissues, compared with the normal control group (Figure 2). Dexamethasone, a positive control, showing prominent anti-inflammatory effect, significantly inhibited mucin secretion and the amount of mucosubstances (acidic mucins) in tracheal tissues. However, AMA stimulated the secretion of airway mucin and mucosubstances (acidic mucins) in tracheal tissues in this rat model. This result can explain, at least in part, the traditional use of AMA as expectorants for controlling various pulmonary inflammatory diseases that are accompanied by mucus hypersecretion. AMA might stimulate the secretion of airway mucus and then remove the mucus from airway by inducing cough reflex. AMA can provoke the expelling of sputum through inducing cough reflex via stimulation of secretion of mucus in airway luminal surface under inflammatory status. Dexamethasone, one of the corticosteroidal compounds used as a remedy for inflammatory diseases, might suppress the overproduction of in vivo airway mucin and resultantly might decrease the amount of secretion of mucin, under inflammatory condition. Since AMA showed the stimulatory action on airway mucin secretion in in vivo model, we tried to investigate which component of AMA can contribute to its pharmacologic activity. Among the twenty one MUC genes coding human mucins reported to date, MUC5AC was mainly expressed in goblet cells in the airway surface epithelium2,20. PMA was reported to stimulate the endogenous activator of protein kinase C (PKC), diacylglycerol21 and to be an inflammatory stimulant that can control a gene transcription22, cell growth and differentiation23. PMA also can induce MUC5AC gene expression in NCI-H292 cells5. PMA activates a type of PKC isoforms. This activates matrix metalloproteinases, which cleave pro-epidermal growth factor receptor (EGFR) ligands from the cell surface to become mature EGFR ligands. These ligands bind to the EGFR, provoking the phosphorylation of its intracellular tyrosine kinase. This leads to activation of MEK leading to ERK activation. Following is the activation of the transcription factor (Sp1) and binding of the factor to specific sites with the MUC5AC gene promoter. Eventually, the promoter is activated and produced the gene transcription and translation to MUC5AC mucin protein22. Based upon these reports, we investigated the effects of AE and 70% EE of Morus alba L. on PMA-induced MUC5AC mucin production from NCI-H292 cells, a human pulmonary mucoepidermoid cell line. As shown in results, AE and 70% EE of Morus alba L. inhibited the production of MUC5AC mucin induced by PMA, respectively (Figure 3). Also, kuwanon E, kuwanon G, mulberrofuran G, and morusin, the natural products derived from Morus alba L., inhibited the production of MUC5AC mucin induced by PMA, respectively (Figure 4). The production and the secretion of airway mucin are known to be regulated through separate signaling steps2. However, based just on the result of this study, we cannot explain the reason AE, 70% EE and the single compounds derived from Morus alba L., kuwanon E, kuwanon G, mulberrofuran G, and morusin, inhibited the production of airway mucin, although AE of Morus alba L. stimulated the secretion of airway mucin. This result might be interpreted that, although AE of Morus alba L. can stimulate the secretion of airway mucin already produced by inflammatory condition, AE and EE and the single compounds derived from it can inhibit de novo production of airway mucin under inflammatory or stimulatory conditions. This interpretation of the result is coincident with the record that AE of Morus alba L. was utilized as a folk remedy which shows an antiinflmmatory activity (decrease in mucin production) and expectorating (increase in mucin secretion) activity6. The underlying mechanisms of action of these single compounds on MUC5AC mucin secretion and production are not clear at present, although we are investigating whether kuwanon E, kuwanon G, mulberrofuran G and morusin act as a potential regulator of the mitogen-activated protein kinase cascade and/or a potential regulator of nuclear factor-kB signaling pathway, in mucin-producing NCI-H292 cells. In summary, these results can explain, at least in part, the folk use of AMA as expectorants and anti-inflammatory agents for controlling various pulmonary inflammatory diseases that are accompanied by hypersecretion of mucus. We suggest it is valuable to find the natural products that have specific regulative effects on mucin production and secretion-in view of both basic and clinical sciences-and the result from this study suggests a possibility of developing kuwanon E, kuwanon G, mulberrofuran G, and morusin as a candidate for the new efficacious mucoregualtors for inflammatory pulmonary diseases, although further studies are essential.
Acknowledgements
This research was supported by a grant (12172KFDA989) from Korea Food & Drug Administration in 2012.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee CJ, Paik SH, Ko KH, Kim KC. Effects of polycationic peptides on mucin release from airway goblet cells: relationship between polymer size and activity. Inflamm Res. 2002;51:490–494. doi: 10.1007/pl00012417. [DOI] [PubMed] [Google Scholar]
- 2.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 3.Heo HJ, Lee HJ, Kim YS, Kang SS, Son KH, Seok JH, et al. Effects of baicalin and wogonin on mucin release from cultured airway epithelial cells. Phytother Res. 2007;21:1130–1134. doi: 10.1002/ptr.2222. [DOI] [PubMed] [Google Scholar]
- 4.Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009;23:1458–1461. doi: 10.1002/ptr.2801. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, et al. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res. 2011;25:1196–1200. doi: 10.1002/ptr.3362. [DOI] [PubMed] [Google Scholar]
- 6.Jang IM. Treatise on Asian herbal medicines. Seoul: Haksul-pyunsu-kwan in Research Institute of Natural Products of Seoul National University; 2003. [Google Scholar]
- 7.Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP. Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: cyclooxygenases and lipoxygenases. Biochem Pharmacol. 2001;62:1185–1191. doi: 10.1016/s0006-2952(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 8.Geng CA, Ma YB, Zhang XM, Yao SY, Xue DQ, Zhang RP, et al. Mulberrofuran G and isomulberrofuran G from Morus alba L.: anti-hepatitis B virus activity and mass spectrometric fragmentation. J Agric Food Chem. 2012;60:8197–8202. doi: 10.1021/jf302639b. [DOI] [PubMed] [Google Scholar]
- 9.Lee JC, Won SJ, Chao CL, Wu FL, Liu HS, Ling P, et al. Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells. Biochem Biophys Res Commun. 2008;372:236–242. doi: 10.1016/j.bbrc.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Mihara S, Hara M, Nakamura M, Sakurawi K, Tokura K, Fujimoto M, et al. Non-peptide bombesin receptor antagonists, kuwanon G and H, isolated from mulberry. Biochem Biophys Res Commun. 1995;213:594–599. doi: 10.1006/bbrc.1995.2173. [DOI] [PubMed] [Google Scholar]
- 11.Pon DJ, van Staden CJ, Boulet L, Rodger IW. Hyperplastic effects of aerosolized sodium metabisulfite on rat airway mucus-secretory epithelial cells. Can J Physiol Pharmacol. 1994;72:1025–1030. doi: 10.1139/y94-143. [DOI] [PubMed] [Google Scholar]
- 12.Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11618–11623. doi: 10.1073/pnas.1534804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hano Y, Fukai T, Nomura T, Uzawa J, Fukushima K. Structure of mulberrofuran I, a novel 2-arylbenzofuran derivatives from the cultivated mulberry tree (Morus bombycis Koidz) Chem Pharm Bull. 1984;32:1260–1263. [Google Scholar]
- 16.Hano Y, Hirakura K, Nomura T, Terada S, Fukushima K. Components of root bark of morus lhou1 1. Structures of two new natural diels-alder adducts, kuwanons N and o. Planta Med. 1984;50:127–130. doi: 10.1055/s-2007-969649. [DOI] [PubMed] [Google Scholar]
- 17.Nomura T, Fukai T, Narita T. Hypotensive constituent, kuwanon H, a new flavone derivative from the root bark of the cultivated mulberry tree (Morus alba L.) Heterocycles. 1980;14:1943–1951. [Google Scholar]
- 18.Nomura T, Fukai T. Constituents of the cultivated mulberry tree. Planta Med. 1981;42:79–88. doi: 10.1055/s-2007-971550. [DOI] [PubMed] [Google Scholar]
- 19.Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 2007;62:153–161. doi: 10.1136/thx.2006.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006;38:116–125. doi: 10.1080/07853890600585795. [DOI] [PubMed] [Google Scholar]
- 21.Hong DH, Petrovics G, Anderson WB, Forstner J, Forstner G. Induction of mucin gene expression in human colonic cell lines by PMA is dependent on PKC-epsilon. Am J Physiol. 1999;277:G1041–G1047. doi: 10.1152/ajpgi.1999.277.5.G1041. [DOI] [PubMed] [Google Scholar]
- 22.Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004;344:683–695. doi: 10.1016/j.jmb.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 23.Park SJ, Kang SY, Kim NS, Kim HM. Phosphatidylinositol 3-kinase regulates PMA-induced differentiation and superoxide production in HL-60 cells. Immunopharmacol Immunotoxicol. 2002;24:211–226. doi: 10.1081/iph-120003751. [DOI] [PubMed] [Google Scholar]