Abstract
Background
Low levels of serum vitamin D is associated with several lung diseases. The production and activation of matrix metalloproteinases (MMPs) may play an important role in the pathogenesis of emphysema. The aim of the current study therefore is to investigate if vitamin D modulates the expression and activation of MMP-2 and MMP-9 in human lung fibroblasts (HFL-1) cells.
Methods
HFL-1 cells were cast into three-dimensional collagen gels and stimulated with or without interleukin-1β (IL-1β) in the presence or absence of 100 nM 25-hydroxyvitamin D (25(OH)D) or 1,25-dihydroxyvitamin D (1,25(OH)2D) for 48 hours. Trypsin was then added into the culture medium in order to activate MMPs. To investigate the activity of MMP-2 and MMP-9, gelatin zymography was performed. The expression of the tissue inhibitor of metalloproteinase (TIMP-1, TIMP-2) was measured by enzyme-linked immunosorbent assay. Expression of MMP-9 mRNA and TIMP-1, TIMP-2 mRNA was quantified by real time reverse transcription polymerase chain reaction.
Results
IL-1β significantly stimulated MMP-9 production and mRNA expression. Trypsin converted latent MMP-2 and MMP-9 into their active forms of MMP-2 (66 kDa) and MMP-9 (82 kDa) within 24 hours. This conversion was significantly inhibited by 25(OH)D (100 nM) and 1,25(OH)2D (100 nM). The expression of MMP-9 mRNA was also significantly inhibited by 25(OH)D and 1,25(OH)2D.
Conclusion
Vitamin D, 25(OH)D, and 1,25(OH)2D play a role in regulating human lung fibroblast functions in wound repair and tissue remodeling through not only inhibiting IL-1β stimulated MMP-9 production and conversion to its active form but also inhibiting IL-1β inhibition on TIMP-1 and TIMP-2 production.
Keywords: Vitamin D, Matrix Metalloproteinase 9, Fibroblasts
Introduction
The role of vitamin D in calcium and bone homeostasis is well described. In the last years, it has been recognized that in addition to this classical function, vitamin D modulates a variety of processes and regulatory systems including host defense, inflammation, immunity, and repair1,2.
Low levels of serum vitamin D is associated with impaired pulmonary function, increased incidence of inflammatory, infectious or neoplastic diseases. Several lung diseases, all inflammatory in nature, may be related to activities of vitamin D including asthma, chronic obstructive pulmonary disease (COPD) and cancer3,4,5. The exact mechanisms underlying these data are unknown, however, vitamin D appears to impact on the function of inflammatory and structural cells of lung.
The aim of the current study therefore is to investigate if vitamin D modulates expression and activation of matrix metalloproteinase (MMP)-9 in human lung fibroblasts (HFL-1) cells, and study the functions of vitamin D on inflammatory and structural cells of lung.
Materials and Methods
1. Materials
25-Hydroxyvitamin D (25(OH)D) or 1,25-dihydroxyvitamin D (1,25(OH)2D) were purchased from TOCRIS Bioscience (Ellisville, MO, USA). Interleukin-1β (IL-1β) was purchased from R&D Systems (Minneapolis, MN, USA). Tissue culture supplements and medium were purchased from GIBCO BRL (Life Technologies, Grand Island, NY, USA). Fetal calf serum (FCS) was purchased from BioFluids (Rockville, MD, USA).
2. Cell culture
Human fetal lung fibroblasts (HFL-1) were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in 100-mm tissue culture dishes (Falcon; Becton Dickinson Labware, Lincoln Park, NJ, USA) with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS, 50 mg/mL of penicillin, 50 mg/mL of streptomycin, and 0.25 mg/mL of Fungizone. The fibroblast were passaged every 3-5 days. Subconfluent fibroblasts were trypsinized (trypsin-EDTA; 0.05% trypsin, and 0.53 mM EDTA-4 Na) and used for collagen gel culture. Fibroblasts used in these experiments were between cell passages 15 and 20.
To assess the effect of vitamin D on fibroblast release of MMPs and tissue inhibitors of metalloproteinases (TIMPs), HFL-1 cells were cast into collagen gels and released into medium. HFL-1 cells in gels then treated with 1 mL per well serum-free DMEM (SF-DMEM), vitamin D, 25(OH)D (100 nM), or 1,25(OH)2D (100 nM) and IL-1β. On day 2, 2 µg trypsin was added into 5 mL gel floating medium to activate MMPs. The gel size of third day was measured in 24 hours after adding trypsin on the cell, media were harvested for enzyme-linked immunosorbent assay (ELISA) (TIMPs) or gelatin zymography EIA (MMPs) as described below. Cells were trypsinized and counted with a Coulter Counter.
3. Preparation of collagen
Type I collagen was extracted from rat tail tendons by a previously published method6,7. Briefly, tendons were excised from rat tails, and the tendon sheath and other connective tissues were carefully removed. After repeated washes with Tris-buffered saline and 95% ethanol, type I collagen was extracted in 4 mM acetic acid at 4℃ for 24 hours. Protein concentration was determined by weighing a lyophilized aliquot from each lot of collagen solution. Sodium dodecyl sulfate polyacrylamide gel electrophoresis routinely demonstrated no detectable proteins other than type I collagen.
4. Preparation of collagen gels
Collagen gels were prepared by mixing the appropriate amounts of rat tail tendon collagen, distilled water, 4× concentrated DMEM, and cell suspension so that the final mixture resulted in 0.75 mg/mL of collagen, 4.5×105 cells/mL, and a physiological ionic strength.
Fibroblasts were always added last to minimize damage during the preparation of the collagen gels. The mixture (0.5-mL aliquots) was cast into each well of 24-well tissue culture plates (Falcon, Franklin Lakes, NJ, USA). Gelation occurred in 20 minutes at room temperature, after which the gels were released and transferred to 60-mm tissue culture dishes containing 5 mL of SF-DMEM and cultured at 37℃ in 5% CO2 for 4-5 days. To demonstrate the effects of cytokines on collagen gel contraction and collagen degradation, cytokines (5 ng/mL of IL-1β), vitamin D, 25(OH)D (100 nM), or 1,25(OH)2D (100 nM) or a combination of both was added to the culture medium. Gel area was measured daily with an image analysis system (Optimax V, Burlington, MA, USA).
5. Hydroxyproline assay
Hydroxyproline, which is directly proportional to type I collagen content, was measured by spectrophotometric determination8,9. Briefly, the medium surrounding the gels was completely removed, and the gels were transferred to a glass tube (KIMAX; Fisher Scientific, St. Louis, MO, USA) with 2 mL of 6N HCl. O2 was removed by ventilation with N2 for 30 seconds. The gels were hydrolyzed at 110℃ for 12 hours. The samples were dried with a vacuum centrifuge and redissolved in distilled H2O before measurement. Hydroxy-proline in the samples was reacted with oxidant (1.4% chloramines T in acetate-citric acid buffer; Sigma, St. Louis, MO, USA) and Ehrlich's reagent (0.4% p-dimethylaminobenzaldehyde; Sigma) in 60% perchloric acid (Fisher Chemical, Fair Lawn, NJ, USA) at 65℃ for 25 minutes, and hydroxyproline content was determined by spectrophotometer at 550 nm.
6. Gelatinase activity assay
To investigate the activity of gelatinase, gelatin zymography was performed. The supernatant-conditioned media were concentrated 10-fold by lyophilization and dissolved in distilled water. Gelatin zymography was performed with a modification of a previously published procedure10,11. Samples were dissolved in 23 electrophoresis sample buffer (0.5 M Tris·HCl, pH 6.8, 10% sodium dodecyl sulfate, 0.1% bromphenol blue, and 20% glycerol) and heated for 5 minutes at 95℃. Forty microliters of each sample were then loaded into each lane, and electrophoresis was performed at 45 mA/gel. After electrophoresis, the gels were soaked with 2.5% (vol/vol) Triton X-100 and gently shaken at 20℃ for 30 minutes. After this, the gels were incubated in the metalloproteinase buffer (0.06 M Tris·HCl, pH 7.5, containing 5 mM CaCl2 and 1 mM ZnCl2) for 18 hours at 37℃. The gels were then stained with 0.4% (wt/vol) Coomassie blue and rapidly destained with 30% (vol/vol) methanol, and 10% (vol/vol) acetic acid.
7. ELISA
The amount of TIMP-1 and TIMP-2 in the cultures was determined by ELISA. Floating collagen gels containing fibroblasts were cultured with or without IL-1β, vitamin D, 25(OH)D (100 nM), or 1,25(OH)2D for 3 days. After incubation, the supernatants surrounding the gels were collected and the concentration of TIMP-1 and TIMP-2 in culture media was also determined by ELISA technique. Ninety-six-well ELISA plates were coated overnight at 4℃ with 100 µL of anti-human TIMP-1 or TIMP-2 antibodies (R&D Systems) diluted in Voler's buffer (pH 9.6). Plates were then washed three times in phosphate buffered saline (PBS) with 0.05% Tween 20 (pH 7.2-7.4) and 100 µL of recombinant human TIMP-1 standards (31.25-4,000 pg/mL) or TIMP-2 standards (15.62-2,000 pg/mL) were added in duplicate. Samples (diluted 1:100 in PBS for TIMP-1 and 1:20-1:50 for TIMP-2) were added in duplicate to individual wells and incubated at room temperature for 2 hours. After three washes, 100 µL of biotinylated anti-human TIMP-1 antibody (R&D Systems) or biotinylated anti human TIMP-2 (R&D Systems) diluted in PBS-Tween were added for 1 hour. After another three washes, 100 µL of horseradish peroxidase-avidin conjugate (Zymed, San Francisco, CA, USA), diluted 1:20,000 in PBS-Tween, were added and incubated for 1 hour in room temperature. After the final three washes, 200 µL of tetramethyl benzidine substrate were added, and color was developed for 30 minutes at room temperature. The reaction was stopped by adding 50 µL of stop solution (1 M H2SO4), and the degree of color generated was determined by measuring the optical density at 450 nm in a microplate reader (Bio-Rad, Hercules, CA, USA).
8. Real-time reverse transcription polymerase chain reaction (RT-PCR)
Fibroblasts were plated into 60-mm dishes and cultured until nearly confluent. Cells were then treated with SF-DMEM ethanol (EOH, 1:1,000), vitamin D, 25(OH)D, or 1,25(OH)2D in the presence or absence of IL-1β. After 24 hours of treatment, total RNA was extracted using Trizol (Invitrogen, Grand Island, NY, USA) following the manufacturer's instructions. Reverse transcription was performed using a commercial kit following the manufacturer's instructions (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems, Invitrogen). Real-time polymerase chain reaction (PCR) was conducted using presynthesized probe and primer sets purchased from Applied Biosystems (Invitrogen) following the manufacturer's instructions. PCR with total volume of 25 mL for each reaction in duplicated assays for each sample was performed with a 7500 Real Time PCR Instrument (Invitrogen).
rRNA was simultaneously tested using TaqMan Ribosomal RNA Control Reagents (Applied Biosystems). Data were normalized by the internal control and expressed as fold change versus ethanol treatment.
9. Statistical analysis
All data are expressed as mean±standard error of the mean (SEM). Statistical comparison of paired data was performed using Student's t-test, whereas multigroup data were analyzed by ANOVA followed by the Tukey's (one-way) or Bonferroni's (two-way) post-hoc analysis using PRISM4 software (GraphPad Prism, San Diego, CA, USA). p<0.05 was considered significant.
Results
1. Effect of vitamin D on collagen gel contraction in the presence of IL-1β
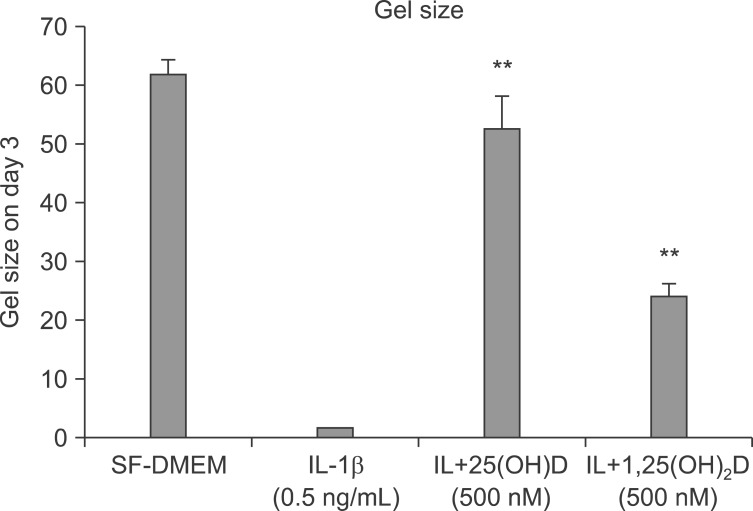
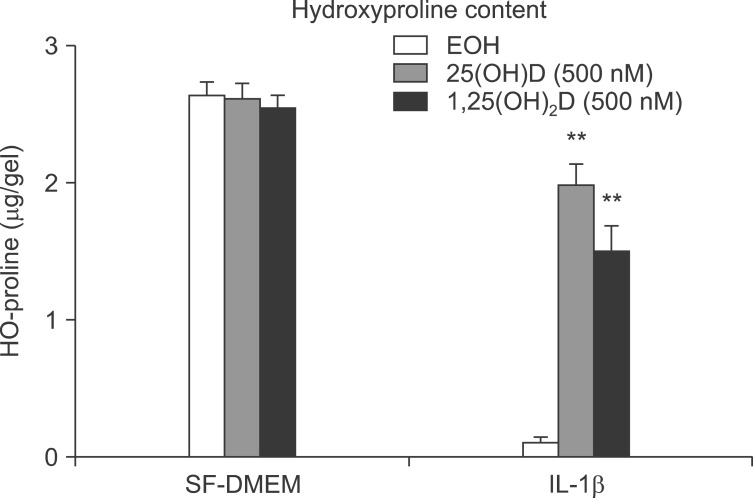
Human lung fibroblasts (HFL-1) cells were cast into three-dimensional collagen gels and stimulated with or without IL-1β (0.5 ng/mL) in the presence or absence of 100 nM 25(OH)D or 1,25(OH)2D for 48 hours. Trypsin was then added into the culture medium in order to activate MMPs. Gel size was measured by an image analyzer. The gel size of third day was measured in 24 hours after adding trypsin on the cell. IL-1β induced collagen gel degradation completely (Figure 1), vitamin D, 25(OH)D, and 1,25(OH)2D significantly inhibited collagen degradation by IL-1β+trypsin as determined by hydroxyproline (0.12±0.07 µg/gel of IL-1β+trypsin vs. 2.00±0.08 µg/gel of IL-1β+trypsin+25(OH)D vs. 1.55±0.35 µg/gel of IL-1β+trypsin+1,25(OH)D; p<0.01) (Figure 2).
Figure 1.
Vitamin D inhibits collagen degradation. HFL-1 cells were cast into collagen gels and released into medium, as shown. On day 2, 2 µg trypsin was added into a 5 mL gel floating medium in order to activate matrix metalloproteinases. After 24 hours of adding trypsin, that is, on day 3, gel size was measured, as shown. Interleukin-1β (IL-1β) induced collagen gel degradation completely, and vitamin D, 25(OH)D, and 1,25(OH)2D inhibited collagen degradation. **p<0.01 compared to IL-1β alone by one-way ANOVA followed by Tukey test. SF-DMEM: serum-free Dulbecco's modified Eagle's medium.
Figure 2.
Gels were harvested and HO-proline amount was quantified, as described. **p<0.01 compared to interleukin-1β (IL-1β) alone by two-way ANOVA followed by Bonferroni test. SF-DMEM: serum-free Dulbecco's modified Eagle's medium.
2. Effect of vitamin D on IL-1β induced production of MMP-9 and MMP-2 in the HFL-1 cells
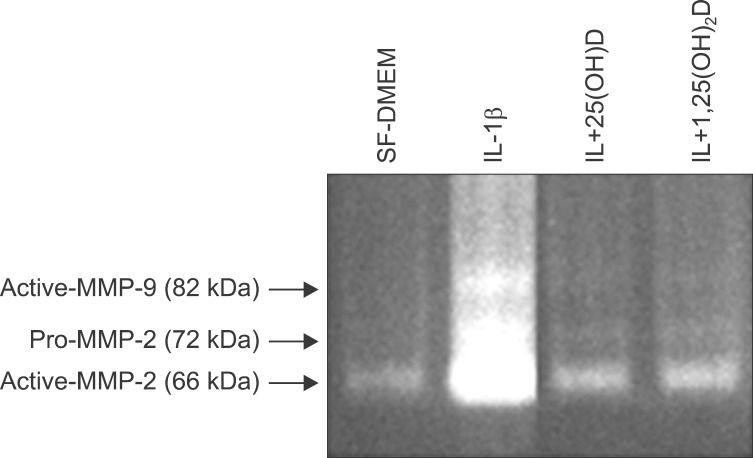
Expression and activity of MMP-2 and MMP-9 (92 kDa) were assessed by gelatin zymography with culture media of collagen gel of fibroblasts in each condition. IL-1β stimulates MMP-2 and MMP-9, trypsin converts latent form of MMP-2 and MMP-9 into active form within 24 hours, vitamin D, 25(OH)D, and 1,25(OH)2D inhibit MMP-2 and MMP-9 production in response to IL-1β, and also inhibits activation of MMPs in induced by trypsin (Figure 3).
Figure 3.
Interleukin-1β (IL-1β) stimulates matrix metalloproteinase (MMP)-9 and MMP-2, and trypsin converts latent form of MMP-2 and -9 into active form. Vitamin D, 25(OH)D, and 1,25(OH)2D inhibit MMP-2 and MMP-9 production in response to IL-1β, and also inhibits activation of MMPs induced by trypsin. SF-DMEM: serum-free Dulbecco's modified Eagle's medium.
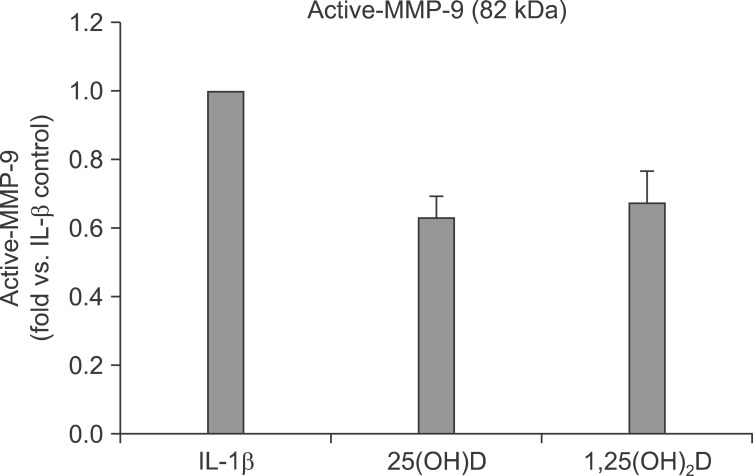
This conversion was significantly inhibited by 25(OH)D (100 nM) and 1,25(OH)2D (100 nM) (62.6±6.5% inhibition by 25(OH)D, and 67.7±9.1% inhibition by 1,25(OH)2D) (Figure 4).
Figure 4.
Active matrix metalloproteinase (MMP)-9 (82 kDa) was determined by scanning densitometry. Interleukin-1β (IL-1β) stimulates MMP-9 and MMP-2, and trypsine converts latent form of MMP-2 and -9 into active form. Vitamin D, 25(OH)D, and 1,25(OH)2D inhibit the activation of MMP induced by trypsin.
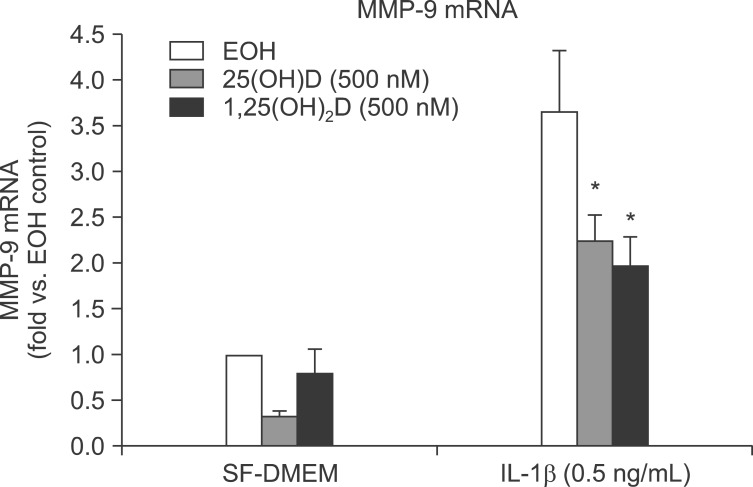
We next examined if vitamin D could inhibit MMP-9 production in response to IL-1β using real-time RT-PCR. HFL-1 cells were treated with vitamin D in presence or absence IL-1β for 24 hours. Total RNA extracted with Trizol. IL-1β significantly stimulated MMP-9 mRNA expression which was partially but significantly blocked by 25(OH)D and 1,25(OH)2D (3.70±0.67 fold vs. ethanol control of IL-1β vs. 1.76±0.31 fold vs. ethonol control of IL-1β+25(OH)D, 1.97±0.35 fold vs. ethanol control of IL-1β+1,25(OH)2D; p<0.05) (Figure 5).
Figure 5.
HFL-1 cells were treated with interleukin-1β (IL-1β) or vitamin D for 24 hours. Total RNA extracted with Trizol. mRNA was measured by real-time reverse transcription polymerase chain reaction. *p<0.05 compared to IL-1β alone by two-way ANOVA followed by Bonferroni test. SF-DMEM: serum-free Dulbecco's modified Eagle's medium. MMP-9: matrix metalloproteinase-9.
3. Effect of vitamin D on TIMP-1 and TIMP-2 production in the presence of IL-1β from HLF-1 cells
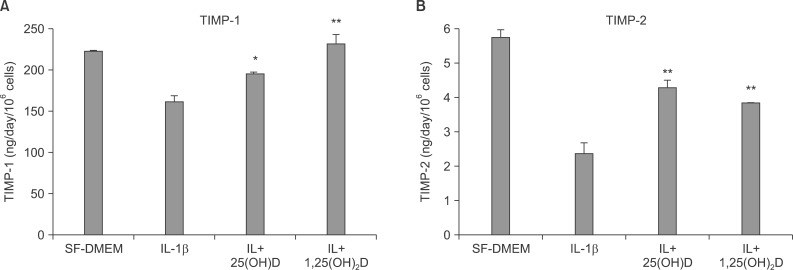
Expression of TIMP-1 and TIMP-2 were assessed by ELISA with culture media of collagen gel of fibroblasts in each condition. IL-1β inhibits TIMP-1 and TIMP-2 production, and vitamin D, 25(OH)D and 1,25(OH)2D significantly blocked IL-1β inhibition on TIMP-1 (161.50±6.51 ng/day/106 cells of IL-1β vs. 194.51±2.06 ng/day/106 cells of IL-1β+25(OH)D, 232.20±11.23 ng/day/106 cells of IL-1β+1,25(OH)2D; p<0.05) (Figure 6A), and TIMP-2 production (2.42±0.32 ng/day/106 cells of IL-1β vs 4.275±0.195 ng/day/106 cells of IL-1β+25(OH)D, 3.82±0.04 ng/day/106 cells of IL-1β+1,25(OH)2D, p<0.05) (Figure 6B).
Figure 6.
Interleukin-1β (IL-1β) inhibits tissue inhibitor of metalloproteinase (TIMP)-1 (A) and TIMP-2 (B) production, and 25(OH)D and 1,25(OH)2D significantly block IL-1β inhibition on TIMP-1 (A) and TIMP-2 (B) production. *p<0.05, **p<0.01 compared to IL-1β alone by one way ANOVA followed by Tukey test. SF-DMEM: serum-free Dulbecco's modified Eagle's medium.
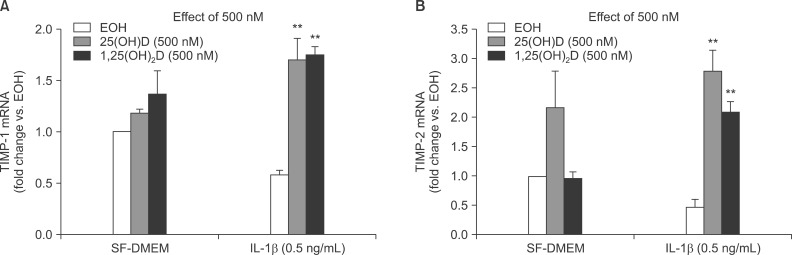
We next examined if vitamin D could inhibit TIMP-1 and TIMP-2 production in response to IL-1β using real-time RT-PCR. HFL-1 cells were treated with vitamin D in presence or absence IL-1β for 24 hours. Total RNA extracted with Trizol. IL-1β inhibits TIMP-1 and TIMP-2 mRNA expression, and vitamin D, 25(OH)D, and 1,25(OH)2D significantly blocked IL-1β inhibition on TIMP-1(0.59±0.04 fold vs. ethanol control of IL-1β vs. 1.705±0.21 fold vs. ethanol control of IL-1β+25(OH)D, 1.74±0.08 fold vs. ethanol control of IL-1β+1,25(OH)2D; p<0.01) (Figure 7A) and TIMP-2 mRNA expression (0.48±0.08 fold vs. ethanol control of IL-1β vs. 2.76±0.37 fold vs. ethanol control of IL-1β+25(OH)D, 2.07±0.19 fold vs. ethanol control of IL-1β+1,25(OH)2D; p<0.01) (Figure 7B).
Figure 7.
(A) TIMP-1 mRNA. (B) TIMP-2 mRNA: **p<0.01 compared to interleukin-1β (IL-1β) treatment by two-way ANOVA followed by Bonferroni test. SF-DMEM: serum-free Dulbecco's modified Eagle's medium.
Discussion
This study demonstrates vitamin D play a role in regulating human lung fibroblast functions in wound repair and tissue remodeling through inhibiting IL-1β stimulated the MMP-2 and MMP-9 production from fibroblasts and inhibiting conversion from latent to active form of MMP-2 and MMP-9. Consistently, vitamin D, 25(OH)D, and 1,25(OH)2D significantly inhibited collagen degradation by IL-1β+trypsin as determined by hydroxyproline assay. Vitamin D, 25(OH)D, and 1,25(OH)2D also significantly blocked IL-1β inhibition on TIMP-1 and TIMP-2 production from fibroblasts.
Emphysema has been believed to develop when mediators of tissue injury exceed protective mechanisms within the lung. Evidence also supports the concept that tissue destruction represents a balance between tissue injury and tissue repair12. If these repair responses can restore normal tissue architecture, function can be preserved. Efforts at repair, however, may result in disruption of normal tissue. In COPD, both in the airways and in the alveolar structures, tissue dysfunction likely results from altered structure due to incompletely effective repair responses13. The net tissue destruction that characterizes emphysema represents an imbalance between tissue destruction and tissue repair processes, analogous to the protease-antiprotease balance. Therefore, the levels and activities of MMPs and TIMPs that are potentially involved in alveolar destruction (emphysema) and extracellular matrix remodeling.
A parallel regulation of IL-1β-induced expression of MMPs and TIMPs was observed in human fibroblasts14 and rat mesangial cells15. An elevated level of IL-1 is one of the key mediators that greatly enhances the biosynthesis and secretion of precursors of these MMPs (pro-MMPs) and prostaglandin E2 from mesenchymal cells at inflammatory sites16. The promotion of wound healing and tissue degradation is considered to be in part due to the production of MMPs by cells stimulated with IL-117.
Fibroblasts play a critical role in tissue repair and remodeling, which is a key feature of COPD and asthma. Fibroblasts modulate tissue repair by producing and modifying extracellular matrix components and by releasing mediators that act as autocrine or paracrine modulators of tissue remodeling18,19.
The culture of fibroblasts in three-dimensional collagen gels has been utilized as an in vitro system to evaluate tissue repair and remodeling20,21. When cultured in three-dimensional gels composed of native type I collagen, fibroblasts orient themselves along the collagen fibers. Both fibroblast proliferation and protein production in three-dimensional collagen gel culture differ markedly from those in routine tissue culture conditions20. Through interactions that depend in part on α2β1-integrins, fibroblasts can exert a tensile force on the collagen fibers. If the gels are unrestrained, for example in floating gel culture, the fibroblasts cause the gels to contract. This contraction can be modified by a variety of exogenous agents, which can either stimulate or inhibit collagen gel contraction22,23.
A recent study showed that vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D binding gene24. There are several factors that could account for vitamin D deficiency in COPD patients: poor diet, a reduced capacity of aging skin for vitamin D synthesis, reduced outdoor activity and therefore sun exposure, an increased catabolism by glucocorticoids, impaired activation because of renal dysfunction, and a lower storage capacity in muscles or fat due to wasting25. Many steps of the vitamin D pathway (intake, synthesis, storage, metabolism) can potentially be disturbed in COPD patients.
Vitamin D belongs to a steroid hormone superfamily of nuclear receptors that has pleiotropic protective effects on several diseases and disorders including asthma and COPD26,27,28. 1,25(OH)2D3 (1,25-dihydroxyvitamin D3) an active metabolite of vitamin D (which binds with nuclear receptor vitamin D receptor [VDR] and interacts other steroid hormone receptors), is a potent regulator of the immune response in Th1 cell-directed diseases29,30.
Vitamin D is a ligand for nuclear hormone VDR, and upon binding it modulates various cellular functions. Vitamin D or VDR deficiency would invoke lung inflammation and alteration in lung function by proteinase/antiproteinase imbalance. Deletion of VDR leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation31.
Vitamin D also to attenuates tumor necrosis factor-α induced upregulation of MMP-9 in keratinocytes32. Vitamin D deficiency may lead to a reduced attenuation of MMP-9 activity resulting in enhanced degradation of lung parenchyma.
In the current study, we have demonstrated that the latent form of MMP-2 and MMP-9 produced in fibroblasts in three-dimensional collagen gels with response to cytokines. Trypsin have a clear effect in converting the MMP-2 and MMP-9 to lower molecular mass forms that corresponded to active MMP-2 and MMP-9. Active form of MMP-2 and MMP-9 from fibroblasts in collagen gel, cultured with IL-1β+trypsin, induced degradation collagen gel completely, however, vitamin D, 25(OH)D, and 1,25(OH)2D significantly inhibited collagen degradation by IL-1β+trypsin (Figure 1) as determined by hydroxyproline assay. Thus the current study supports the concept that cytokines such as IL-1β can induce the production of MMPs but that maximal collagen degradation is achieved only in the presence of an activator such as trypsin.
Vitamin D, 25(OH)D, and 1,25(OH)2D inhibit MMP-2 and MMP-9 production in response to IL-1β, and also inhibits activation of MMPs in induced by trypsin (Figures 3, 4) as a function of modulator of extracellular matrix homeostasis. Using real-time RT-PCR, this study also showed IL-1β significantly stimulated MMP-9 mRNA expression which was partially but significantly blocked by vitamin D, 25(OH)D, and 1,25(OH)2D in real-time RT-PCR (Figure 5). IL-1β inhibits TIMP-1 and TIMP-2 production from fibroblasts and vitamin D, 25(OH)D, and 1,25(OH)2D also significantly blocked IL-1β inhibition on TIMP-1 and TIMP-2 production in ELISA assay (Figure 6). IL-1β inhibits TIMP-1 and TIMP-2 mRNA expression, and vitamin D, 25(OH)D, and 1,25(OH)2D significantly blocked IL-1β inhibition on TIMP-1 and TIMP-2 mRNA expression (Figure 7).
In summary, we have demonstrated that vitamin D, 25(OH)D, and 1,25(OH)2D play a role in regulating human lung fibroblast functions in wound repair and tissue remodeling through not only inhibiting IL-1β stimulated MMP-2 and MMP-9 production and conversion from latent to active form of MMP-2 and MMP-9, but also inhibiting IL-1β inhibition on TIMP-1 and TIMP-2 production.
Our findings that vitamin D modulates cytokine induced MMP synthesis and collagen degradation by human lung fibroblasts, suggest that vitamin D may regulate fibroblast-mediated lung tissue repair and remodeling. This provides a potential mechanism whereby vitamin D could modify lung structure and function related to inflammation such as COPD.
Acknowledgements
This paper was supported by Wonkwang University in 2012.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 2.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. J Immunol. 1986;136:2734–2740. [PubMed] [Google Scholar]
- 3.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 6.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996;32:427–433. doi: 10.1007/BF02723005. [DOI] [PubMed] [Google Scholar]
- 8.Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem. 1963;35:1961–1965. [Google Scholar]
- 9.Edwards CA, O'Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 10.Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 1994;218:325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, McCluskey K, Fujii K, Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998;161:3071–3076. [PubMed] [Google Scholar]
- 12.Snider GL, Failing LJ, Rennard SI. Chronic bronchitis and emphysema. In: Murray JF, Nadel JA, editors. Textbook of respiratory medicine. 2nd ed. Philadelphia: W.B. Saunders; 1994. pp. 1331–1397. [Google Scholar]
- 13.Niewoehner DE. Anatomic and pathophysiological correlations in COPD. In: Baum GL, Crapo JD, Celli BR, Karlinsky JB, editors. Textbook of pulmonary diseases. Philadelphia: Lippincott-Raven; 1998. pp. 823–842. [Google Scholar]
- 14.Murphy G, Reynolds JJ, Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985;260:3079–3083. [PubMed] [Google Scholar]
- 15.Eberhardt W, Beeg T, Beck KF, Walpen S, Gauer S, Bohles H, et al. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000;57:59–69. doi: 10.1046/j.1523-1755.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- 16.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 17.Dinarello CA. The biology of interleukin-1. Chem Immunol. 1992;51:1–32. doi: 10.1159/000319075. [DOI] [PubMed] [Google Scholar]
- 18.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med. 2008;178:248–260. doi: 10.1164/rccm.200706-929OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennard SI, Wachenfeldt K. Rationale and emerging approaches for targeting lung repair and regeneration in the treatment of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011;8:368–375. doi: 10.1513/pats.201102-019RM. [DOI] [PubMed] [Google Scholar]
- 20.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dans MJ, Isseroff R. Inhibition of collagen lattice contraction by pentoxifylline and interferon-alpha, -beta, and -gamma. J Invest Dermatol. 1994;102:118–121. doi: 10.1111/1523-1747.ep12371743. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HY, Gharaee-Kermani M, Phan SH. Regulation of lung fibroblast alpha-smooth muscle actin expression, contractile phenotype, and apoptosis by IL-1beta. J Immunol. 1997;158:1392–1399. [PubMed] [Google Scholar]
- 24.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 26.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert CR, Arum SM, Smith CM. Vitamin D deficiency and chronic lung disease. Can Respir J. 2009;16:75–80. doi: 10.1155/2009/829130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsiligianni IG, van der Molen T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir Res. 2010;11:171. doi: 10.1186/1465-9921-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223:230–233. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 30.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011;406:127–133. doi: 10.1016/j.bbrc.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010;222:729–737. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]