Abstract
Introduction
In the second part of this paper, concerning the use of invisible near infrared light (NIR) fluorescence with indocyanine green (ICG) and methylene blue (MB) in urology, other possible uses of this new technique will be presented. In kidney transplantation, this concerns allograft perfusion and real time NIR–guided angiography; moreover, perfusion angiography of tissue flaps, NIRF visualization of ureters, NIR–guided visualization of urinary calcifications, NIRF in male infertility and semen quality assessment. In this part, we have also analysed cancer targeting and imaging fluorophores as well as cost benefits associated with the use of these new techniques.
Material and methods
PubMed and Medline databases were searched for ICG and MB use in urological settings, along with data published in abstracts of urological conferences.
Results
Although NIR–guided ICG and MB are still in their initial phases, there have been significant developments in a few more major domains of urology, including 1) kidney transplantation: kidney allograft perfusion and vessel reconstruction; 2) angiography perfusion of tissue flaps; 3) visualization of ureters; 4) visualization of urinary calcifications; and 5) NIRF in male infertility and semen quality assessment.
Conclusions
Near infrared technology in urology is at its early stages. More studies are needed to assess the true potential and limitations of the technology. Initial studies show that this pioneering tool may influence various aspects of urology.
Keywords: near infrered light, fluorescence, indocyanine green
INTRODUCTION
In the second part of this paper about the usage of invisible near infrared light (NIR) fluorescence with indocyanine green (ICG) and methylene blue (MB) in urology we have analysed other possible non–oncological uses of this new technique. We have presented the use of this technique in the case of kidney transplantation, as well as allograft perfusion and real time NIR–guided angiography and perfusion angiography of tissue flaps. Moreover NIRF visualization of ureters and NIR–guided visualization of urinary calcifications were analysed. This new technique can also be used in male infertility and semen quality assessment. Additionally, we have also analysed the cost benefits associated with the use of these new techniques.
Kidney transplantation: kidney allograft perfusion
Ever since Joseph Murray performed the first successful kidney transplant in 1954, this procedure has been modified and developed to become one of the most common types of transplantation performed worldwide. However, the challenge still remains to effectively assess organ perfusion; in clinical practice, it is frequently performed subjectively by the surgeon. Only postoperatively, perfusion evaluation using US with Doppler can reveal features of reduced perfusion. A novel approach using NIR–guided ICG was illustrated by Hoffmann et al. [1] in 10 patients, where the authors were able to evaluate renal perfusion intraoperatively immediately after transplantation. By using the NIR system, differences in the brightness of fluorescence were identified in vascular areas of the transplanted organ. This heterogeneity of fluorescence was due to maximum light absorption by deoxygenated hemoglobin compared to oxygenated hemoglobin and water.
Kidney transplantation: Real–time NIR–guided angiography
Although NIR angiography was initially developed for cardiac surgery [2], it has been further applied to kidney and liver transplant surgery. This technique has the benefit of allowing for the assessment of organ vascularity, as well visualization of the number of proximal blood vessels and their location, and possible impediments to resection. Sekijima et al. [3] described 15 patients who underwent kidney transplantation; intraoperative assessment of organ perfusion was performed after completion of vascular anastomosis, during which ICG was given. The advantage of this technique was the simplicity in evaluating the perfusion of the transplanted organ, as the ICG fully revealed the reconstructed vessels in all patients. Furthermore, this method was deemed especially useful when the transplanted organ had more than one artery and as evaluation of anastomosess can be performed immediately thereafter.
Perfusion angiography of tissue flaps
Golijanin et al. [4] applied the NIR–guided imaging technique to demonstrate the ability to evaluate tissue vascularization in three male children undergoing repair of complex hypospadiasis with a local tissue flap. NIR–guided ICG allowed visualization of small vessels, which facilitated in planning the actual volume of the tissue flap. The use of ICG and NIRF in tissue flap perfusion angiography was a less invasive and easier method than radiological assessment using contrasts.
NIRF visualization of ureters
A common task in any uro–abdominal surgery is the identification and isolation of the ureters, as iatrogenic injuries can lead to a number of problems [5]. Additionally, iatrogenic injuries are on the rise as more procedures are performed laparoscopically [6]. A further difficulty in the identification of the ureters is the adipose tissue surrounding the ureters, which is particularly developed in obese individuals. As intraoperative identification and prevention of ureteral injuries reduce patient morbidity and medical costs, a number of methods have been devised, including computed urologic tomography, IV pyelography, ureteral stents in conjunction with palpation, or illuminated catheter and X–ray fluoroscopy using iodine contrast [7]. Unfortunately, the common problem with these techniques is that, rather than simplifying the procedure they can lead to additional complications. Tanaka et al. [8] presented in their study the ability to visualize the ureters using NIR–guided CW800–CA dye and ICG in animal models. First demonstrated in rat models, CW800–CA was administered by IV, while ICG was administered directly and by retrograde manner into the ureters.
CW800–CA was able to detect the ureter after 3 to 5 minutes. The experiment was repeated using a porcine model with CW800–CA and in humans with ICG. During these studies, it was found that the NIR–guided procedure could aid in detecting even small (2.5 mm) foreign bodies located in the urinary tract, as well as in localizing ureteral injury. It was also possible to visualize the peristaltic movements of the ureters using the NIR camera.
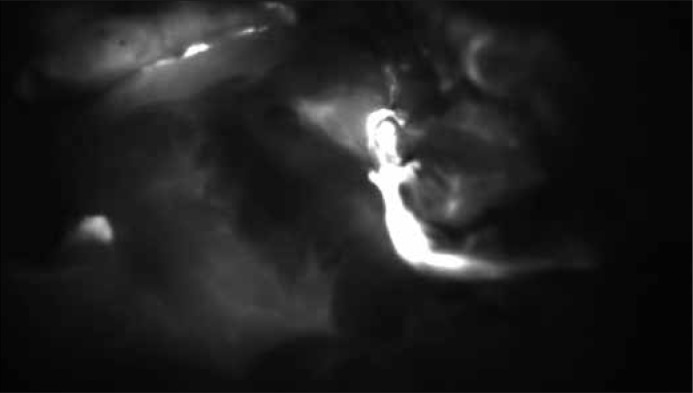
Matsui et al. [9], in their study, presented the possibility of using NIR–guided MB to identify the ureters. This was possible because, unlike ICG – which is mainly cleared via the liver and bile – MB is excreted via the kidneys [10, 11]. The study was conducted on the porcine model, proving that micromolar concentrations of adequately diluted MB turn into a colorless form, leucomethylene blue (LMB), and demonstrate very good fluorescence in the NIR camera. A test of porcine urine showed a typical concentration of 1% in pH 7.4 not visible with NIR light because of quenching. When diluted, MB shows good fluorescence (Figure 1).
Figure 1.
Ureter visualization in NIR light after ICG administration during surgery.
NIR–guided visualization of urinary calcifications
An important aspect of urological diagnosis includes determining the location of urolithiasis, stenosis, and peristaltic movements of the ureters. NIR is usefull in all these cases. Currently, there are a variety of methods to detect stones in the urinary tract, including noncontrast CT, radiographic fluoroscopy and US. Figueiredo [12] illustrated a method for intraoperative detection of calcium urolithiasis using fluorescent probes OS680 and OS750. These fluorophores are diphosphonate imaging agents that have been used in prior studies for the visualization of microcalcification and bone remodeling in vivo [13]. Once administered, these fluorophores bind with hydroxyapatite, which is the main product of both osteoblasts and calcifying vascular cells, and has high binding affinity for phosphonates. The phosphonate component of the fluorophore contains the group PO(OH)2, which is a chelating agent for calcium and magnesium. The binding of diphosphonate to kidney stones was originally discovered when a bone scan was performed using technetium–99m methylene diphosphonate [14]. These fluorophores made it possible to detect various calcium calculi of mixed composition, and to visualize the stone in the renal pelvis or within the renal parenchyma itself.
Cancer targeting and imaging fluorophores
Future imaging of tumors using NIR fluorophores should aim to improve patient outcomes, while reducing procedural costs. One approach has been to develop novel fluorophores capable of improved chemo– and photo–stability, and a greater intensity of fluorescence with more favorable physical properties. Furthermore, NIR fluorophores will become more specific as they are being developed to specifically target cancer cells. These are based on the strategy of combining cancer–targeted ligands such as small organic molecules, peptides, proteins, antibodies, DNA, siRNA, or aptamers to fluorescent dyes such as cyanine dyes, squaraines, phthalocyanines, porphyrin derivatives, and BODIPY analogues. An example of such a complex compound has been developed for use with urological cancer and is a combination of NIR dyes: CTT–Cy7 54.2 to cancer cell, or Cy5.5 ligand to YC–27 cancer molecular ligand for the target molecule, which is PSMA–specific to prostate cancer cells [15, 16]. Cy7 and Cy5.5 are polymethines, which are able of combining with ligands of cancer. Other methods of connecting nanoprobes to specific cancer cells include specific binding with tumor–associated ligands, such as tumor cell receptors, tumor extracellular matrix, and enzymes. NIR fluorophores, capable of activation, for cancer targeting and imaging, can be created as there are a number of differences between cancer and normal cells, such as the presence of specific enzymes or the difference in pH between these cells. In vitro, these molecules do not exhibit fluorescence or only weak fluorescence. They fluoresce only after specific retention and activation of the fluorophore at the target site and reaction with specific enzymes or by alteration of the pH in vivo. A number of different NIR probes that are capable of activation have previously been described in the literature [17–22].
NIRF in male infertility and semen quality assessment
NIRF technique has also been used in the assessment of semen quality in men, and in cases of male infertility. Younan et al. used this technique to examine 40 Egyptian men for the determination of the prevalence of chromosomal anomalies in spermatoses using fluorescence in situ hybridisation [23]. Cortes–Gutierrez et al used NIRF investigating constitiutive alkali–labile sites (ALSs) with the use of DNA breakage detection plus fluorescence in situ hybridization (DBD–FISH) and alkaline single – cell gel electrophoresis (SCGE or comet assay) in cases of spermatozoa in fertile and infertile men [24].
Cost benefits
As NIR guided surgical procedures are rather novel, detailed cost analyses are not widely available. However, in colorectal surgery NIRF costs could be reduced because of the reduction of surgical revisions from 18 to 4 cases (78% reduction). The cost of each revision is about 25000 EUR which means total savings of 350000 EUR. A comparison of total costs and savings per year for 60 surgical procedures was also done. For NIRF systems using ICG – the cost per surgery was estimated to be 6000 EUR. Technetium 99m – 24.000 EUR and Technetium 99m + Blue Dye – 28.500 EUR. Savings comparing with the use of Technetium 99m was 18.000 EUR, Technetium 99m + Blue Dye 22.600 EUR. Cost per patient analysis was also performed showing that the price with the use of ICG and NIRF is <100 EUR, Technetium 99m is 300–450 EUR and for Technetium 99m + Blue Dye is 450–500 EUR. [25]. An important element is the reduction of costs associated with a shorter patient hospital stay, proven with the use NIRF systems [26]. Cost reduction when using different tools and methods is common and very important not only in surgery, but in many other medical areas [27].
CONCLUSIONS
Near infrared–guided ICG and MB surgery are novel techniques which are still in development. There have been significant developments not only in oncological urology, but in other urological settings such as kidney transplantation, angiography, perfusion of tissue flaps, ureter and urinary calcification visualization or infertility. These techniques provide pioneering alternatives and useful tools to current urological image–guided surgery.
There are still many challenges to overcome, but it may in the near future become an important tool allowing for safer, more accurate, and more precise diagnosis and treatment in different urological conditions.
References
- 1.Hoffmann C, Compton F, Schäfer JH, Steiner U, Fuller TF, Schostak M, et al. Intraoperative Assessment of Kidney Allograft Perfusion by Laser– Assisted Indocyanine Green Fluorescence Videography. Transplant Proc. 2010;42:1526–1530. doi: 10.1016/j.transproceed.2010.01.069. [DOI] [PubMed] [Google Scholar]
- 2.Taggart DP, Choudhary B, Anastasiadis K, Abu–Omar Y, Balacumaraswami L, Pigott DW. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann Thorac Surg. 2003;75:870. doi: 10.1016/s0003-4975(02)04669-6. [DOI] [PubMed] [Google Scholar]
- 3.Sekijima M, Tojimbara T, Sato S, Nakamura M, Kawase T, Kai K, et al. An Intraoperative Fluorescent Imaging System in Organ Transplantation. Transplant Proc. 2004;36:2188–2190. doi: 10.1016/j.transproceed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Golijanin DJ, Singer EA, Madeb RR, Wood RW, Dogra VS, Hulbert WC, Jr, et al. Evaluation of flap perfusion during complex hypospadiasis repair using near infrared fluorescence of intravenous indocyanine green. Can Urol Assoc J. 2007;1:305–332. [Google Scholar]
- 5.Elliot SP, McAninch JW. Ureteral injuries: external and iatrogenic. Urol Clin North Am. 2006;33:55. doi: 10.1016/j.ucl.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240–2246. doi: 10.1016/s0022-5347(17)31650-6. [DOI] [PubMed] [Google Scholar]
- 7.Chahin F, Dwivedi AJ, Paramesh A, Chau W, Agrawal S, Chahin C, Kumar A, et al. The implications of lighted ureteral stenting in laparoscopic colectomy. JSLS. 2002;6:49–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka E, Ohnishi S, Laurence RG, Choi HS, Humblet V, Frangioni JV. Real–Time Intraoperative Ureteral Guidance Using Invisible Near–Infrared Fluorescence. J Urol. 2007;178:2197–2202. doi: 10.1016/j.juro.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui A, Tanaka E, Choi HS, Kianzad V, Gioux S, Lomnes SJ, Frangioni JV. Real–time, near–infrared, fluorescence–guided identification of ureters using methylene blue. Surgery. 2010;148:78–86. doi: 10.1016/j.surg.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Santo AR, Wagner JG. Pharmacokinetics of highly ionized drugs. I. Methylene bluewhole blood, urine, and tissue assays. J Pharm Sci. 1972;61:598–602. doi: 10.1002/jps.2600610422. [DOI] [PubMed] [Google Scholar]
- 11.Di Santo AR, Wagner JG. Pharmacokinetics of highly ionized drugs. II. Methylene blueabsorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci. 1972;61:1086–1090. doi: 10.1002/jps.2600610710. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo JL, Passerotti CC, Sponholtz T, Nguyen HT, Weissleder R. A Novel Method of Imaging Calcium Urolithiasis Using Fluorescence. J Urol. 2008;179:1610–1614. doi: 10.1016/j.juro.2007.11.100. [DOI] [PubMed] [Google Scholar]
- 13.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. In vivo nearinfrared fluorescence imaging of osteoblastic activity. Nat Biotechnol. 2001;19:1148. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 14.Wolf JS, Jr, Irby PB, 3rd, Shields A, Yuen-Green MS, Hattner RS, Stoller ML. Urolithoscintygraphy: preliminary report of a new imaging modality of urolithiasis. J Endourol. 1994;8:133. doi: 10.1089/end.1994.8.133. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Wu LY, Hopkins MR, Choi JK, Berkman CE. A targeted low molecular weight nearinfrared fluorescent probe for prostate cancer. Bioorg Med Chem Lett. 2010;20:7124–7126. doi: 10.1016/j.bmcl.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Dhara S, Banerjee SR, Byun Y, Pullambhatla M, Mease RC, Pomper MG. A low molecular weight PSMA–based fluorescent imaging agent for cancer. Biochem Biophys Res Commun. 2009;390:624–629. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CH, Cheng SH, Wang YJ, Yu–Ching Chen YC, Chen NT, Souris J, et al. Near–infrared mesoporous Silica nanoparticles for optical imaging: characterization and in vivo biodistribution. Adv Funct Mater. 2009;19:215–222. [Google Scholar]
- 18.He X, Wu X, Wang K, Shi B, Hai L. Methylene blue–encapsulated phosphonate– terminated silica nanoparticles for simultaneous in vivo imaging and photodynamic therapy. Biomaterials. 2009;30:5601–5609. doi: 10.1016/j.biomaterials.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Tung CH. Fluorescent peptide probes for in vivo diagnostic imaging. Pept Sci. 2004;76:391–403. doi: 10.1002/bip.20139. [DOI] [PubMed] [Google Scholar]
- 20.Tung C-H, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60:4953–4958. [PubMed] [Google Scholar]
- 21.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, et al. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 22.Matsui A, Tanaka E, Choi HS, Kianzad V, Gioux S, Lomnes SJ, Frangioni JV, et al. A novel method for imaging apoptosis using a caspase–1 near–infrared fluorescent probe. Neoplasia. 2004;6:95–105. doi: 10.1593/neo.03214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younan D, Sorour A, Genedy R. Aneuploidy frequency in spermatozoa of Egyptian men with normal and abnormal semen parameters usingfluorescence in situ hybridisation. Andrologia. 2014 Feb 27; doi: 10.1111/and.12251. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Cortés–Gutiérrez EI, Dávila–Rodríguez MI, Cerda–Flores RM, Fernández JL, López–Fernández C, Aragón Tovar AR, Gosálvez J. Localisation and quantification of alkali–labile sites in human spermatozoa by DNA breakage detection–fluorescence in situ hybridisation. Andrologia. 2014 Feb 27; doi: 10.1111/and.12250. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25. www.pulsion.com.
- 26. www.fluoptics.com.
- 27.Comploj E, Mian M, Koen M, Becker T, Berger C, Riccabona M. Effectiveness and cost analysis of different methods of anti–refluxive operations in VUR grade 3 in a single institution. Paediatric Urology. doi: 10.5173/ceju.2010.03.art5. [DOI] [Google Scholar]