Abstract
Background
Stargazin is the first transmembrane protein known to regulate synaptic targeting of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors. Yet, it is unclear whether regulation of the surface delivery of spinal AMPA receptor subunits by stargazin contributes to postoperative pain development.
Methods
Western blot analysis was used to examine changes in the surface delivery of AMPA receptor subunits GluR1 and GluR2 in rat dorsal horn. The interaction between stargazin and GluR1 and GluR2 was examined by coimmunoprecipitation. Expression of stargazin was suppressed by intrathecal administration of small interfering ribonucleic acid (siRNA)311.
Results
Membrane-bound GluR1, but not GluR2, in ipsilateral dorsal horn was increased at 3 h (1.49±0.15-fold of β-tubulin, mean±SEM) and 1 day (1.03±0.25) after incision, as compared to that in control rats (naïve, 0.63±0.23, P < 0.05, n=6/group). The amount of GluR1 coimmunoprecipitated with stargazin was greater at 3 h after incision (1.48±0.31-fold of input) than in control animals (0.45±0.24, P < 0.05, n=6/group). Importantly, the increase in membrane GluR1 at 3 h after incision was normalized to near control level (0.72±0.20-fold of β-tubulin) by pretreatment with intrathecal stargazin siRNA311 (0.87±0.09), but not scrambled siRNA (1.48±0.24) or vehicle (1.25±0.13, P < 0.05, n=6/group). Stargazin siRNA311 pretreatment prevented the increase in stargazin–GluR1 interaction and decreased postoperative pain after incision.
Conclusions
This study suggests a critical role of stargazin-mediated surface delivery of GluR1 subunit in the development of postoperative pain. A better therapeutic strategy for postoperative pain may involve selectively downregulating spinal stargazin to inhibit synaptic targeting of GluR1 subunit.
Introduction
Postoperative pain remains a significant medical problem.1 Opioids are commonly used for pain management but are often associated with severe side effects such as nausea, vomiting, and respiratory depression. Moreover, the etiology of postoperative pain is likely different from that of other clinical pain conditions such as rheumatoid arthritis and fibromyalgia.2 Consequently, the treatment is likely to differ also. To date, developing safe and effective analgesics remains a priority for improving postoperative pain management.
To mimic postsurgical pain manifestations in humans, Brennan et al.3 developed a rodent model of postoperative pain that is induced by an incision in the plantar aspect of the hind paw in rats. Several lines of evidence have shown that epidural or intrathecal administration of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate receptor antagonists produces analgesia in this model, suggesting an important role of spinal AMPA receptors in postoperative pain.4;5 AMPA receptors are multimeric assemblies of four subunits, GluR1–4. Receptor function can be regulated by the phosphorylation and trafficking of these subunits.6;7 AMPA receptors that lack the edited GluR2 subunit are permeable to Ca2+, as editing of this subunit after transcription into messenger RNA results in the introduction of a positive charge in the pore-forming region.8–10 Under physiologic conditions, most AMPA receptors are impermeable to Ca2+, owing to the presence of the GluR2 subunit. Changes in GluR1 and GluR2 subunit trafficking, which lead to rapid alterations in the composition of synaptic AMPA receptors, may play an important role in pain hypersensitivity that develops after tissue or nerve injury.11–14 Our recent study showed that plantar incision may selectively increase the surface delivery of GluR1, but not GluR2, in the dorsal horn at 3 h after incision.15 This finding suggests that an increase in Ca2+-permeable AMPA receptors may contribute to postoperative pain by strengthening the excitatory synaptic transmission in dorsal horn.
Stargazin is an important member of the AMPA receptor regulatory protein family that binds to GluR1, 2, and 4 at sites other than PDZ [Postsynaptic density (PSD)-95/SAP90, Dlg, ZO-1] target motifs.16 Binding of stargazin’s C-terminal tail to PDZ domains of synaptic scaffolding proteins, such as PSD-95, may mediate the proper targeting of AMPA receptors to synaptic membrane.17 AMPA receptors fail to traffic to the plasma membrane or synapse in stargazer mutant cerebellar cells, suggesting that stargazin is indispensable for the plasma membrane expression of AMPA receptor subunits.18;19 Further, overexpression of a dominant negative stargazin construct reduces synaptic AMPA receptor function in hippocampal neurons.20 Accordingly, we hypothesize that stargazin may play an important role in incision-induced membrane trafficking of AMPA receptors in dorsal horn neurons and hence contribute to postoperative pain. Conversely, disrupting the interaction between stargazin and GluR1 in dorsal horn might decrease postoperative pain. We used intrathecal delivery of small interfering RNAs (siRNAs), a selective and effective gene-silencing method, to determine whether downregulation of spinal stargazin in vivo attenuates postoperative pain in rats by inhibiting the membrane surface delivery of GluR1 and/or GluR2 subunit in the dorsal horn.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 280–300 g were used for all studies. All animal procedures were approved by the Ethical Committee of Beijing Chaoyang Hospital, Capital Medical University (Beijing, China), and were performed in accordance with the Guidelines for Animal Experimentation of the International Association for the Study of Pain. To avoid selection bias, we assigned animals randomly to different experimental groups by a computer-generated randomization list. We determined the sample size for each experiment based on similar studies of our previous work.
Plantar incision
The plantar incision model was carried out as previously described.3 Briefly, a 1-cm longitudinal incision was made through plantar skin and fascia of the right hind paw of rats under isoflurane anesthesia (1.5–1.8%). The underlying flexor muscle was elevated with forceps and incised longitudinally. The wounded skin was closed and covered with antibiotic ointment. Sham-operated rats underwent all procedures but were not incised. Rats were subjected to behavioral testing and then sacrificed at 3 h, 1 day, and 3 days after plantar incision.
Behavioral testing
Behavioral tests were performed by an experimenter blind to surgery and drug treatment conditions. The cumulative pain scores were evaluated based on guarding behavior, and mechanical sensitivity was tested with calibrated von Frey filaments (North Coast Medical, Inc., San Jose, CA). In brief, the animals were placed on an elevated mesh floor and observed closely for a 1-min period every 5 min for 1 h. Based on the extent of weight-bearing on the incised foot during the scoring period, the rat was given a score of 0, 1, or 2. The sum of the 12 scores established the cumulative pain score.3;21 To measure the mechanical withdrawal threshold, we applied a series of filaments vertically to an area adjacent to the wound near the medial heel for 6 s or until the animal withdrew the paw. A positive response included rapid lifting, shaking, or licking of the incised paw. The mechanical withdrawal threshold was determined by an up-down method as described previously.22;23
Western blot analysis, subcellular membrane fractionation, and detection of GluR1 and GluR2 subunits
We used Western blot analysis to determine the expression of stargazin and to examine cellular trafficking of GluR1 and GluR2 in dorsal horn during the period of postoperative pain. Subcellular membrane fractionation and protein detection were carried out as described previously.13;24 The dorsal lumbar spinal cord (L3–L6) ipsilateral and contralateral to the incised paw was separated and stored at −70°C. Tissues were homogenized and centrifuged to separate cytosolic and membrane fractions. Protein concentrations were measured with a bicinchoninic acid kit (Pierce Biotechnology Inc., Rockford, IL, USA). Proteins were loaded and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Pan-cadherin, a plasma membrane marker, was used as a control for the membrane fractions. After electrophoresis, proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane at 4°C. The membrane was blocked and incubated overnight with primary rabbit antibodies to N-cadherin, GluR1, or GluR2 (1:1000, Millipore, Billerica, MA). Then, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000; Amersham, Piscataway, NJ). Finally, the membranes were exposed to reagents from the Enhanced Chemiluminescence (ECL) Detection Kit (PerkinElmer Life Science, Waltham, MA) and x-ray film for visualization of protein bands. Band intensity was quantified with imaging software; β-tubulin was used as the loading control.
Whole-cell homogenates
The spinal cord tissues were homogenized and centrifuged at 14,000 rpm for 15 min at 4°C. The protein concentration of the supernatant was determined with the bicinchoninic acid kit. Protein (60 μg) was separated by SDS-PAGE and then transferred onto a PVDF membrane. The membrane was blocked and then incubated with primary rabbit antibody to stargazin (1:1000 dilution) at 4°C overnight. Then the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000) for 1 h. Bands were visualized by ECL and exposure to x-ray film. Immunostaining of β-tubulin was used as a loading control.
Immunofluorescence
Rats were perfused transcardially with 10 mM phosphate-buffered saline followed by 4% formaldehyde. L3–L6 spinal cord segments were mounted and frozen at −80°C and then transversely cut to a 20-μm thickness. Spinal cord sections were blocked and dual stained for stargazin and GluR1. The tissues were incubated overnight in monoclonal rabbit anti-stargazin (1:50; Cell Signaling Technology, Danvers, MA) with mouse anti-GluR1 (1:50; Novus, Littleton, CO). The sections were then incubated for 1 h in tetramethylrhodamine isothiocyanate-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate-conjugated donkey anti-mouse IgG (1:200) and mounted in VectaShield medium (Vector Laboratories, Burlingame, CA). Confocal microscopy was performed, and images were prepared with the confocal software and Adobe Photoshop software (San Jose, CA). All images were taken from the right dorsal horn of the normal rats.
Coimmunoprecipitation
Coimmunoprecipitation was carried out as described previously with some modification.25 The affinity-purified rabbit stargazin antibody (3 μg) was incubated with 30 μL of protein G-sepharose slurry for 1 h. The complex was spun down at 2,000 rpm for 4 min. The total protein from spinal cord homogenates (500 μg) was added to the sepharose beads and the mixture incubated for 2 to 3 h at 4°C. After the mixture was washed, the proteins were separated by SDS-PAGE and transferred onto PVDF membrane. The membrane was blocked and then incubated overnight with primary antibodies to stargazin, GluR1, or GluR2 (1:1000, Millipore). As a positive control (input), 50 μg of total protein was loaded onto the gel. The proteins were detected with horseradish peroxidase-conjugated secondary antibody and visualized by ECL and exposure to x-ray film. The intensity of the protein bands was quantified with densitometry.
Intrathecal catheter implantation
An intrathecal catheter was placed into the lumbar spinal region as described in our previous study.15 Briefly, a 20-gauge blunt guide cannula was advanced into the narrow space between the L5 and L6 spinous processes until a tail flick or paw retraction was observed. A sterile, polyethylene-10 catheter was threaded into the guide cannula. At 2–3 days after catheter implantation, successful intrathecal drug delivery was confirmed by infusing lidocaine (10 μl, 2%, Hospira, Lake Forest, IL) through the catheter. If the catheter is placed properly, the lidocaine induces a temporary motor paralysis of the lower limbs.
Suppression of stargazin expression with siRNA
Purified siRNA duplexes targeting rat stargazin were chemically synthesized by Genechem Inc. (Shanghai, China). We used three different siRNA sequence pairs as follows: pair 1, 5′-CCCAUUCCGGAUUAUGGAGtt-3′ and 5′-CUCCAUAAUCCGGAAUGGGtt-3′; pair 2, 5′-CCGCAGAGUAUUUCCUCAGtt-3′ and 5′-CUGAGGAAAUACUCUGCGGtt-3′; and pair 3, 5′-UCGGGAUCAUCGUGUAUAUtt-3′ and 5′-AUAUACACGAUGAUCCCGAtt-3′. Clustal alignment indicates that the sequences are to nucleotides 209 to 227, 311 to 329, and 485 to 503, respectively, of the rat stargazin messenger RNA sequence (GenBank Accession number: NM_053351).
To identify the interference efficiency of the three siRNA duplexes and screen the optimal siRNA duplexes for in vivo experiments, we first carried out co-transfection experiments in vitro. Human embryonic kidney 293T cells were cultivated under standard conditions as described previously.26 Co-transfection experiments were carried out with a pcDNA3.1/stargazin-green fluorescent protein (GFP) plasmid (0.3 μg) and two concentrations of siRNA (0.4 or 0.8 μg). Selective stargazin siRNA or scrambled siRNA was mixed in 200 μL of OptiMEM I low-serum media with 6 μL of Lipofectamine reagent (Invitrogen, Grand Island, NY). Complexes were allowed to form for 20 min before being added to cells. Cells were incubated at 37°C with gentle rocking for 48 h and then harvested. Protein expression was analyzed by Western blotting. The stargazin-GFP fusion protein was detected with anti-GFP serum (Invitrogen).27
The rats were injected intrathecally with vehicle, stargazin siRNA, or scrambled siRNA (negative control, 2.6 μg in 10 μL) in transfection reagent (at a ratio of 1:5, w:v) twice daily for 3 consecutive days before plantar incision. All injections were followed by a 10 μL saline flush to clear the catheter. The selected doses of siRNA were based on our preliminary data.
Statistical analysis
Quantitative analysis for immunoblotting was performed after scanning of the x-ray film with the Gel Doc 2000 imaging system (Bio-Rad Laboratories Inc., Hercules, CA). The relative optical density of each band was normalized against the corresponding β-tubulin or glyceraldehyde-3-phosphate dehydrogenase. We analyzed expression time courses of GluR1, GluR2, and stargazin by one-way analysis of variance (ANOVA) followed by all pair-wise multiple comparison procedures using the Bonferroni test. In coimmunoprecipitation experiments, comparisons of immunoprecipitated GluR1 and GluR2 between control and the 3 h time point were made with Student’s t-test for independent samples. For comparisons of input and immunoprecipitation on immunoblots of GluR1, GluR2, and stargazin among control, sham-operated, and incision groups, we used ANOVA followed by Bonferroni multiple comparison tests. The data from cell culture and pain behavioral tests, and the expression of GluR1 and GluR2 in cytosolic and membrane fractions of control, sham-operated, and incision groups were also analyzed with ANOVA followed by Bonferroni multiple comparison tests. Because we had open outcome expectations for these experiments (i.e., possible changes in both directions), we used two-tailed/two-sided tests. Data are expressed as mean ± SEM. P < 0.05 was regarded as significant. SPSS 11.5 (SPSS Inc., Chicago, IL) was used for data analysis.
Results
Throughout all experiments, no data were excluded, missing, or lost to observation. Six rats were used per group for each time point, except where noted otherwise.
Plantar incision increases the surface delivery of GluR1, but not GluR2, in ipsilateral dorsal horn
At 3 h after plantar incision, GluR1 level in the plasma membrane fraction was significantly increased in ipsilateral dorsal horn, as compared to that in the control group (P < 0.01; Fig. 1A). In contrast, cytosolic GluR1 was significantly decreased in ipsilateral dorsal horn (P < 0.01; Fig. 1B). The cytosolic GluR1 remained downregulated for 3 days after incision (P < 0.05). GluR2 expression did not change in the plasma membrane or cytosolic fraction after incision (Fig. 1, A and B). Expression of neither GluR1 nor GluR2 changed in the contralateral dorsal horn after incision in either the plasma membrane or cytosolic compartment (data not shown). The integrin N-cadherin, a plasma membrane-specific protein, was present only in the plasma membrane fraction, confirming the fractionation procedure (Fig. 1A).
Fig. 1. Plantar incision increases the surface delivery of AMPA receptor subunit GluR1, but not GluR2, in the dorsal horn.
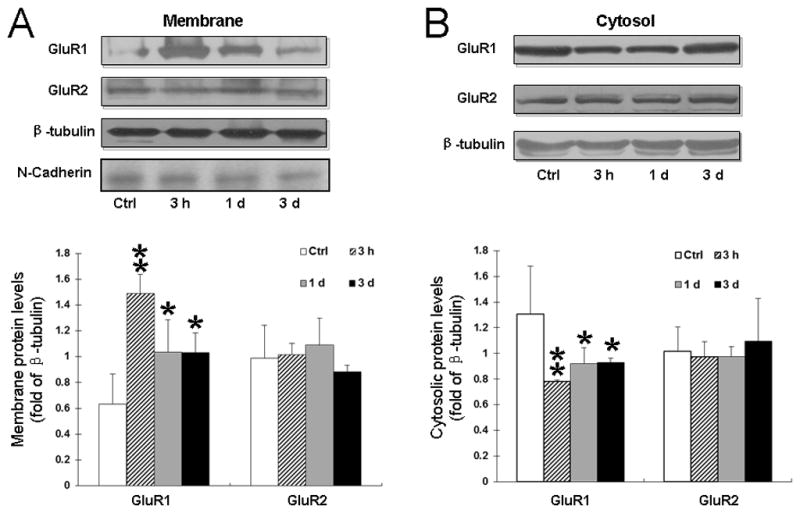
(A) Representative Western blots and quantitative analysis of band density show GluR1 and GluR2 protein levels in the plasma membrane fraction of ipsilateral dorsal horn in rats at 3 h, 1 day, and 3 days after plantar incision. β-tubulin was used as an internal reference. (B) Representative Western blots and quantitative analysis of GluR1 and GluR2 protein levels in the cytosolic fraction of different groups. Ctrl: Control group (naïve). *P < 0.05, **P < 0.01 compared to the control group. n = 6 rats/time point.
Plantar incision does not alter the expression of stargazin in ipsilateral dorsal horn
Stargazin is required for the trafficking of AMPA receptor GluR1 and GluR2 subunits. However, we found no significant change in stargazin expression in the ipsilateral dorsal horn at 3 h, 1 day, or 3 days after incision (Fig. 2, A and B). Expression of stargazin in contralateral dorsal horn was also unchanged at these time points (data not shown).
Fig. 2. Plantar incision does not significantly change the expression of stargazin in dorsal horn.
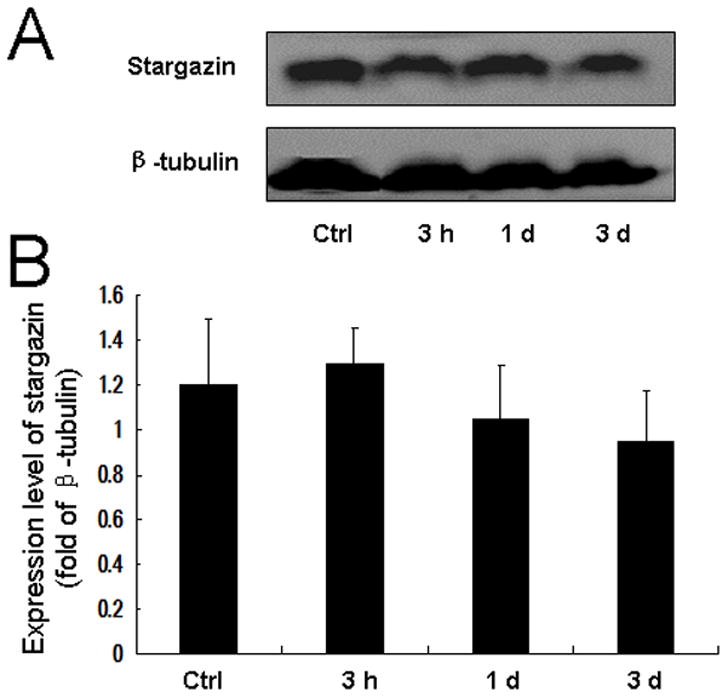
(A) Representative Western blot shows the expression of stargazin in the ipsilateral spinal cord dorsal horn in rats at different time points after plantar incision. β-tubulin was used as an internal reference. (B) Quantitative analysis shows that surgical incision did not significantly alter the expression of stargazin in the ipsilateral spinal cord dorsal horn, as compared to that in the control group (P > 0.05, n = 6 rats/time point). Ctrl, control.
Stargazin colocalizes with GluR1 in dorsal horn neurons
A previous study showed that stargazin colocalizes with the GluR2 subunit in spinal cord dorsal horn.25 Our double-labeling immunofluorescence study showed that stargazin and GluR1 subunits are also highly colocalized in dorsal horn of naïve rats (Fig. 3, A–F).
Fig. 3. Colocalization of stargazin and GluR1 in rat dorsal horn.
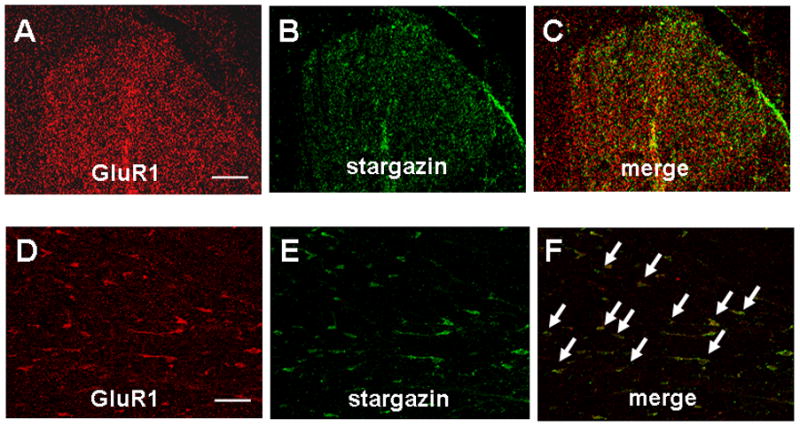
Representative confocal images of GluR1 (A and D, red) and stargazin (B and E, green) immunoreactivity in the dorsal horn of L5–L6 spinal segment. Colocalization (merge) is shown in yellow/orange (C and F). Double-labeled neurons are indicated by arrows. A, B, and C are shown in lower magnification. D, E, and F are shown in higher magnification. Scale bars: 100 μm (A) and 20 μm (D).
Plantar incision enhances the interaction between stargazin and GluR1 subunits
To determine if stargazin differentially regulates the surface delivery of GluR1 and GluR2 in the dorsal horn after plantar incision, we examined the interactions between stargazin and each subunit by coimmunoprecipitation. Both GluR1 and GluR2 were coimmunoprecipitated by stargazin antibody in naïve animals. At 3 h after plantar incision, the amount of GluR1 subunit that was coimmunoprecipitated with stargazin in the ipsilateral dorsal horn of rats was significantly increased compared to that of the control group (Fig. 4, A and B). However, the amount of GluR2 coimmunoprecipitated remained unchanged. Neither GluR1 nor GluR2 subunit was coimmunoprecipitated by IgG (Fig. 4A).
Fig. 4. Interaction between stargazin and GluR1 subunit in the ipsilateral dorsal horn is enhanced at 3 h after plantar incision.
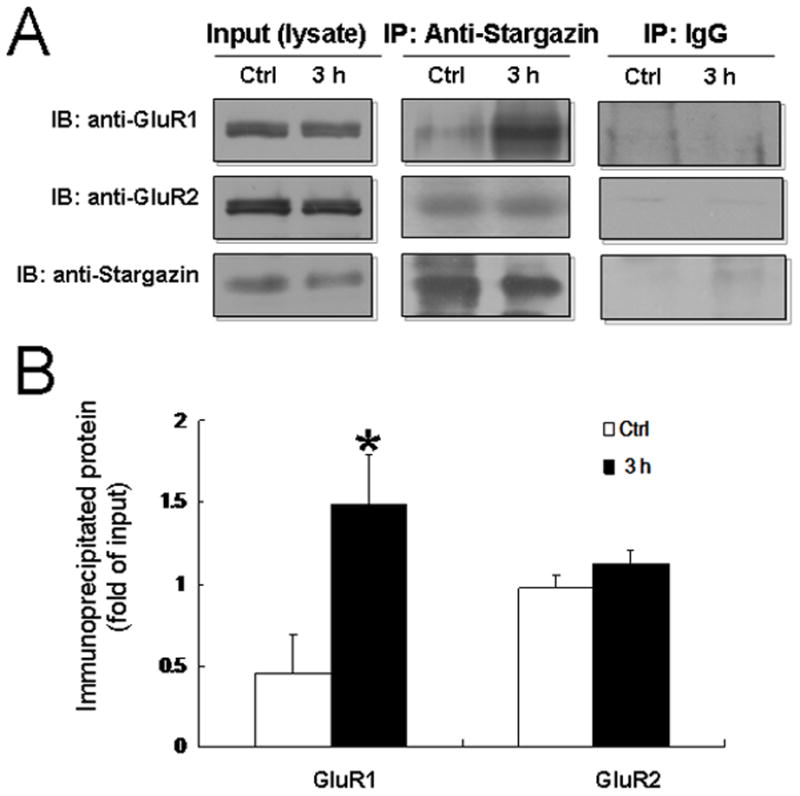
(A) Ipsilateral dorsal horn tissue was collected from rats at 3 h after plantar incision of the right hind paw and from control (naïve) rats. Representative immunoblots illustrate total GluR1 and GluR2 protein (Input) and GluR1 and GluR2 coimmunoprecipitated by stargazin antibody (IP: anti-stargazin) or IgG (negative control). (B) Quantitative analysis shows that GluR1 and GluR2 were both coimmunoprecipitated by stargazin antibody in the control group. The association between stargazin and GluR1 was significantly elevated at 3 h after incision. Neither GluR1 nor GluR2 was coimmunoprecipitated with IgG. IP, immunoprecipitation; IB, immunoblotting; Ctrl, control. *P < 0.05 compared to the control group. n = 6 rats/group.
Identification of the optimal siRNA for knocking down stargazin expression in vitro
We screened three siRNAs in cultured human embryonic kidney 293T cells to identify the one most effective for inhibiting stargazin expression. siRNA209, siRNA311, and siRNA485 all dose-dependently inhibited the expression of stargazin-GFP (Fig. 5A). siRNA311 induced a greater inhibition than the other two constructs at the lower dose (0.4 μg) and completely inhibited expression at the higher dose (0.8 μg, Fig. 5B). Therefore, we chose siRNA311 to downregulate the expression of stargazin in the subsequent in vivo experiments. The scrambled control siRNA did not influence stargazin-GFP protein expression in the dose range investigated.
Fig. 5. Identification of small interfering RNA (siRNA) for the knockdown of stargazin in cell culture.
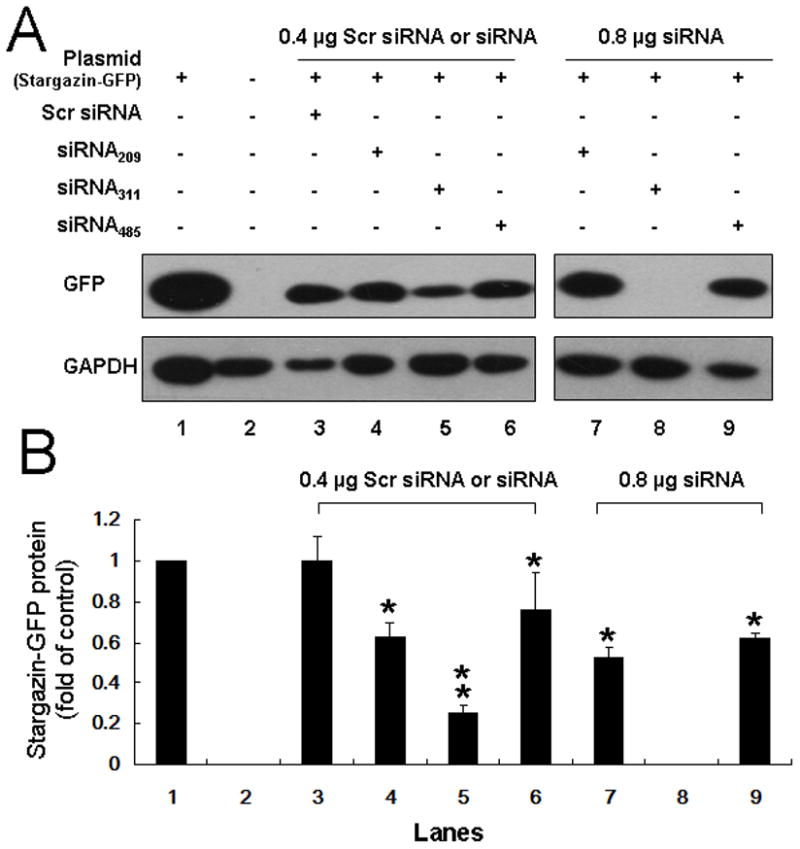
(A) Representative immunoblots show the effects of three stargazin siRNA constructs on the expression of stargazin-green fluorescent protein (GFP) fusion protein. Lane 3 shows the effect of scrambled (Scr) siRNA. (B) Quantitative analysis shows that siRNA209 (lanes 4, 7), siRNA311 (lanes 5, 8), and siRNA485 (lanes 6, 9) each dose-dependently inhibited the expression of stargazin in cultured cells. siRNA311 completely silenced stargazin-GFP expression at a dose of 0.8 μg. The scrambled control siRNA did not affect stargazin-GFP expression. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; *P < 0.05, **P < 0.01 compared to the control group (lane 1). n = 6/group.
Pretreatment with intrathecal siRNA311 attenuates postoperative pain in rats
Twenty-seven of 30 rats that received intrathecal catheters were confirmed to have successful drug delivery and showed no motor deficit from surgical complications. We divided them equally into three experimental groups (n=9/group) that received intrathecal vehicle, scrambled siRNA, or siRNA311. Plantar incision significantly increased cumulative pain scores (P < 0.05; Fig. 6A) compared to those in naïve and sham-operated rats. Likewise, plantar incision decreased the paw withdrawal threshold to mechanical stimulation (P < 0.05; Fig. 6B). Intrathecal pretreatment with siRNA311 significantly reduced the cumulative pain scores and increased the paw withdrawal threshold in rats at 3 h post-incision, as compared to vehicle pretreatment (P < 0.05; Fig. 6). Pain inhibition was only partial, as cumulative pain scores and paw withdrawal thresholds of rats pretreated with siRNA311 remained significantly different from those of the control group (P < 0.05). Pretreatment with scrambled siRNA did not attenuate incision pain, as compared to vehicle pretreatment (P > 0.05; Fig. 6, A and B). No significant differences in the cumulative pain scores or paw withdrawal thresholds were observed in the contralateral hind paw among the different groups (data not shown).
Fig. 6. Intrathecal pretreatment of rats with small interfering RNA (siRNA) targeting stargazin decreases cumulative pain scores and mechanical hypersensitivity at 3 h after plantar incision.
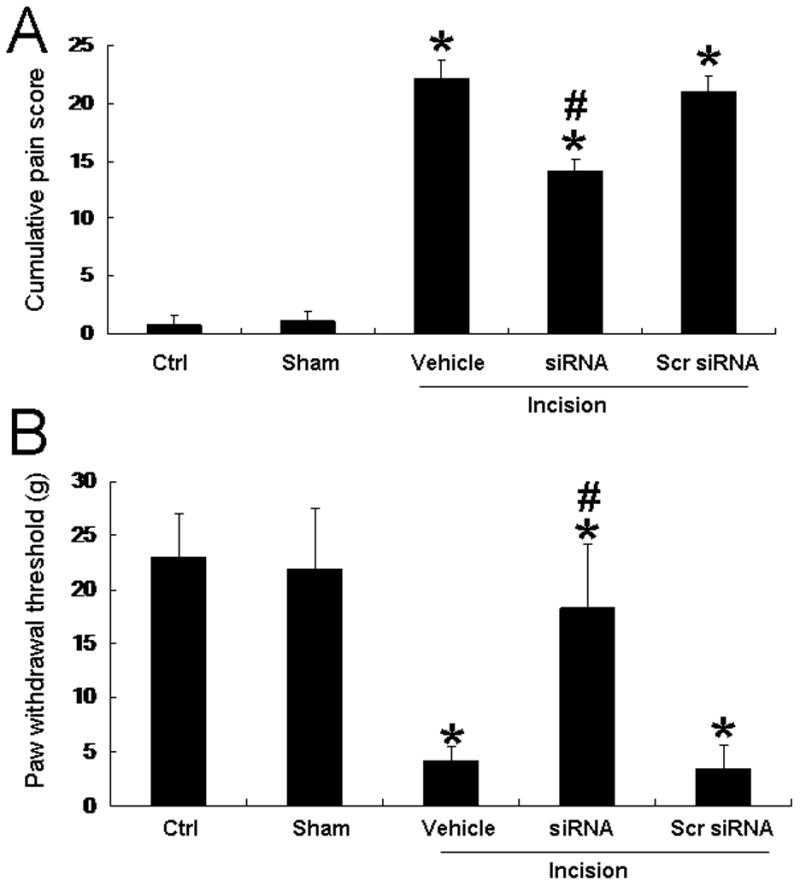
In rats pretreated with intrathecal vehicle, plantar incision significantly increased ipsilateral cumulative pain scores (A) and decreased ipsilateral paw withdrawal threshold to mechanical stimulation (B) as compared to values in the naïve control (Ctrl) group. Pretreatment with intrathecal siRNA311, but not scrambled (Scr) siRNA, significantly reduced the cumulative pain scores and increased the paw withdrawal threshold of rats at 3 h post-incision as compared with vehicle pretreatment. *P < 0.05 compared to the control group; #P < 0.05 compared to the vehicle-pretreated, incision group. n=9 rats per group.
Intrathecal pretreatment with siRNA311 inhibits the increased interaction between stargazin and GluR1 and the surface delivery of GluR1 after plantar incision
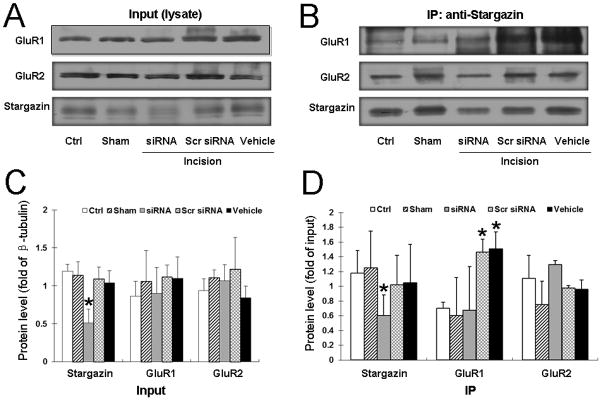
After completing the behavioral tests, we randomly selected 6 rats from each group and harvested spinal cord tissue for Western blot analysis. At 3 h after plantar incision, stargazin expression was significantly lower in the ipsilateral dorsal horn of rats pretreated with siRNA311 than in that of control rats (P < 0.05; Fig. 7, A and B). Stargazin expression did not differ significantly among the control group, sham-operated group, and incision groups pretreated with vehicle and scrambled siRNA (Fig. 7C). Additionally, total GluR1 and GluR2 protein levels did not differ between groups. Compared with that in the control group, the amount of GluR1 protein coimmunoprecipitated by stargazin antibody in the ipsilateral dorsal horn was significantly higher in incision groups pretreated with vehicle or scrambled siRNA (P < 0.05; Fig. 7D), suggesting an increased interaction between stargazin and GluR1 after incision. Pretreatment with siRNA311 prevented the increase in GluR1 coimmunoprecipitated by stargazin antibody (Fig. 7D). There was no significant difference in the interaction between stargazin and GluR2 among groups (P > 0.05; Fig. 7D).
Fig. 7. Intrathecal pretreatment of rats with small interfering RNA (siRNA)311 prevents the increase in stargazin–GluR1 interaction after plantar incision.
Representative immunoblots illustrate (A) total GluR1 and GluR2 protein (Input) and (B) GluR1 and GluR2 coimmunoprecipitated by stargazin antibody (IP: anti-stargazin) in the ipsilateral dorsal horn tissue of different groups. (C) Quantitative analysis shows that intrathecal pretreatment with siRNA311 significantly decreased stargazin expression in the ipsilateral dorsal horn of rats at 3 h after plantar incision. The stargazin levels did not differ significantly among the other groups. The total GluR1 and GluR2 protein (Input) levels were not significantly different among the groups. (D) Coimmunoprecipitation results show that plantar incision significantly increased the interaction between stargazin and GluR1 in the dorsal horn compared to the level of interaction in the naïve control (Ctrl) and sham-operated groups. However, pretreatment with siRNA311 reduced the level of stargazin-GluR1 interaction such that it was no different from the level in the control and sham-operated groups. Stargazin–GluR2 interaction was similar in all groups. IP: immunoprecipitation; IB: immunoblotting; Scr siRNA, scrambled siRNA. *P < 0.05 compared to the control group. n = 6 rats per group.
In the incision group pretreated with vehicle or scrambled siRNA, surface delivery of GluR1, but not of GluR2, was significantly increased in the ipsilateral dorsal horn at 3 h post-incision, as compared to control (P < 0.05; Fig. 8A). Pretreatment of incised rats with siRNA311 inhibited the increase of GluR1 in the membrane fraction (Fig. 8A) and also prevented the corresponding decrease of GluR1 in the cytosolic fraction in the ipsilateral dorsal horn (Fig. 8B).
Fig. 8. Intrathecal pretreatment of rats with small interfering RNA (siRNA)311 inhibits the enhanced surface delivery of GluR1 after plantar incision.
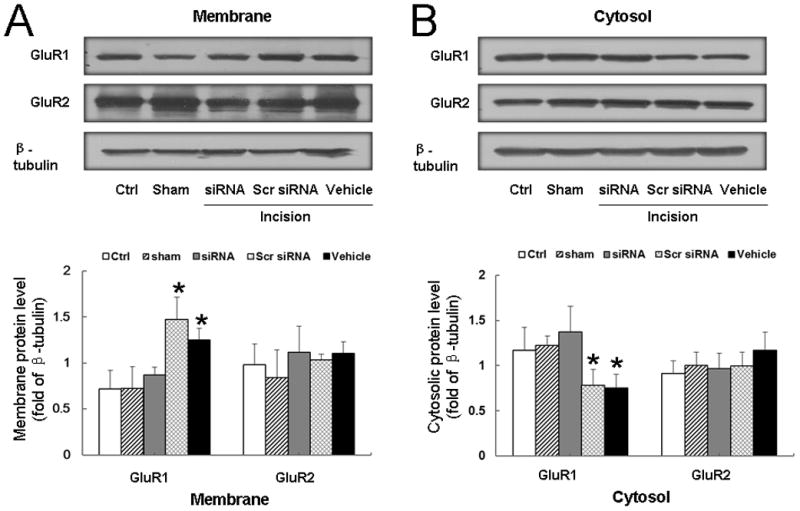
(A) Representative Western blots and quantitative analysis of band density show GluR1 and GluR2 protein levels in the plasma membrane fraction of ipsilateral dorsal horn tissue from different groups. β-tubulin was used as an internal reference. Pretreatment of rats with intrathecal siRNA311, but not scrambled (Scr) siRNA or vehicle, prevented the increase in GluR1 expression in the plasma membrane fraction of ipsilateral dorsal horn at 3 h after plantar incision. (B) Representative Western blots and quantitative analysis of band density show GluR1 and GluR2 protein levels in the cytosolic fraction in different groups. Ctrl, control (naïve) group. *P < 0.05 compared to the control group. n = 6 rats/group.
Discussion
The current study showed that surgical incision enhances the interaction between stargazin and AMPA receptor subunit GluR1, but not GluR2, and increases the surface delivery of GluR1 in dorsal horn neurons. Importantly, downregulation of stargazin with intrathecal siRNA significantly inhibited incision-induced interaction between stargazin and GluR1, and attenuated the surface delivery of GluR1 in dorsal horn. These actions of stargazin siRNA may contribute to its ability to inhibit guarding pain behavior and mechanical hypersensitivity produced by plantar incision in rats.
AMPA receptors present distinctive characteristics in trafficking from the cytosol to the plasma membrane in hippocampus and dorsal horn neurons.6;28 Changes in the number and composition of membrane AMPA receptors may affect synaptic strength and fast excitatory synaptic transmission. Tissue injury and noxious stimulation may induce the insertion of Ca2+-permeable AMPA receptors into plasma membranes in dorsal horn neurons.11;13;14;24;29 Acute nociceptive stimuli induced by capsaicin, carrageenan, and formalin injection increase membrane GluR1 level without affecting GluR2 trafficking in the dorsal horn.13;24;29 In contrast, in a persistent inflammatory pain model induced by an intraplantar injection of complete Freund’s adjuvant, in which the pain hypersensitivity may take hours to days rather than minutes to develop, membrane GluR2 level decreases, but membrane GluR1 level is unchanged.11;14;30;31 Thus, different pathological pain conditions may be associated with different patterns of AMPA receptor trafficking. The current findings suggest that plantar incision induces a time-dependent increase in membrane insertion of GluR1, but not GluR2, in dorsal horn neurons. Our previous studies showed that the level of total GluR1 protein in dorsal horn does not change significantly after plantar incision.15;32 Together, these findings suggest that painful incisional stimuli may enhance the surface delivery of GluR1 without increasing total GluR1 expression in dorsal horn. This compositional switch from Ca2+-impermeable to Ca2+-permeable AMPA receptor subunits in the plasma membrane might strengthen excitatory synaptic transmission in dorsal horn. Accordingly, our study supports the possibility that an increase in Ca2+-permeable AMPA receptors in synaptic membrane may contribute to postoperative pain.
Stargazin acts as an auxiliary subunit of AMPA receptor and controls the number of AMPA receptors at the synapse by regulating the surface delivery of different AMPA receptor subunits to the plasma membrane.33;34 Stargazin initially interacts with AMPA receptor subunits in the endoplasmic reticulum compartment and then assists in the surface delivery of these subunits. Finally, stargazin/AMPA receptor complexes in the plasma membrane move to the postsynaptic density (lateral diffusion) through interaction between the C terminus of stargazin and PSD-95.6;35 Increasing evidence suggests that functional regulation of AMPA receptors in the dorsal horn is important to the development and maintenance of postoperative pain.15;32 Because stargazin is required for the surface delivery of both GluR1- and GluR2-containing AMPA receptors, it plays a critical role in the compositional switch of AMPA receptors in the plasma membrane. We provide novel evidence that, by regulating AMPA receptor trafficking, stargazin may be implicated in postoperative pain. Although the total stargazin protein level in the ipsilateral dorsal horn was not significantly changed after plantar incision, coimmunoprecipitation experiments indicated that surgical incision enhanced the interaction between stargazin and GluR1-containing AMPA receptors in dorsal horn neurons. In contrast, the interaction between stargazin and GluR2 was unchanged. Additionally, immunofluorescence showed that stargazin and GluR1 were highly colocalized in the dorsal horn of naïve animals. This morphological evidence suggests a physiological basis for the interaction between stargazin and GluR1 in the same cell. In conjunction with findings from the stargazin siRNA experiments, our study suggests that stargazin may contribute to the increased plasma membrane delivery of GluR1-containing AMPA receptors in dorsal horn that leads to the development of postoperative pain. Because membrane delivery of GluR1 peaked at 3 h post-incision and pain hypersensitivity in rats also reaches a maximum at this time,32 we chose 3 h post-incision as the representative time to investigate stargazin–AMPA receptor interaction in coimmunoprecipitation and siRNA studies. As we have not examined later post-incision time points, data interpretation is limited to the early postoperative period.
Differences in interaction between stargazin and GluR1 and GluR2 subunits after plantar incision may ultimately increase the membrane GluR1/GluR2 ratio, which is indicative of increased insertion of Ca2+-permeable AMPA receptors that enhance spinal nociceptive transmission. Other anchoring proteins, such as A-kinase anchoring protein (AKAP), may also regulate the trafficking of AMPA receptors and play a role in pain sensitization.36 By recruiting cyclic adenosine monophosphate-dependent protein kinase (PKA) and protein phosphatase 2B-calcineurin to membrane-associated and guanylate kinase-linked AMPA receptors, AKAP may affect AMPA receptor phosphorylation and synaptic plasticity. However, our previous study showed that protein levels of GluR1 phosphorylated at serine-845 (the primary phosphorylation site for PKA) did not change during the period of postoperative pain. Accordingly, AKAP and PKA might not be important to the surface delivery of GluR1 in the incisional pain model.32
We showed that suppression of stargazin with RNA interference produced an analgesic effect, suggesting that stargazin is involved in the development of postoperative pain. In an inflammatory pain model, intrathecal administration of stargazin antisense oligonucleotide attenuated central sensitization.25 Compared to antisense oligonucleotide, however, RNA interference with siRNA is thought to be more efficient. Interestingly, Nissenbaum et al.37 recently demonstrated that polymorphisms in the human stargazin gene, CACNG2, were associated with chronic pain in a cohort of cancer patients who underwent breast surgery. These data support the notion that CACNG2 is a pain susceptibility gene and that stargazin is a critical player in pain modulation in humans.38
The switch in the composition of synaptic AMPA receptors may greatly affect the efficacy of AMPA receptor-mediated fast excitatory synaptic transmission.35 Noxious stimuli may induce rapid alterations in the composition of synaptic AMPA receptors in the spinal cord dorsal horn. For example, Galan et al.24 showed that the expression ratio of synaptic GluR1 to GluR2 increases in a visceral pain model. Furthermore, GluR1-deficient mice exhibited fewer Ca2+-permeable AMPA receptors and a decrease in AMPA current density in dorsal horn neurons together with a loss of nociceptive plasticity and decreased inflammatory hyperalgesia.39 Conversely, GluR2-deficient mice exhibited an increase in spinal Ca2+-permeable AMPA receptors that augmented nociceptive plasticity and long-term inflammatory hyperalgesia.39 Furthermore, the long-term potentiation of primary afferent neurotransmission is also enhanced in GluR2-deficient mice.40 In line with these findings, we showed that knockdown of stargazin protein with siRNA reversed the incision-induced interaction between stargazin and GluR1 and inhibited GluR1 trafficking into plasma membrane. In contrast, interaction between stargazin and GluR2 and membrane insertion of GluR2 were unchanged. Thus, stargazin siRNA may cause a reduction in the number of Ca2+-permeable AMPA receptors in the plasma membrane of dorsal horn neurons, which, in turn, decreases synaptic efficacy and leads to analgesic action. Electrophysiological investigations are warranted to help confirm the functional role of stargazin in the development of postoperative pain.
In summary, our study suggests that surgical incision promotes the delivery of AMPA receptor subunit GluR1, but not GluR2, to the plasma membrane through enhanced interaction between stargazin and GluR1 in spinal cord dorsal horn. Our data also show that knockdown of stargazin inhibits the development of incisional pain. These findings provide evidence that targeting the transmembrane AMPA receptor regulatory protein in spinal cord could have a potential role in the treatment of postoperative pain.
Acknowledgments
The authors thank Claire F. Levine, MS, ELS (scientific editor, Department of Anesthesiology/CCM, Johns Hopkins University, Baltimore, Maryland, USA), for editing the manuscript. This work was supported by the National Natural Science Foundation of China (81171055/H0903, Beijing, China), Natural Science Foundation of Beijing (7112054, Beijing, China), New Century Excellent Talents Program from the Ministry of Education, China (NCET-10-0014, Beijing, China), and a grant from the National Institutes of Health to Y.G. (NS70814, Bethesda, Maryland, USA).
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Reference List
- 1.Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: A systematic update of the evidence. Anesth Analg. 2007;104:689–702. doi: 10.1213/01.ane.0000255040.71600.41. [DOI] [PubMed] [Google Scholar]
- 2.Zahn PK, Brennan TJ. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology. 1998;88:143–56. doi: 10.1097/00000542-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Pogatzki-Zahn EM, Brennan TJ. The effect of the AMPA/kainate receptor antagonist LY293558 in a rat model of postoperative pain. J Pain. 2006;7:768–77. doi: 10.1016/j.jpain.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114:499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–9. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33:1354–6. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- 8.Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–31. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- 9.Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–8. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- 10.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–98. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 11.Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, Ito S. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci. 2008;27:3161–70. doi: 10.1111/j.1460-9568.2008.06293.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Petralia RS, Takamiya K, Xia J, Li YQ, Huganir RL, Tao YX, Yaster M. Preserved acute pain and impaired neuropathic pain in mice lacking protein interacting with C Kinase 1. Mol Pain. 2011;7:11. doi: 10.1186/1744-8069-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–53. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–19. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wu J, Guo R, Zhao Y, Wang Y, Zhang M, Chen Z, Wu A, Yue Y. Surgical incision induces phosphorylation of AMPA receptor GluR1 subunits at Serine-831 sites and GluR1 trafficking in spinal cord dorsal horn via a protein kinase Cgamma-dependent mechanism. Neuroscience. 2013;240:361–70. doi: 10.1016/j.neuroscience.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Diaz E. Regulation of AMPA receptors by transmembrane accessory proteins. Eur J Neurosci. 2010;32:261–8. doi: 10.1111/j.1460-9568.2010.07357.x. [DOI] [PubMed] [Google Scholar]
- 17.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–7. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shevtsova O, Leitch B. Selective loss of AMPA receptor subunits at inhibitory neuron synapses in the cerebellum of the ataxic stargazer mouse. Brain Res. 2012;1427:54–64. doi: 10.1016/j.brainres.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Jackson AC, Nicoll RA. Stargazin (TARP gamma-2) is required for compartment-specific AMPA receptor trafficking and synaptic plasticity in cerebellar stellate cells. J Neurosci. 2011;31:3939–52. doi: 10.1523/JNEUROSCI.5134-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuadra AE, Kuo SH, Kawasaki Y, Bredt DS, Chetkovich DM. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J Neurosci. 2004;24:7491–502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Brennan TJ. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology. 2010;112:153–64. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 23.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 24.Galan A, Laird JM, Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain. 2004;112:315–23. doi: 10.1016/j.pain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Tao F, Skinner J, Su Q, Johns RA. New role for spinal Stargazin in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated pain sensitization after inflammation. J Neurosci Res. 2006;84:867–73. doi: 10.1002/jnr.20973. [DOI] [PubMed] [Google Scholar]
- 26.Mise-Omata S, Montagne B, Deckert M, Wienands J, Acuto O. Mammalian actin binding protein 1 is essential for endocytosis but not lamellipodia formation: functional analysis by RNA interference. Biochem Biophys Res Commun. 2003;301:704–10. doi: 10.1016/s0006-291x(02)02972-8. [DOI] [PubMed] [Google Scholar]
- 27.Christoph T, Grunweller A, Mika J, Schafer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–43. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 28.Tao YX. AMPA receptor trafficking in inflammation-induced dorsal horn central sensitization. Neurosci Bull. 2012;28:111–20. doi: 10.1007/s12264-012-1204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson M, Broman J. Translocation of GluR1-containing AMPA receptors to a spinal nociceptive synapse during acute noxious stimulation. J Neurosci. 2008;28:7084–90. doi: 10.1523/JNEUROSCI.5749-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Sun YN, Wu X, Sun Q, Liu FY, Xing GG, Wan Y. Role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor subunit GluR1 in spinal dorsal horn in inflammatory nociception and neuropathic nociception in rat. Brain Res. 2008;1200:19–26. doi: 10.1016/j.brainres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Atianjoh FE, Yaster M, Zhao X, Takamiya K, Xia J, Gauda EB, Huganir RL, Tao YX. Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund’s adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. Pain. 2010;151:226–34. doi: 10.1016/j.pain.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Mu X, Wu J, Wu A, Fang L, Li J, Yue Y. Differential roles of phosphorylated AMPA receptor GluR1 subunits at Serine-831 and Serine-845 sites in spinal cord dorsal horn in a rat model of post-operative pain. Neurochem Res. 2011;36:170–6. doi: 10.1007/s11064-010-0288-y. [DOI] [PubMed] [Google Scholar]
- 33.Milstein AD, Nicoll RA. TARP modulation of synaptic AMPA receptor trafficking and gating depends on multiple intracellular domains. Proc Natl Acad Sci USA. 2009;106:11348–51. doi: 10.1073/pnas.0905570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessels HW, Kopec CD, Klein ME, Malinow R. Roles of stargazin and phosphorylation in the control of AMPA receptor subcellular distribution. Nat Neurosci. 2009;12:888–96. doi: 10.1038/nn.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Wu J, Wu Z, Lin Q, Yue Y, Fang L. Regulation of AMPA receptors in spinal nociception. Mol Pain. 2010;6:5. doi: 10.1186/1744-8069-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanderson JL, Dell’Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–36. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nissenbaum J, Devor M, Seltzer Z, Gebauer M, Michaelis M, Tal M, Dorfman R, Abitbul-Yarkoni M, Lu Y, Elahipanah T, delCanho S, Minert A, Fried K, Persson AK, Shpigler H, Shabo E, Yakir B, Pisante A, Darvasi A. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Res. 2010;20:1180–90. doi: 10.1101/gr.104976.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nissenbaum J. From mouse to humans: Discovery of the CACNG2 pain susceptibility gene. Clin Genet. 2012;82:311–20. doi: 10.1111/j.1399-0004.2012.01924.x. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44:637–50. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Youn DH, Royle G, Kolaj M, Vissel B, Randic M. Enhanced LTP of primary afferent neurotransmission in AMPA receptor GluR2-deficient mice. Pain. 2008;136:158–67. doi: 10.1016/j.pain.2007.07.001. [DOI] [PubMed] [Google Scholar]