Abstract
Glutamate neurotransmission via the N-methyl-D-aspartate receptor (NMDAR) is thought to mediate the synaptic plasticity underlying learning and memory formation. There is increasing evidence that deficits in NMDAR function are involved in the pathophysiology of cognitive dysfunction seen in neuropsychiatric disorders and addiction. NMDAR subunits confer different physiological properties to the receptor, interact with distinct intracellular postsynaptic scaffolding and signaling molecules, and are differentially expressed during development. Despite these known differences, the relative contribution of individual subunit composition to synaptic plasticity and learning is not fully elucidated. We have previously shown that constitutive deletion of GluN2A subunit in the mouse impairs discrimination and re-learning phase of reversal when exemplars are complex picture stimuli, but spares acquisition and extinction of non-discriminative visually cued instrumental response. To investigate the role of GluN2A containing NMDARs in executive control, we tested GluN2A knockout (GluN2AKO), heterozygous (GluN2AHET) and wild-type (WT) littermates on an attentional set-shifting task using species-specific stimulus dimensions. To further explore the nature of deficits in this model, mice were tested on a visual discrimination reversal paradigm using simplified rotational stimuli. GluN2AKO were not impaired on discrimination or reversal problems when tactile or olfactory stimuli were used, or when visual stimuli were sufficiently easy to discriminate. GluN2AKO showed a specific and significant impairment in ventromedial prefrontal cortex-mediated set-shifting. Together these results support a role for GluN2A containing NMDAR in modulating executive control that can be masked by overlapping deficits in attentional processes during high task demands.
Keywords: Executive Function, NMDAR, Set-shifting, GluN2A, Mouse models
Introduction
The ability to efficiently alter non-rewarding behaviors is essential to adapt in a complex environment. The cortico-striatal circuits underlying this behavioral flexibility are highly conserved across species (Middleton and Strick, 1996). The ventromedial (vmPFC) and orbitofrontal (OFC) prefrontal cortex mediate complementary but dissociable forms of behavioral flexibility, while choice learning is supported by regions of the basal ganglia in rodents (Graybiel, 2008, Schoenbaum et al., 2009, Balleine and O’Doherty, 2010, Bissonette et al., 2013). Reversal learning, which requires a shift in response to a previously unrewarded stimulus, is mediated by the OFC across stimulus modalities (Dias et al., 1996, Chudasama and Robbins, 2003, Moore et al., 2009, Rudebeck and Murray, 2011, Rudebeck et al., 2013). In contrast, the rodent vmPFC mediates the top-down control of attention required to shift between stimulus dimensions during attentional set-shifting (Ragozzino et al., 1999, Birrell and Brown, 2000b, Bissonette et al., 2008a, Floresco et al., 2008). The vmPFC can be further subdivided into distinct functional regions with the anterior cingulate cortex (ACC) mediating associative learning by directing attention to stimulus features, and the prelimbic cortex (PrL) and infralimbic cortex (iL) maintaining and facilitating strategy shifting, respectively (Bussey et al., 1997, Bissonette et al., 2008b, Rich and Shapiro, 2009, Oualian and Gisquet-Verrier, 2010).
The ability to rapidly shift actions is thought to require the induction of plasticity within these cortical subregions, although questions remain regarding the specific neurochemical substrates for this plasticity. N-methyl-D-aspartate receptors (NMDAR), which are essential for mediating certain forms of synaptic plasticity during memory formation, are a strong candidate for the molecular mechanism underlying efficient behavioral flexibility (Bannerman et al., 2006). Loss of NMDAR function impairs cortically-mediated cognitive processes in rodents, further supporting a role for these receptors (Lebel et al., 2006). NMDARs, heterotetramers comprising two obligatory GluN1 subunits and varying combinations of GluN2A-2D subunits, gain distinct physiological and secondary signaling properties from their subunit composition (Cull-Candy et al., 2001, Kohr et al., 2003). GluN2A and GluN2B are the primary subunits in the adult cortex, with GluN2A predominantly expressed at two postnatal weeks in the rodent. The precise contribution of each subunit to plasticity and behavioral flexibility is still being elucidated.
Global knockout of GluN2A (GluN2AKO) impairs hippocampal LTP, spatial memory (Sakimura et al., 1995, Ito et al., 1996), contextual learning and motor learning (Kishimoto et al., 1997, Kiyama et al., 1998). We have previously shown that GluN2AKO mice have difficulty discriminating between complex visual stimuli, but are unimpaired on visually cued instrumental learning, suggesting these mice have specific difficulty in successfully differentiating rewarded and unrewarded stimuli (Brigman et al., 2008). To further explore the role of GluN2A in learning and executive control behaviors we tested GluN2AKO mice and controls on an attentional set-shifting task (ASST) that required discriminative learning, reversal, and set-shifting of species-specific stimulus domains (Birrell and Brown, 2000a). To investigate whether previously reported associative learning deficits were specific to sensory modality or due to task difficulty, we tested GluN2AKO on a visual discrimination reversal task that utilized easily discriminable stimuli.
Materials and Methods
Subjects
GluN2A were generated as previously described (Sakimura et al., 1995, Brigman et al., 2008). Briefly, the GluN2A null mutation was backcrossed into the C57BL/6J strain for >10 generations to produce a congenic C57BL/6J genetic background. Analysis of 150 SNP markers at B15–20 megabase intervals across all autosomal chromosomes confirmed 499% C57BL/6J congenicty in the mutant line (Boyce-Rustay and Holmes, 2006). To avoid potential phenotypic abnormalities resulting from genotypic differences in maternal behavior and early life environment knock-out (GluN2AKO), heterozygous (GluN2AHET) and wild-type controls (WT) WT mice were all generated from HET x HET matings. Mice were bred at the University of New Mexico Health Sciences Center from breeding pairs graciously provided from the Holmes lab at NIAAA. Mice were housed in same sex groupings of 2–4 per cage in a temperature- and humidity- controlled vivarium under a reverse 12 h light/dark cycle (lights off 0800 h) and tested during the dark phase. Separate cohorts of male and female GluN2AKO, GluN2AHET and WT littermates were tested on Attentional Set-Shifting (n=5–6 per sex/genotype; 10–11 total per genotype) and visual discrimination reversal (n=6 per sex/genotype; 12 total per genotype) to avoid order effects. Mice, aged 8 weeks at onset of testing, were slowly reduced and then maintained at 85% free-feeding body weight to ensure motivation to work for food reward. Prior to testing, mice were acclimated to the 14 mg pellet food reward by provision of 3 pellets/mouse in the home cage for 7 days while in the testing room. Mice were counterbalanced to dimension in simple discrimination or visual stimuli and number of mice per experiment is given in figure legends. All experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee.
Attentional Set-Shifting
Testing was conducted in an acrylic apparatus measuring 30 × 18 × 12 cm. Two ceramic digging bowls (4.5 × 2.5 cm) were placed on platforms (11 × 5 cm) in each quarter section and were separated by a clear acrylic panel (Figure 1A). Access to digging bowls was limited by a removable divider. Scented medium was made by mixing 150 g of cob bedding with 20 crushed 14 mg dustless precision pellets (#F0568, BioServ, Frenchtown, NJ) and 3 g of commercially available powdered spices: ginger, nutmeg, garlic, coriander, thyme and cinnamon (McCormick & Company, Sparks, MD). Approach platforms were manufactured to size from commercially available materials in house and included sandpaper, wood, neoprene, metal wire, tile and a plastic fiber sponge.
Figure 1. Impaired extradimensional set-shifting in GluN2AKO.
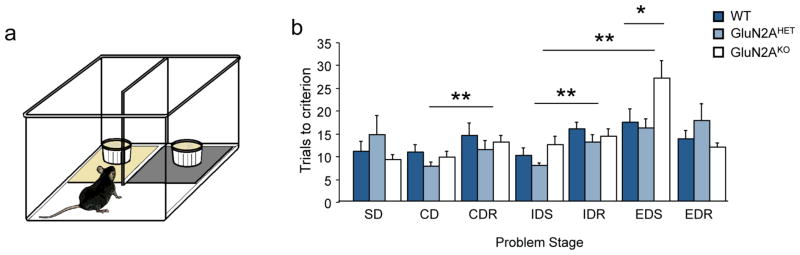
(A) Cartoon of approach platform version of the ASST task. (B) GluN2AKO mice were not significantly different than GluN2AHET or WT controls on problems that required either discrimination (SD, CD, IDS) or reversal performance (CDR, IDR, EDR). Mice of all genotypes required significantly more trials to complete the CDR and IDR versus the preceding CD and ID problem. All mice showed formation of attentional set as measured by significantly increased trials to perform the EDS regardless of starting dimension. GluN2AKO required significantly more trials to perform the EDS problem versus GluN2AHET or WT controls. *=P<.01 main effect of genotype, **=P<.01 main effect of problem.
ASST was conducted as previously described (Young et al., 2010, Young et al., 2011). Briefly, on day one mice were acclimated to the testing chamber and trained to dig in unscented cob medium for food reward. Initially, three pellets were available on the floor of the chamber and in the bottom of empty digging bowls. The remaining trials consisted of pellets available in digging bowls only, starting with 3 pellets per side. Digging medium was incrementally increased while successively burying one additional pellet each trial. When the digging bowls were full of medium and all pellets were buried under the digging cob, pellets were restricted to a single bowl, randomly assigned per trial. Pellet number was decreased with every proceeding trial, with the final 9 trials containing only one pellet. A total of fifty pellets were received on day one. Trials were timed from divider lifting until all pellets were consumed. Mice who ceased digging for pellets were returned to their home cage for 45 min before resuming at last attempted trial.
Day two training introduced the mice to each odor and platform combination that they may encounter during day three testing (Table 1). A single food pellet was placed approximately ¾ of the way below the digging medium surface in only one bowl, assigned randomly between trials. Placement of a pellet was mimicked in the empty bowl to “sham bait” and prevent mice learning experimenter related cues. A total of 24 pellets were received.
Table 1.
Testing stages and stimulus combinations for the mouse attentional set-shifting paradigm with odor as initially-trained dimension. Starting dimension was counterbalanced across genotypes. Stimulus exemplars for odor and platform dimension are listed in the text.
| Problem Stages | Dimensions
|
Exemplars
|
||
|---|---|---|---|---|
| Relevant | Irrelevant | S+ | S− | |
| Simple Discrimination (SD) | Odor | n/a | O1 | O2 |
|
| ||||
| Compound Discrimination (CD) | Odor | Platform | O1/P1 | O2/P2 |
|
| ||||
| O1/P2 | O2/P1 | |||
|
| ||||
| Compound Discrimination Reversal (CDR) | Odor | Platform | O2/P1 | O1/P2 |
|
| ||||
| O2/P2 | O1/P1 | |||
|
| ||||
| Intradimensional Shift (ID) | Odor | Platform | O3/P2 | O4/P3 |
|
| ||||
| O3/P3 | O4/P2 | |||
|
| ||||
| Intradimensional Shift Reversal (IDR) | Odor | Platform | O4/P2 | O3/P3 |
|
| ||||
| O4/P3 | O3/P2 | |||
|
| ||||
| Extradimensional Shift (EDS) | Platform | Odor | P5/O5 | P6/O6 |
|
| ||||
| P5/O6 | P6/O5 | |||
|
| ||||
| Extradimensional Shift Reversal (EDR) | Platform | Odor | P6/O5 | P5/O6 |
|
| ||||
| P6/O6 | P5/O5 | |||
On day 3 mice were tested in succession with no inter-session-breaks on seven discrimination tasks (Table 1). In the simple discrimination (SD) mice were initially trained to discriminate two exemplars in either the odor or platform dimension. Upon reaching criterion mice were moved to the compound discrimination (CD) during which the second, non-rewarded dimension, was added. Mice were still required to respond in accordance to the first discrimination learned. For the first four trials of the SD and CD stages mice were allowed to dig in the incorrect bowl without consequence, although an error was recorded. In all other trials the opposite passageway was blocked upon an error. In successful trials, the mouse was allowed to collect the pellet before continuing to the next trial. If the mouse did not dig in either bowl by 2 min the trial was recorded as ‘no decision’ and the mouse proceeded to the next trial. Criterion was set to six consecutive correct responses. Upon completion of the CD the rewarded exemplar in the initially rewarded dimension was reversed to form a compound discrimination reversal (CDR).
Following the CDR, a novel set of exemplars in each dimension was introduced and mice were rewarded for responding to one exemplar in the initially learned dimension (intra-dimensional shift (IDS)). Next, the intra-dimensional reversal (IDR) reversed the correct stimuli within the same dimension. Following criterion performance of the IDR a second novel set of exemplars in both dimensions were introduced in the extra-dimensional shift (EDS). This time, the rewarded exemplar was in the previously irrelevant dimension. Finally, the correct exemplar within the newly learned dimension was reversed to form an extra-dimensional reversal (EDR) problem.
Trials to criterion and errors were recorded for each stage. Trial latencies to respond were measured from the time the barrier was raised till digging was initiated. A dig was defined as the moment when the mouse’s nose or paw broke the surface of the cob-digging medium. Three GluN2AKO and 1 WT mouse that required more than 60 trials in a single stage or exceeded 120 trials total were omitted from the analysis.
Discrimination Reversal
All operant behavior was conducted in a chamber measuring 21.6 × 17.8 × 12.7 cm (model # ENV-307W, Med Associates, St. Albans, VT) housed within a sound- and light-attenuating box (Med Associates, St. Albans, VT). The standard grid floor of the chamber was covered with a solid acrylic plate to facilitate ambulation. A pellet dispenser delivering 14 mg dustless pellets (#F05684, BioServ, Frenchtown, NJ) into a magazine, a house-light, tone generator and an ultra-sensitive lever was located at one end of the chamber. At the opposite end of the chamber there was a touch-sensitive screen (Conclusive Solutions, U.K.) covered by a black acrylic aperture plate allowing two 2 × 5 cm touch areas separated by 0.5 cm and located at a height of 6.5 cm from the floor of the chamber. Stimulus presentation in the response windows and touches were controlled and recorded by the KLimbic Software Package (Conclusive Solutions, U.K.).
Discrimination reversal learning of simple stimuli was assessed as previously described (Brigman et al., 2010). Briefly, mice were habituated to the operant chamber and to eating out of the pellet magazine by being placed in the chamber for 30 min with pellets available in the magazine. Mice retrieving 10 pellets within 30 min were moved onto pre-training. Mice were given a three-stage pre-training regimen. First, mice were trained to obtain reward by pressing a lever within the chamber on an FR1 schedule. Mice pressing and collecting 30 rewards in under 30 minutes were moved to touch training. Here, a lever press led to the presentation of a white (variously-shaped) stimulus in 1 of the 2 response windows (spatially pseudorandomized). The stimulus remained on the screen until a response was made. Touches in the blank response window had no response while a touch to a white stimulus resulted in reward delivery concomitant with a tone and the illumination of the magazine light. Mice initiating, touching and retrieving 30 pellets within 30 min were moved to the final stage of pre-training. This stage was identical to touch-training except that responses at a blank window during stimulus presentation now produced a 15 sec timeout (signaled by illumination of the house light) to discourage indiscriminate screen responding. Errors on this and subsequent stages were followed by correction trials in which the same stimulus and left/right position was presented until a correct response was made. Mice making ≥75% (excluding correction trials) of their responses at a stimulus-containing window over a 30-trial session were moved onto discrimination.
Following pre-training all mice were tested on a pairwise discrimination-reversal paradigm. For discrimination learning, 2 novel approximately equiluminescent stimuli were presented in a spatially pseudorandomized manner over 30-trial sessions (5 sec ITI; Figure 2A). Responses at 1 stimulus (horizontal lines) resulted in reward; responses at the other stimulus (vertical lines) resulted in timeout followed by a correction trial. Mice were trained to a criterion of ≥85% correct responding excluding errors made on correction trials (categorized as correction errors) over 2 consecutive sessions. Reversal training began on the session after discrimination criterion was attained. Here, the designation of stimuli as correct versus incorrect was reversed for each mouse. Mice were trained on 30-trial daily sessions (as for discrimination) to a criterion of 85% correct responding (excluding correction trials) over 2 consecutive sessions.
Figure 2. GluN2AKO show intact visual discrimination learning with simplified stimuli.
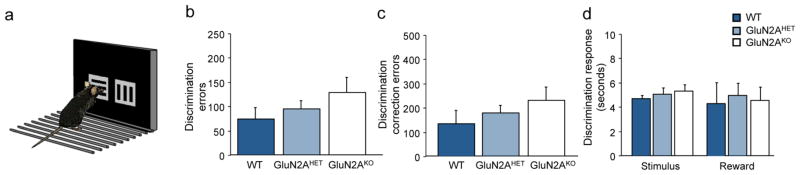
(A) Cartoon of touch-screen learning task with rotational line stimuli. (B) GluN2AKO did not require significantly more errors to attain discrimination criterion versus GluN2AHET or WT controls. (C) GluN2AKO did not make significantly more correction errors during discrimination learning when compared to GluN2AHET or WT controls. (D) There were no significant differences across genotypes on stimulus response or reward retrieval latencies during discrimination learning.
Statistical analysis
For ASST, trials to criterion were analyzed using a repeated measures two-factor ANOVA with stage (SD, CD, CDR, ID, IDR, ED, and EDR) as a within-subjects factor and sex, genotype and initial dimension as between-subjects factors. As no main effect of sex was seen and sex did not significantly interact with any other dimension groups, all mice were combined for analysis. Since genotype and stage did not significantly interact with initial dimension (ANOVA: ns), it was not analyzed further. Planned repeated measures ANOVA were conducted for individual stages measuring discrimination (SD, CD, IDS), reversal (CDR, IDR, EDR) and set-shifting (EDS) believed to be mediated by different neural substrates (Birrell & Brown, 2000). For discrimination and reversal, analysis of variance (ANOVA) was used to analyze performance by genotype on the following measures: correct trials, errors, and correction errors to criterion. Additionally, trial reaction time (=time from trial initiation to touchscreen response), and magazine latency (=time from touchscreen response to reward retrieval) were analyzed for each problem.
Results
Attentional Set-Shifting Task
No significant main effect (ANOVA: ns) of genotype or initial dimension was observed for the ASST problem series. There was a main effect of stage (F6,6=7.49; p<.001) for all animals and a significant interaction (F6,12=1.86; p<.05) for stage x genotype. Post-hoc tests revealed that all genotypes required significantly more trials to complete both the compound reversal (CDR) and intradimensional reversal (IDR) versus the proceeding CD and ID problem respectively (p<.05). Additionally, all genotypes required significantly more trials to perform the EDS versus IDS, confirming the formation of an attentional set (Figure 1B). Analysis of stages that required only pairwise discrimination found no differences in trials to criterion by genotype (F2,20=1.23; p=.32) or stage (F2,20=1.50; p=.23) and no significant stage x genotype interaction (F2,20=2.01; p=.18). No significant differences were seen in trial latencies by genotype or discrimination stage indicating motivation to work for food reward did not decline over the problem series. Similarly, when the reinforcement contingencies of the previous stage were reversed in the following stage (CDR, IDR and EDR) no differences were observed for genotype (F2,20=0.39; p=.68) or stage (F2,20=0.50; p=.60) and no significant stage x genotype interaction (F2,20=1.27; p=.30). Trial latencies did not differ by genotype or stage on reversal stages Comparison of trials to criterion on EDS stage revealed a significant main effect of genotype (F2,20=3.81; p<.05). Fisher’s protected least significant difference post hoc test revealed that GluN2AKO required significantly more trials (27.00±3.85) than GluN2AHET (16.13±2.0) or WT mice (17.25±3.06) to complete the EDS (p<.05; Figure 1B). No significant main effect of genotype, dimension, or stage was seen for trial latency and no significant interaction was seen between variables. For the EDS stage where performance varied significantly by genotype, there was no significant difference in trial latency across genotypes (F2,20=0.35; p=.71).
Touchscreen Discrimination and Reversal Learning Performance
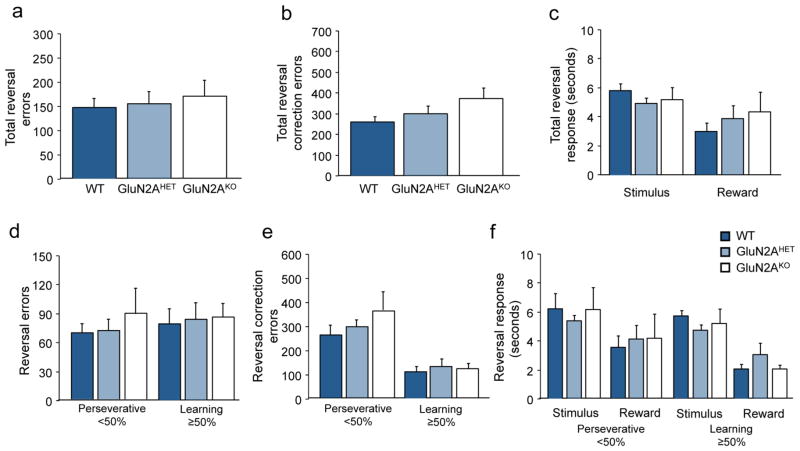
Analysis of pre-training stage revealed no significant differences between genotypes (Stage 1: F2,26=1.68; p=.20; Stage 2: F2,26=0.98; p=.39; Stage 3: F2,26=0.59; p=.56). For visual discrimination learning there was no significant effect of genotype on either on trials, errors or correction errors to attain criterion performance, as measured by number of errors (F2,26=1.43; p=.25; Figure 2B) or correction errors (F2,26=.94; p=.40, Figure 2C) to criterion. No difference in motivation to respond or collect reward as measured by stimulus response or reward retrieval was seen across genotypes (Figure 2D). Analysis of discrimination and reversal performance with repeated measures ANOVA revealed a significant main effect of problem for both errors (F2,1=20.67; p<.001) and correction errors (F2,1=33.57; p<.001) while post-hoc tests revealed that the reversal was significantly more difficult for all genotypes. On the reversal, no significant differences were seen across genotypes as measured by errors (F2,26=0.57; p=.57; Figure 3A) or correction errors (F2,26=0.94; p=.40; Figure 3B) across the problem. Analysis of performance during early, perseverative reversal (sessions <50% correct) versus the late, learning phase (sessions ≥50% correct) found no significant differences across genotypes (ANOVA: ns) on errors (Perseverative: F2,26=0.48; p=.62; Learning: F2,26=0.52; p=.25; Figure 3D) or correction errors (Perseverative: F2,26=0.99; p=.39; Learning: F2,26=0.18; p=.89; Figure 3E) on either early perseverative or later learning stages. Stimulus response time and reward retrieval also did not significantly differ across the problem (Figure 3C), or by stage (Figure 3F).
Figure 3. GluN2AKO show intact reversal learning with simplified stimuli.
(A) GluN2AKO did not require significantly more errors or (B) correction errors across the entire reversal problem versus GluN2AHET or WT controls. (C) There were no significant differences across genotypes on stimulus response or reward retrieval latencies across reversal learning. (D) Analysis of early perseverative (sessions <50% correct) and later learning (sessions ≥50% correct) revealed no significant difference in error responses across genotypes. (E) GluN2AKO did not make significantly more correction errors on either stage of reversal learning compared to control. (F) Stimulus response and reward retrieval latencies did not differ across genotypes on either stage of the reversal.
Discussion
Here we show that brain-wide loss of GluN2A-containing NMDAR leads to a specific impairment in attentional set-shifting on an olfactory and tactile based task while heterozygous loss results in no apparent deficit. GluN2AKO have previously been reported to have deficits in associative learning on a visual discrimination-reversal paradigm. In the current study however, no deficits in discrimination learning, within-dimension reversals or attentional set formation were observed. To determine if the intact learning behavior seen in the current study was due to dimension modality or problem difficulty, we tested GluN2AKO and control mice on a visual touchscreen discrimination-reversal problem using simplified rotational line stimuli. Our results show that GluN2AKO mice exhibit no impairment in discrimination or reversal learning using these simplified stimuli.
Previous work in rodents demonstrated that the behavioral flexibility required to successfully perform an EDS during attentional set-shifting is mediated by distinct subregions of the vmPFC. Targeted lesion of the PrL or iL was sufficient to impair maze based attentional set-shifting, with in vivo electrophysiology implicating strategy maintenance and strategy switch initiation for these regions, both being necessary to successfully complete set-shifting (Rich and Shapiro, 2009, Oualian and Gisquet-Verrier, 2010). Specifically, targeted lesion of PrL and iL cortices impaired EDS by increasing trials to criterion (Ng et al., 2007a). Preclinical studies have also suggested that NMDARs, with their role in synaptic plasticity, may play a unique role in mediating flexible behavior. Systemic administration of NMDAR antagonists impaired both acquisition and set-shifting in a maze based operant paradigm, perhaps through actions on cortical and hippocampal regions, while administration targeting the PrL and iL subregions of vmPFC selectively impaired set-shifting in operant and odor-tactile paradigms in rats (Stefani et al., 2003, Stefani and Moghaddam, 2005, Dahlin et al., 2008). However, few studies have parsed apart the roles of specific subunits of NMDAR in mediating behavioral flexibility. Studies examining the role of GluN2B in behavioral flexibility have found that loss of the subunit impaired performance on operant based reversal and set-shifting tasks (Dalton et al., 2011), while other studies found that loss of GluN2B function actually improved behavioral flexibility (Kos et al., 2011, Brigman et al., 2013). These studies suggest that GluN2B may not be mediating set-shifting specifically but modulating general behavioral flexibility and learning. Previous work in GluN2AKO failed to find an effect on perseverative reversal performance (Brigman et al., 2008), and to our knowledge no studies have previously examined the role of GluN2A in set-shifting behaviors. The current study shows that brain-wide loss of GluN2A selectively impairs EDS performance, suggesting that GluN2A containing NMDAR, in the vmPFC, may play a unique role in mediating executive control of top-down behavioral flexibility required for optimal set-shifting.
Intriguingly, we failed to see any effect on discrimination stages of the set-shifting task even though it has been previously shown that whole-brain GluN2AKO impaired learning on a touchscreen based paradigm (Brigman et al., 2008). This is despite the fact that power-analysis determined sample sizes used in both visual discrimination reversal and ASST experiments replicate those used in previous touch-screen experiments that detected impaired discrimination learning in the GluN2A mutant mice. It is possible that previous deficits and the intact performance reported here are due to the differences in sensory modalities used on the problems. However, there is evidence that subregions of the vmPFC, which seems to be uniquely altered in GluN2AKO, may also be involved in attentional processes required to attend to and discriminate stimuli based on their features. Selective lesions of the ACC impair performance on IDS problems (Ng et al., 2007b) and visual discriminations. Importantly, loss of ACC function only impairs visual learning when the stimuli are difficult to attend to, suggesting this region plays a role in directing attention under high conflict situations within a single modality (Bussey et al., 1997). To test the hypothesis that GluN2A loss in the vmPFC may be driving both the reported EDS deficit and previous impairments on complex visual stimuli, we tested GluN2AKO and controls on a discrimination reversal task using simplified visual stimuli compared to previous reports. We found that GluN2AKO mice were able to learn a simple discrimination and successfully complete a reversal of rotated visual stimuli at comparable rates to controls. This finding suggests that rather than impairing modality specific learning, loss of GluN2A may disrupt attentional focus to stimulus features through a deficit in the ACC. Thus, GluN2A loss spares performance when stimuli are easy to discriminate, such as olfactory, tactile and simple visual, while impairing learning performance when stimuli require a high level of attention to discriminate, as in previous touchscreen learning studies.
In light of numerous studies showing that hippocampal NMDARs are necessary for associative learning using spatial cues, it should be pointed out that both the ASST and touch-screen discrimination reversal paradigm actively minimize spatial learning by never making spatial location informative. Both tasks require trial-to-trial working memory to integrate learning across trials however, and the role of hippocampal GluN2A in these behaviors cannot be ruled out.
Interestingly, although GluN2AKO had elevated errors and correction errors across the problem, we failed to see an effect of GluN2A loss on reversal performance, a measure of behavioral flexibility mediated selectively by the OFC in rodents, when olfactory, tactile or simple visual stimuli were used (Schoenbaum et al., 2002, Chudasama and Robbins, 2003, Izquierdo et al., 2013). Given that genetic or pharmacological loss of GluN2B is sufficient to impair reversal in the mouse (Brigman et al., 2013), the data presented here suggest that GluN2A in OFC may not play a significant role in mediating reversal learning. Alternatively, other, non-NMDAR mediated pathways may compensate for NMDAR-dependent associative learning and reversal in the absence of GluN2A while the higher task demands of attentional set-shifting cannot be compensated. One such mechanism may be through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), as targeted blockade in the vmPFC indicates AMPARs are required for both optimal set-formation and shifting, while NMDAR are required selectively for set-shifting (Stefani et al., 2003).
Interestingly GluN2A heterozygous mice, expressing only 50% of GluN2A subunits showed performance similar to WT throughout testing (Sakimura et al., 1995). Previously, gene-dose dependent effects have been observed in the attentional (Young et al., 2007) and simple learning (Young et al., 2012) capabilities of alpha 7 nicotinic acetylcholine receptor mutant mice. Here however, we were able to determine that reduced functioning of the GluN2A subunit did not impact any aspect of discrimination learning or behavioral flexibility. Although it appears there are gene-dose dependent effects on errors in simple visual discrimination (Figure 2B and C), these effects were not significant. Larger sample sizes or adding an additional NMDAR antagonist insult may have significantly teased apart the effect of receptor levels.
Brain-wide knockout of GluN2A resulted in a specific executive control impairment of vmPFC mediated set-shifting. This finding is the first evidence implicating a distinct requirement for the GluN2A subunit in cortically driven executive functioning. Our data support the conclusion that GluN2A is required for optimal set-shifting and attentional processing by vmPFC and ACC, respectively. Glutamatergic dysfunction and resulting hypofrontality are thought to play a large role in executive control deficits in many neuropsychiatric disorders, such as addiction and schizophrenia (Goldberg and Weinberger, 1988). Given the role of NMDAR in in learning and memory (Malenka and Bear, 2004), as well as initial evidence of its role in executive function processes, understanding the unique role that NMDAR subunits play is essential to furthering our understanding of these disorders and for providing potential therapeutic targets.
Acknowledgments
Supported by National Institutes of Health grants 1K22AA020303-01. We are grateful to Andrew Holmes for providing mice for these experiments and Rahul Sigdel for his assistance genotyping.
Footnotes
All authors have no conflict of interest to declare.
References
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, Good MA. The drugs don’t work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology (Berl) 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nature Neuroscience. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learning & memory. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Ma LM, Phillips AG, Floresco SB. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology. 2011;216:525–535. doi: 10.1007/s00213-011-2246-z. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Probing prefrontal function in schizophrenia with neuropsychological paradigms. Schizophrenia bulletin. 1988;14:179–183. doi: 10.1093/schbul/14.2.179. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annual review of neuroscience. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Darling C, Manos N, Pozos H, Kim C, Ostrander S, Cazares V, Stepp H, Rudebeck PH. Basolateral amygdala lesions facilitate reward choices after negative feedback in rats. J Neurosci. 2013;33:4105–4109. doi: 10.1523/JNEUROSCI.4942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Kirino Y, Kadotani H, Nakamura Y, Ikeda M, Yoshioka T. Conditioned eyeblink response is impaired in mutant mice lacking NMDA receptor subunit NR2A. Neuroreport. 1997;8:3717–3721. doi: 10.1097/00001756-199712010-00012. [DOI] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, Jensen V, Koester HJ, Mihaljevic AL, Utvik JK, Kvello A, Ottersen OP, Seeburg PH, Sprengel R, Hvalby O. Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci. 2003;23:10791–10799. doi: 10.1523/JNEUROSCI.23-34-10791.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos T, Nikiforuk A, Rafa D, Popik P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology (Berl) 2011;214:911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel D, Sidhu N, Barkai E, Quinlan EM. Learning in the absence of experience-dependent regulation of NMDAR composition. Learning & memory. 2006;13:566–570. doi: 10.1101/lm.276606. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar output influences non-motor function. Mol Psychiatry. 1996;1:429–433. [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB. Effects on executive function following damage to the prefrontal cortex in the rhesus monkey (Macaca mulatta) Behav Neurosci. 2009;123:231–241. doi: 10.1037/a0014723. [DOI] [PubMed] [Google Scholar]
- Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007a;27:12123–12131. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CW, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. J Neurosci. 2007b;27:12123–12131. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oualian C, Gisquet-Verrier P. The differential involvement of the prelimbic and infralimbic cortices in response conflict affects behavioral flexibility in rats trained in a new automated strategy-switching task. Learning & memory. 2010;17:654–668. doi: 10.1101/lm.1858010. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature reviews Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behavioral neuroscience. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behavioral neuroscience. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in alpha7-nicotinic acetylcholine receptor knockout mice. Genes, brain, and behavior. 2011;10:720–733. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacology. 2012;62:1381–1390. doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA, Jeste DV, Risbrough VB. The mouse attentional set-shifting task: A method for assaying successful cognitive aging? Cognitive, Affective & Behavioral Neuroscience. 2010;10:243–251. doi: 10.3758/CABN.10.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]