Abstract
Humans readily learn and remember new motor skills, a process that likely underlies adaptation to changing environments. During adaptation, the brain develops new sensory-motor relationships, and if consolidation occurs, a memory of the adaptation can be retained for extended periods. Considerable evidence exists that multiple brain circuits participate in acquiring new sensory-motor memories, though the networks engaged in recalling these and whether the same brain circuits participate in their formation and recall has less clarity. To address these issues, we assessed brain activation with functional MRI while young healthy adults learned and recalled new sensory-motor skills by adapting to world-view rotations of visual feedback that guided hand movements. We found cerebellar activation related to adaptation rate, likely reflecting changes related to overall adjustments to the visual rotation. A set of parietal and frontal regions, including inferior and superior parietal lobules, premotor area, supplementary motor area and primary somatosensory cortex, exhibited non-linear learning-related activation that peaked in the middle of the adaptation phase. Activation in some of these areas, including the inferior parietal lobule, intra-parietal sulcus and somatosensory cortex, likely reflected actual learning, since the activation correlated with learning after-effects. Lastly, we identified several structures having recall-related activation, including the anterior cingulate and the posterior putamen, since the activation correlated with recall efficacy. These findings demonstrate dynamic aspects of brain activation patterns related to formation and recall of a sensory-motor skill, such that non-overlapping brain regions participate in distinctive behavioral events.
Keywords: visual-motor adaptation, learning, recall, event-related functional MRI
Sensory-motor adaptation occurs when an environmental perturbation alters performance to create a mismatch between predictions and outcomes. To restore normal function, the brain creates or updates new sensory-motor relationships based on error signals and stores them for future use. A common method to assess behavioral and brain correlates of sensory-motor adaptation entails using visual-motor adaptation to induce a discrepancy between movement direction and its visual representation (Krakauer et al. 2005), which yields rapid improvements in accuracy, followed by more graduate accuracy improvements, and finally in automaticity. To determine whether new sensory-motor relationships have formed, one can measure after-effects, characterized by reaching errors opposite to the original perturbation (Shadmehr and Mussa-Ivaldi 1994) or savings, by comparing performance during recall to that observed during initial learning (Krakauer et al. 2005).
How the brain mediates adaptation to perturbations to maintain or to restore performance and how it recalls acquired motor memories remains incompletely specified, especially at the systems level. Typically, brain representations of a memory shift across brain regions during its formation and evolution towards automaticity (Frankland and Bontempi 2005; Kelly and Garavan 2005). Although, several brain areas have clear involvement in sensory-motor adaptation, including cerebellar and basal ganglia structures and fronto-parietal cortices (Doyon and Benali 2005; Kelly and Garavan 2005; Shadmehr and Krakauer 2008), a comprehensive accounting of brain activation related to the formation and recall of motor memories related to visual-motor adaptation has not emerged. Specifically, can brain regions that mediate sensory-motor adaptation, much like those engaged during learning and rehearsing arbitrary visual-motor associations (e.g., Bédard and Sanes 2009; Eliassen et al. 2003), become differentiated by their role(s) across dynamic processes that mediate initial adaptation, or error reduction, short-term rehearsal, and immediate recall?
Prior work has implicated cerebellar processing during an initial error reduction phase but also upon recalling acquired memories (Debas et al. 2010; Shadmehr and Holcomb 1997; but see Nezafat et al. 2001), while others have demonstrated basal ganglia involvement in recall but not learning (Bédard and Sanes 2011; Marinelli et al. 2009). The posterior parietal cortex (PPC, Della-Maggiore et al. 2004) and primary motor cortex (M1, Orban de Xivry et al. 2011) appear to become engaged during a period when errors have declined substantially and rehearsal has likely just begun, thus, after initial formation of new rules to sensory perturbations. These studies showed that disrupting processing in PPC or M1 with transcranial magnetic stimulation (TMS) early in learning, when errors are high, had little effect on adaptation; however, applying TMS later in learning, when errors are low, yielded less adaptation. Collectively, these findings suggested existence of variegated brain networks that become engaged as humans pass through various phases of sensory-motor adaptation.
Despite the existing knowledge, it remains unclear whether M1 and PPC have similar involvement during visual-motor adaptation relying on processes other than force-field adaptation (Diedrichsen et al. 2005; Rabe et al. 2009). Furthermore, other brain areas might have more involvement during rehearsal than during initial rule formation. Finally, few studies have examined brain processes when humans recall visual-motor adaptations. Thus, prior work has either not completely assessed sensory-motor adaptation at the systems level or has not assessed whether individual brain regions have involvement during specific phases of early adaptation, rehearsal or recall.
We used functional MRI to measure whole brain activation during formation and immediate recall of a visual-motor adaptation memory. We hypothesized that cerebellar structures would have involvement in error reduction, exhibiting activation function that would adhere to an exponential or power trend, and thus, cerebellar activity should correlate with adaptation rates. By contrast, we reasoned that M1 and PPC will have maximal activation after the initial error reduction phase occurred, reflecting the formation of a new sensory-motor memory as showed in prior TMS studies (Della- Maggiore et al. 2004; Orban de Xivry et al. 2011). Finally basal ganglia structures would show activation changes during recall (Bédard and Sanes 2011; Marinelli et al. 2009).
Method
Participants
We recruited 14 participants from the local community (mean age of 24 ± 4 yr; seven females) all right-handed as assessed by a modified handedness scale (Oldfield 1971). No participant had a history of neurological or sensory-motor disorder. All participants provided written informed consent according to established and approved Institution Review Board guidelines for human participation in experimental procedures at Brown University. We adhered to the principles of the Declaration of Helsinki. Participants received modest monetary compensation.
Tasks, Apparatus and Procedures
Participants performed a goal-directed, center-out movement task (Girgenrath et al. 2008; Graydon et al. 2005; Seidler et al. 2006) consisting mainly of wrist movement using an MRI-compatible joystick (Mag Design and Engineering, Sunnyvale, CA). They used their right hand while laying supine in the MRI system with the right arm, in a semi-prone, fully extended position beside their right side. Participants wore a set of headphones for ear protection and communication with the experimenter. All visual stimuli appeared on a projection screen positioned at the rear of the MRI system for viewing stimuli using an angled mirror mounted on the head coil of the MRI system. The mirror was located ~15 cm from a participant s eyes. Joystick displacements controlled a black cursor (0.75 cm diameter; 2.86° of visual angle) on the visual display. Participants always initiated their movements from a start position that consisted of a black annulus (1.25 cm diameter; visible throughout the experiment; 4.76° of visual angle) located in the center of the visual display; participant would then move toward targets that consisted of a black dot (1 cm diameter; 3.8° of visual angle) that appeared either up, right, down or left of the home position at 5.5 cm of eccentricity from the home position (Fig. 1A). Thus, the peripheral targets subtended an angle of 5.2° (2.6° left and right) from the center of the home position. Targets appeared in order (up, right, down, and left), one at a time and remained visible throughout a trial. We designed an event-related experiment with a trial onset asynchrony (TOA) based on fractions of the fMRI acquisition time (i.e., TR = 2.72 sec see below). We used steps of one-quarter of a TR, 0.68 sec, thus yielding TOAs of 3.4, 4.08, 4.76, 5.44, or 6.12 sec jittered across sets of five trials. We choose a variable TOA to reduce anticipation and promote rapid adaptation (Bock et al. 2005; Huang and Shadmehr 2007). We used the Windows version of PsychToolbox v.2.54 (www.psychtoolbox.org, Brainard 1997; Pelli 1997) for Matlab 6.5 (Mathworks, Natick, MA) to generate visual stimuli and record the joystick position at 500 Hz.
Fig. 1.
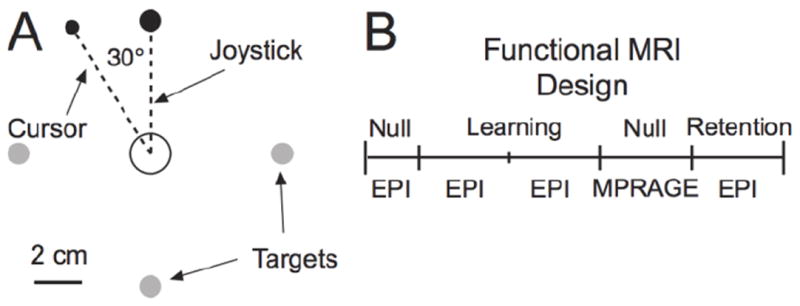
Task schematic. A: Targets appeared one at a time at random jittered times. In the visual perturbation condition, the cursor trajectory was deviated by 30° CCW from the intended joystick trajectory. B: Experimental design illustrating the sequence of trial types. Additional details in Methods.
Using “normal” visual conditions and before entering the MRI system, each participant received training to acquire the target using a rapid ballistic movement without on-line movement corrections or substantial curved trajectories. Participants repositioned the cursor at the home position after each movement. We exhorted participants not to implement on-line movement corrections since these type of corrections do not necessarily improve adaptation in healthy individuals (Tseng et al. 2007) and on-line control relies on some of the brain structures thought to have involvement in learning, such as the cerebellum (Desmurget et al. 2001; Diedrichsen et al. 2005; Lee and van Donkelaar 2006). Note that Debas et al. (2010) reported cerebellar activation at recall using a task with on-line corrections. We believe that our procedures reduced the possible confounds of on-line correction (see text below on percentages of “corrected” trials).
The experiment had two main conditions. In a Null condition, the cursor accurately represented the joystick movements without distortion such that forward joystick movements displaced the cursor upward and backward movements displaced the cursor downward and right and left joystick movements displaced the cursor right and left. For the perturbation condition (Fig. 1A), the direction of the cursor was rotated 30° counterclockwise (CCW) from the joystick trajectory about the home target. Participants practiced Null trials outside the MRI system for ~40 trials and again inside the MRI system for another ~40 trials before acquisition of functional MRI data. While we did not specifically assess training adequacy, we visually ensured that participants executed the movements as instructed.
The experiment was divided in five segments, each separated by a short break of less than 1 min (Fig. 1B). In the first segment, participants performed 40 Null trials. In each of the second and third segments, they performed 80 trials of the visual perturbation condition (cursor rotated CCW by 30°). In the fourth segment, participants performed 80 Null trials and in the fifth segment, participants performed 80 trials in the perturbation condition. We acquired functional MR images during all but the fourth segment.
MR Imaging
We used a 3T TIM Trio MRI system (Siemens Medical Solutions, Erlangen, Germany) to acquire anatomical and functional MR images. Participants lay supine inside the magnet bore with the head resting inside a receive-only eight-channel volume head coil used for radio frequency reception; the body coil transmitted radio frequency signals. Cushioning and mild restraint reduced head movements. The magnetic field was shimmed before MR data acquisition. For the functional data, we generated T2*-weighted gradient echo planar images (EPI) using the blood oxygenation level-dependent mechanism (Kwong et al. 1992) with TR = 2.72 sec, TE = 28 msec, field of view = 192 mm, image matrix = 64 × 64, flip angle = 90°, 3 mm slice thickness for 3 mm isotropic voxels, and acquired 46 slices per volume to cover the whole brain. The MRI system acquired the functional images in an interleaved, ascending manner and did not collect any data for the first two EPI volumes of each segment because of T1 saturation effects. We also acquired a high resolution three-dimensional anatomical image consisting of 160 1 mm sagittal slices (magnetization prepared rapid acquisition gradient echo sequence, MPRAGE), with TR = 1900 msec, TE = 2.98 msec, 1 mm isotropic voxel, 256 mm field of view. We acquired EPI data during the Null (segment 1), Learning (segments 2 and 3), and Recall (segment 5) collecting 80, 145, 145, and 145 volumes, respectively (Fig. 1B). We acquired the anatomical images during segment 4 (while participants performed 80 Null trials).
Behavioral data analysis
We calculated the cursor trajectory by taking the square root of the sum of squared x and y coordinates and filtered the data with a low-pass Butterworth filter using a 8 Hz cut-off. We differentiated the position of the cursor to yield tangential velocity and determined movement onset when the cursor velocity increased beyond 1% of its peak and movement end when the cursor velocity declined below 1% of its peak. We measured reaction time (RT) as the time elapsed between target presentation and movement onset and movement time (MT) as the time elapsed between movement onset and its end. We measured movement accuracy by calculating the angle between a line that joined the start position (central circle in Fig 1A.) to the target with a line that joined the position of the cursor at movement onset to the position of the cursor at peak velocity. CCW errors were deemed positive and clockwise (CW) errors negative.
We also aimed to reduce potential confounds related to on-line control since, as noted above, some brain areas may have common involvement in learning and on-line corrections. To address this potential confound, we implemented a kinematic analysis to identify trials that participants likely implemented on-line movement corrections. After reorienting the x and y coordinates to the straight ahead target, we differentiated them separately to yield direction (x) and extent (y) velocity profiles, respectively, with the extent component as the main movement axis. We then used these velocity profiles to determine characteristics of the movement, particularly whether corrections occurred. We considered a movement having an on-line correction as one that had either a changed sign in velocity or with a second velocity burst after the main peak velocity, and further, if the change in velocity (1) occurred at least 120 msec after movement initiation, (2) for which the corrective movement lasted at least 50 msec, and (3) covered a distance of at least 5 cm (for a similar analysis see Bédard and Proteau 2001; 2004; Chua and Elliott 1993; Khan et al. 2006; Meyer et al. 1988; van Donkelaar and Franks 1991). With these criteria, we viewed changes in velocity profiles before 120 msec as reflecting pre-programmed movement intent rather than sensory-based corrections (Desmurget and Grafton 2000; Saunders and Knill 2003; 2004). By contrast, small velocity changes occurring at the end of a movement would more likely have relation to stopping movements (Plamondon and Alimi 1997). Therefore, while some movements did indeed have curved trajectories, we viewed them as not likely reflecting on-line corrections, but rather reflecting a planned curved trajectory. Across the group, participants corrected 3.93 ± 4.6% movements across the experiment, thus, a very small percentage of the total number of movements. We discarded these corrected trials from all further behavioral and fMRI analyses.
We grouped trials into bins of 20 trials, yielding two Null blocks, eight Learning blocks, and four Recall blocks, and computed the means and within-participant variability of reaching error for each block, using the standard deviation of actual error. We binned trials in blocks of 20 to increase the strength of the estimated fMRI signal (see below). The binning procedure retained the characteristics of the learning curve (Fig. 3) reported by others with a similar paradigm but using fewer trials per blocks. Note, we did not include the first eight trials of the second Learning segment (learning block 5), and the first eight trials of the Recall segment (Recall block 1) in the behavioral and functional MRI analyses, thereby yielding 12 trials (instead of 20) for the fifth Learning block and the first Recall block. The rationale to exclude these trials related to transitory increases in error after pauses in the experimental procedures as participants adapt to visual-motor rotations (Krakauer et al. 2005; Seidler et al. 2006; Reis et al. 2009). Omission of these ‘initial’ trials after pauses has precedence in work investigating behavioral mechanisms of sensory-motor adaptation (Shadmehr and Holcomb 1997; Caithness et al. 2004; Krakauer et al. 2005). We used Matlab 7.4 and the R project (www.r-project.org) for statistical analyses of motor behavior.
Fig. 3.
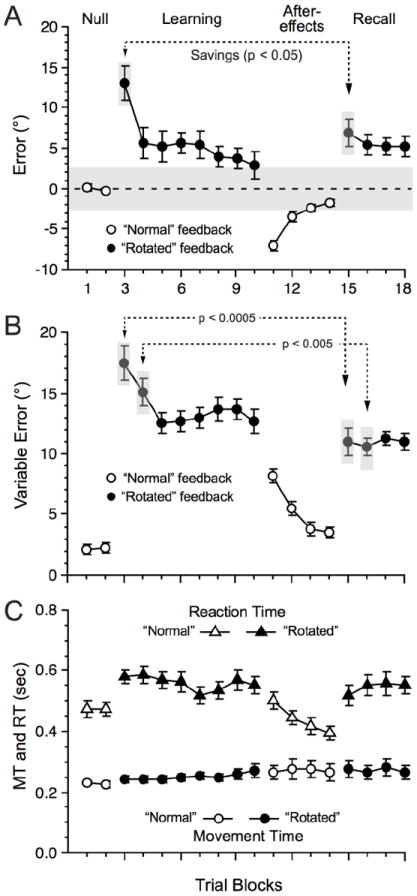
Behavioral performance across the experiment averaged over blocks of 20 trials (mean ± s.e.m. of all 14 participants). A: Reaching error. A repeated measures, one-way ANOVA across the blocks of the Null and Learning phase yielded a significant main effect: F(9, 117) = 10.12, p < 0.0001. Post-hoc tests with the Newman-Keuls procedure (p ≤ 0.05), corrected for multiple comparisons revealed significant differences between each of block 1 and 2 and blocks 3, 4, 5, 6, 7 and also between block 3 and 4. At Recall, error (Fig 3A) was slightly, but significantly, greater (pooled across the four blocks) than Learning (last two blocks; t(13) = 3.56, p ≤ 0.005). B: Reaching variability (standard deviation). A one-way ANOVA across the blocks of the Null and Learning phase was significant, F(9, 117) = 42.9, p < 0.0001. Post-hoc tests revealed significant differences between each of block 1 and 2 compared to blocks 3 to 10; also between block 3 and blocks 4 to 10. At Recall, reaching variability was, conversely to error (Fig 3A), slightly but significantly lower at Recall than during Learning (t(13) = 3.79, p ≤ 0.005). C: MT and RT. MT remained relatively constant across all experimental phases, but the one-way ANOVA was significant, F(9, 117) = 2.55, p = 0.01. Post-hoc tests only revealed significant difference between the Null blocks and the last Learning block. Note that during the Learning phase, MT increased slightly from block 3 to block 10 (means and s.e.m. of 243 ± 10 msec vs. 268 ± 22 msec, respectively) but this increase was not statistically significant (t(13) = 1.15, p > 0.27). Concerning RT, the one-way ANOVA was significant, F(9, 117) = 7.18, p < 0.0001, and RT was lower during the Null blocks than during the Learning blocks. But there was no significant difference from late Learning to Recall (t(13) = 1.32, p > 0.21). During the learning phase RT remained constant from block 3 to block 10 (t(13) = 1.19, p > 0.26). The grey shaded area in A indicates the angle subtended by the target relative to the start position.
MRI signal processing and statistical analysis
We used AFNI (Analysis of Functional NeuroImages; Medical College of Wisconsin, National Institutes of Health: http://afni.nimh.nih.gov/afni, Cox 1996; Cox and Hyde 1997) and FSL (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl, Smith et al. 2004) software packages to process, analyze and visualize the MR images. We first scaled each of the EPI time series by their mean and multiplied by 100 to yield percentage signal change values. We then concatenated these time series and used a six-parameter rigid-body cubic polynomial interpolation (3dvolreg tool in AFNI) to motion correct these referring to the third image acquired and adjusted for slice timing offsets. Baseline drift was removed with a quadratic polynomial during the regression procedures for each MRI segment separately (see below). We then co-registered and normalized the anatomical and functional data sets to the MNI152 template (FLIRT tool in FSL) and finally spatially smoothed the functional data set with a 6 mm full-width half-maximum Gaussian kernel.
For statistical analysis, we initially implemented a first-level analysis (participant-level, fixed effect). Events of interest comprised the time of the target presentation for each trial in each of the Null, Learning, and Recall blocks that we then convolved with a gamma variate function (Cohen 1997) to yield an impulse response function. We then used these reference functions and the six motion correction parameters as inputs to a multiple regression analysis (3dDeconvolve tool in AFNI) to estimate the β weights in the functional MRI data from each block of trials separately. We then used these β weights in a second-level group analysis with participants as a random factor to determine brain representations of (1) error reduction, (2) learning and (3) recall. To assess learning-related changes, we first subtracted the estimated β weights obtained during the two Null blocks (pooled together) from that of each of the eight learning blocks to yield a more appropriate measure of learning.
For the first analysis, we aimed to identify brain areas that potentially coded for the rate of decreasing reaching error during learning. To do this, for each participant, we first averaged reaching error in blocks of eight trials, thus yielding 20 eight-trial blocks, and then fitted these reaching error blocks with a power function, y = a * xr, with y representing the reaching error across blocks (n = 20) and x representing the blocks (EzFit toolbox in Matlab). We used eight-trial blocks as a compromise between fitting the learning curve using all the data, which commonly exhibits extreme trial-by-trial variability, and a running average that reduces variability across trials while preserving the behavioral phenotype. This power function explained on average 45% ± 20 of the variance across participants, more so than an exponential function, y = a * exp(r*x), which explained 30.2% ± 19 or a double exponential, 42% ± 24. We then correlated the resulting rate (r) of each participant with their brain activation obtained during Learning (pooled across all eight learning blocks). We thresholded the resulting maps by retaining voxels that satisfied a probability threshold of p ≤ 0.005 corresponding to an R ≥ 0.661. Then, we corrected for multiple comparisons by first estimating the spatial structure of the noise in the functional MRI time series using the 3dFWHMx tool in AFNI by setting as an input the residuals of the time series after the GLM procedures. Using a threshold of p ≤ 0.05 at the cluster level, an activation cluster needed to include at least 51 contiguous voxels (alphasim tool in AFNI). The correction procedure used Monte Carlo simulations to calculate the probability of having a cluster of a certain size being due to chance (noise) alone. Thus, it computes the probability of a random field of noise producing a cluster of a given size after the noise is thresholded at a given level.
For the second analysis, we assessed learning-related brain activation. As stated in Introduction, we aimed to determine whether brain areas exhibited activation patterns that peaked as performance approached a plateau, as would be predicted from the PPC stimulation study (Della-Maggiore et al. 2004). Della-Maggiore et al. (2004) found that TMS directed to PPC disrupted reaching when performance approached a plateau, suggesting that brain activation in PPC actively contributed to that particular phase of learning and not during other aspects of the learning process. Our behavioral results showed that reaching error and reaching variability (Fig. 3A, B) attained a plateau at block 5-6 (block 3-4 of the Learning phase). If brain activation peaks as performance reaches a plateau, we reasoned that a linearly increasing or a quadratic function (with an inverted “U” shape) could fit the data reasonably well. Therefore, we used regression methods with linear and quadratic functions, run independently across all eight learning blocks (3dRegAna tool in AFNI; note that the program computes increasing and decreasing linear functions as well as quadratic functions with an “U” or an inverted “U” shape). We report on those voxel activation clusters that satisfied a probability threshold of p ≤ 0.005, F (1, 110) ≥ 8.2, and corrected for multiple comparisons at p ≤ 0.05 for 51 contiguous voxels (alphasim tool in AFNI).
Finally for the last analysis, we assessed recall-related brain activation. Specifically, we assessed whether the brain activation observed during learning would shift to different brain areas when participants recalled the memory developed during learning. To assess this potential outcome, we used t-tests to contrast the activation obtained during Recall (pooled across the four blocks) to that obtained during the last two blocks of Learning (Shadmehr and Holcomb 1997). Here, either an increase or decrease of activation from Learning to Recall would reveal a shift in activation. We then thresholded these statistical maps on a voxel basis (p ≤ 0.005, t(13) ≤ or ≥ 3.37) and corrected for multiple comparisons at p ≤ 0.05 for 51 contiguous voxels. Overall, we used the brain atlas of Duvernoy (1991), the cerebellum atlas of Schmahmann et al. (1999), a navigable web-based human brain atlas (https://www.msu.edu/~brains/brains/human/index.html), and tools in FSL to localize activation to brain areas. We have found that these atlases provide more accurate anatomical localization than the built-in tools of brain imaging platforms.
We also implemented an ROI based analysis focussing only on specific brain areas, that is the right cerebellum and left putamen, for which we had strong a priori hypothesis about their role in reducing error and memory recall, respectively. For the cerebellum analysis, we used the whole right hemisphere with a voxel threshold of p ≤ 0.005 and corrected for multiple comparisons at p ≤ 0.05 for 24 contiguous voxels. We correlated the brain activation during the learning phase with the rate of the power function. For the putamen analysis, because we found a cluster that also encompassed regions of the thalamus and insula, we decided to restrict the analysis to include only the putamen itself. We used a p ≤ 0.005 and corrected for multiple comparisons at p ≤ 0.05 for 6 contiguous voxels. We correlated the activation of this regions with the recall success and savings. We used tools in AFNI to draw the cerebellar and putamen ROI. In each of the two analyses, we determined the criteria for the minimum number of voxels contained within a cluster by using the same procedure as mentioned earlier.
Results
Behavioral performance
Figures 2 and 3 illustrate the behavioral outcomes observed across all the experimental phases. Since the current results have substantial similarity to prior data using the visual-motor adaptation paradigm (e.g., Krakauer et al. 2005), we describe the current results succinctly and refer the reader to the figures for details of the inferential statistical tests. Reaching error and variability (Fig. 3A-B, respectively) remained low in the Null phase, increased upon introducing the perturbation (block 3) but rapidly decreased to attain an asymptote level at block 4 (block 5 for variability). At Recall, error (Fig. 3A) was slightly, but significantly, greater (pooled across the four blocks) than during Learning (last two blocks), while reaching variability (Fig. 3B) was slightly but significantly lower at Recall than during Learning. Finally, we assessed savings by by taking the difference in accuracy between the first and second block of Learning and Recall (Krakauer et al. 2005) and only found significant effects for the first block (t(13) = 2.63, p < 0.05; t(13) = 0.33, p = 0.75) along with lower variability for the first two Recall blocks compared to the first two Learning blocks (t(13) = 4.99, p < 0.0005 and t(13) = 3.36, p < 0.005). The gradual improvement in reaching accuracy and decrease of variability and the presence of after-effects suggest that participants adapted successfully to the perturbation and formed a new motor memory, while the presence of savings and the essential similarity of reaching and variable errors between Recall and late Learning suggested that participants had good recall of the motor memory that they developed during Learning, thereby supporting the claim of successful visual-motor adaptation.
Fig. 2.
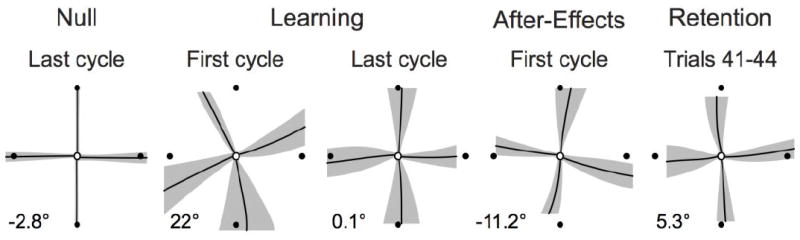
Averaged trajectories across all participants in the Null, Learning, Null, and Recall conditions. The black lines represent the average and the grey regions represent the variability of the joystick trajectories across participants (mean ± s.e.m. of all 14 participants). Note the improved trajectory from the first to the last cycle of the Learning phase. Also note that the trajectories at Recall resembled those occurring during late Learning. The numerical value (lower left of each section) reflects the group mean error for each set of trials).
Next, we assessed whether MT or RT (Fig. 3C circles and triangles, respectively) may, by themselves, have confounded the findings related to reaching error and variability. MT remained relatively constant across all experimental phases with no significant difference from the Null to the Learning phase or from late Learning to Recall with no difference across the eight Learning blocks. Concerning RT, there was a significant increase from the Null to the Leaning phase but with no significant difference from late Learning to Recall. During the learning phase RT remained constant from block 3 to block 10. To formally assess whether a speed-accuracy tradeoff mechanism occurred, which might trivially explain the neuroimaging results, we regressed MT over reaching error for each participant across the eight Learning blocks and found no significant relationship between reaching error and MT (t-test on the slopes coefficients, t(13) = 0.43, p = 0.67). We also applied robust regression methods to address the possibility that outlying data points may have prevented rejection of the null hypothesis; this procedure failed to reveal any significant effects. Concerning RT and reaching error, the same analysis revealed no significant relationship (t(13) = 0.60, p = 0.56). The fact that RT increased from the Null to the Learning phase suggested that participants had more difficulty in performing movements under the visually perturbed conditions. However, since RT remained constant across the Learning phase, an alternative explanation related to task difficulty would not likely explain the functional MRI results (see below). Similarly, since RT and MT did not differ between late Learning and Recall any potential difference in activation between these two task phases would not likely relate solely to RT or MT. These behavioral results appear to suggest that while participants formed and recalled new motor memories upon visual-motor adaptation, they did so without trading speed for accuracy.
While performance changed only slightly after block 4 and 5, the prolonged practice most likely enhanced memory formation via an over-learning effect stipulating that even after attaining an asymptotic level of performance prolonged practice enhances long-term retention (Melnick 1971; Joiner and Smith 2008).
Brain Activation
Learning
We first aimed to identify brain areas that may code for the rate of decreasing reaching error during learning. Therefore, we correlated the rate of decreasing reaching error, as measured by a power function, with brain activation during learning (see Methods). We found no area that exhibited a significant correlation between error and activation using our statistical thresholds (see Methods). To address the possibility that we had insufficient statistical power and because we had strong a priori hypothesis about the role of the cerebellum in reaching error reduction, we restricted our analysis to the right cerebellum (see Methods; Debas et al. 2010). We found a cluster in a region of the right cerebellum encompassing portions of Crus-VIIB and Crus-II with a non-negligible trend toward a learning effect (mean R = 0.74, max R = 0.80; x = 30, y = -63 z = -42; 22 voxels, p = 0.07). Fig. 4B depicts the relationship between the functional MRI signal in the cerebellum and the learning rate obtained from each participant. To address the question whether outlying data points may have unduly affected the results, we used robust regression methods and still rejected the null hypothesis of no correlation between activation and learning rate (p ≤ 0.01). We also used a Spearman rank correlation rank test and found a significant correlation for the cerebellum (R = 0.56, p ≤ 0.05). Finally, we also re-analyzed the data removing the data points associated with the participant that showed a low learning rate and recalculated the correlation coefficient, finding a significant correlation for the cerebellar cluster (p ≤ 0.05); thus we believe that these results have robustness. As can be seen, higher learning rates, that is, faster declines in reaching error, yielded greater brain activation. Although this outcome strongly suggests a relationship between brain activation and decreasing error, it could also reflect formation of a new motor memory specifically related to the perturbation. If the activation in the cerebellum related to learning, rather than merely decreasing error, then the observed activation should correlate with at least one measure of the quality of a motor memory, such as after-effects or savings magnitude. However, activation in the cerebellum did not correlate with after-effects defined as the error in the first cycle (i.e., first four trials) of that phase (p > 0.25, R < 0.33; note we obtained similar results when using 2, 3 or 4 cycles or even the first block of 20 trials of after-effects). Similarly, the cerebellum activation did not correlate with savings, defined as the difference in error between the first block of the Learning and Recall phases (p > 0.24, R < 0.34; note that we obtained similar results when using the first two trial blocks). Thus, it does not appear that the activation related to the learning rate reflected formation of new motor memory; instead, the observed cerebellar activation during Learning seemed more consistent with changes in behavioral performance, that is, decreased error magnitude or variability.
Fig. 4.
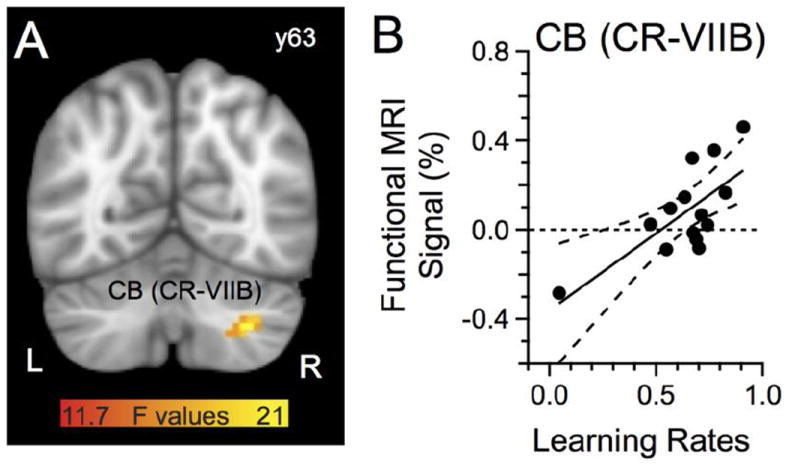
Functional MRI signal (%) correlated with rates of decreasing error. A: Cerebellum region with activation that correlated with a power function. B: Functional MRI signal (%) relationship with rates of decreasing error; one data point per subject. The dashed lines represent the 95% confidence band of the regression. The color bar represents the F statistics of the regression. L, left hemisphere. The anatomical underlay in this and subsequent figures is the MNI template provided by FSL.
We next asked whether brain responses during Learning conformed to a linear or a quadratic fit aiming to provide additional clarity on activation patterns observed during learning (see Methods for rationale). We failed to find activated clusters with a significant linear fit across the trials blocks. However, we identified nine clusters with activation conforming to a significant quadratic fit; all of these clusters exhibited an inverted U-shaped activation pattern (Fig. 5A, Table 1). We did not find clusters having the reverse relationship, that is, a U-shaped function, even when using a generously relaxed cluster threshold of p = 0.2. The activation clusters with the inverted U-shaped activation pattern appeared in the right parietal cortex involving mostly the inferior parietal lobule (IPL), the left superior parietal lobule (SPL), the left intra-parietal sulcus (IPS), the left superior temporal gyrus (STG), the right superior frontal gyrus (SFG) that also included a region that most likely corresponded to the pre-supplementary motor area (pre-SMA), a region encompassing the left primary somatic sensory cortex (S1) and the precuneus on the medial cortical wall (S1/precuneus), the right S1, left middle occipital gyrus (MOG), and the right prefrontal cortex (PFC). Fig. 5B depicts the functional MRI signal for these clusters across the eight learning blocks.
Fig. 5.
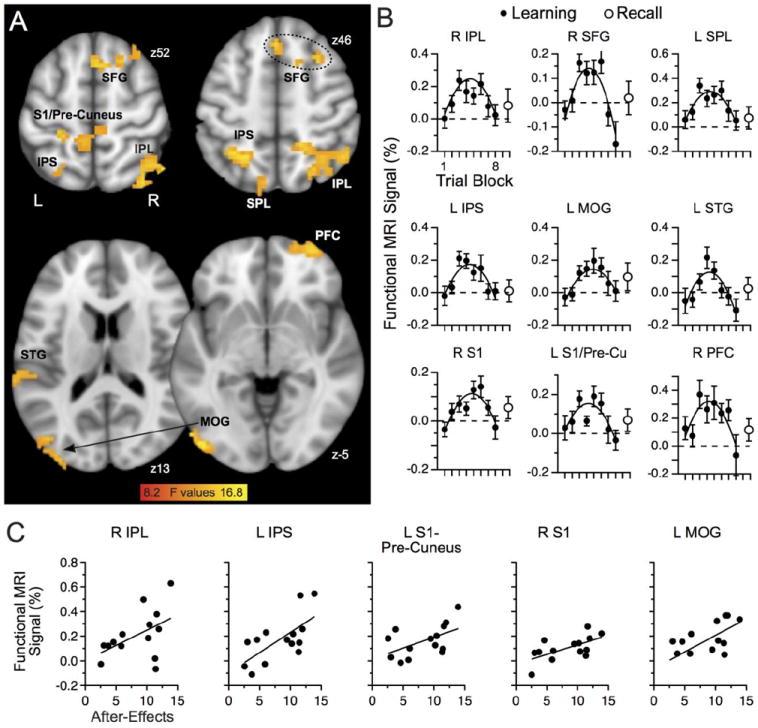
Learning-related activation. A: Regions with quadratic fit to observed activation included frontal and parietal cortices. B: Functional MRI signal (%) across the Learning phase (black circles) and Recall phase (open circles) for regions with a significant quadratic fit. C: Regions having activation correlated with the magnitude of after-effects; one data point per participant. The color bar represents the F statistics of the regression. See Table 1 for more details.
Table 1.
Activation clusters during learning.
| F Values | MNI Corrdinates | |||||
|---|---|---|---|---|---|---|
| Brain Regions | Volume | Mean | Max | X | Y | Z |
| R IPL | 8154 | 10.08 | 16.25 | 52 | -58 | 43 |
| L SPL | 2511 | 9.77 | 16.07 | -15 | -67 | 39 |
| L IPS | 1377 | 10.49 | 14.41 | -21 | -58 | 46 |
| R SFG | 2025 | 10.30 | 14.49 | 30 | 16 | 53 |
| R PFC | 1377 | 10.96 | 16.90 | 33 | 62 | -7 |
| L S1/Precuneus | 2079 | 9.4 | 12.40 | -24 | -37 | 50 |
| R S1 | 1674 | 10.28 | 15.48 | 21 | -31 | 70 |
| L STG | 1377 | 9.79 | 13.3 | -67 | -34 | 8 |
| L MOG | 4806 | 10.1 | 15.01 | -49 | -83 | -12 |
F values represent the F statistics of the quadratic regression. See text for details about analyses. IPL: inferior parietal lobule, SPL: superior parietal lobule, IPS: intra-parietal sulcus, SFG: superior frontal gyrus, PFC: prefrontal cortex, S1: somatosensory cortex, STG: superior temporal gyrus, MOG: middle occipital gyrus.
To complement this analysis, we assessed whether learning-related brain activation in these areas may have corresponded to metrics of motor memory formation in the context of visual-motor adaptation, such as after-effects or savings; note that for this analysis we used the functional MRI signal occurring (and pooled) during blocks 3 to 6 since other blocks exhibited brain activation indistinguishable from baseline. For analyzing and illustrating the after-effects, we used the absolute value (i.e., positive values) of the observed error for intuitive clarity. A regression analysis revealed that the right IPL, left IPS, left S1-precuneus, right S1 and and left MOG exhibited activation with a significant positive correlation to the magnitude of after-effects (Fig. 5C; all p ≤ 0.05, R > -0.48). This positive correlation indicated that more brain activation was associated with larger after-effects, a metric of visual-motor memory formation (Shadmehr and Mussa-Ivaldi 1994). We failed to find a significant correlation between brain activation and savings. This outcome suggested that these structures had a greater role than others in developing new motor memories related to visual-motor adaptation, but not necessarily in recalling these memories. We also found that the structures with this non-linear activation profile showed little evidence of any change in activation during Recall (Fig. 5B, open circle to right of activation profile).
Cognitive strategies
We also asked whether the longer RT observed during Learning compared to that occurring during the Null phase reflected the implementation of a cognitive strategy during Learning. One might expect that if the higher RT during Learning related to implementation of a cognitive strategy, then we would observe a statistically significant relationship between brain activation and RT, either directly, such as finding correlated activation with RT, perhaps reflecting different brain processing to implement the cognitive strategy. When correlating the functional MRI signal of the clusters activated during Learning with the concurrent observed RT, we found only a single cluster with a significant negative activation-RT correlation, located in the superior frontal gyrus (R = -0.66, p ≤ 0.01; SFG in Fig. 5A). The negative sign of the correlation indicates that higher brain activation was associated with lower RT, which might have inconsistency with the simple minded notion of higher activation signifying implementation of a cognitive strategy, which typically would yield higher RT. Furthermore, since RT did not decline once participants learned the visual rotation, that is, in the final Learning trials when performance stabilized, also argues against participants implementing a cognitive strategy.
In summary, the analysis on learning rates (Fig. 4 and associated text above) assessed how the dynamics of behavioral changes occurring during visual-motor adaptation influenced brain activation. As noted, this analysis suggested that the activation in the cerebellum related more to error reduction than to formation of a new motor memory, perhaps consistent with prior reports that reaching error can yield activation in brain areas thought to have involvement in motor learning (Diedrichsen et al. 2005; Grafton et al. 2008). The subsequent analyses (Fig. 5 and associated text) identified regions with specific activation trends related to learning. Differences in behavioral measures (Fig. 3) between the Null and Learning phases are unlikely to explain these results as they differed markedly from a quadratic activation pattern. Additionally, our methods and analyses reduced considerably the confound of on-line corrections.
Recall
We next assessed brain activation related to recall by contrasting the functional MRI signal obtained during Recall to that acquired late in Learning (Fig. 6A-B; Table 2). This analysis revealed a cluster in frontal cortex straddling the right ACC and the frontopolar gyrus, a cluster that included the mid cingulate gyrus and paracentral lobule, a cluster bilaterally in the cerebellum (CR IV), a cluster that included the right insula and the temporal gyrus (inferior to superior portions), and a cluster in the left putamen that also included part of the insula and thalamus (labelled putamen in Fig. 6A-B and in Table 2). We found no brain area that exhibited less activation during Recall than during Learning. To examine this negative result further, we assessed the contrast of Learning > Recall without using a cluster thresholding procedure, only finding one activation cluster of two voxels, located in the left insula, with greater activation during Learning than Recall; thus, we would conclude that processes related to Recall only yielded relative increases in activation compared to Learning.
Fig. 6.
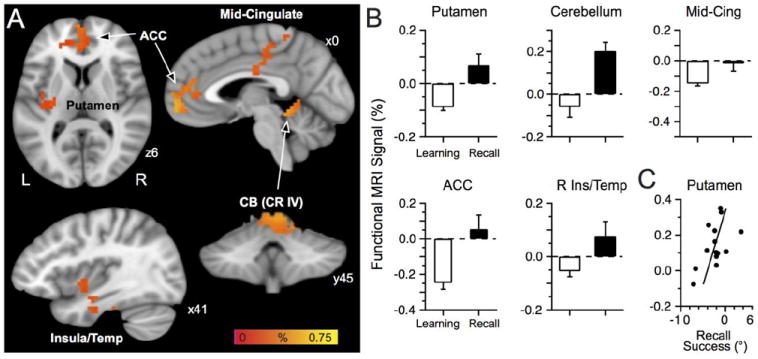
Recall-related activation. A: Regions with more activation at Recall than late learning included the ACC, mid-cingulate, putamen, and cerebellum. The color bar represents the percent signal of the contrast Learning vs. Recall. B: Functional MRI signal during the late Learning phase (last two blocks pooled) and Recall (all four blocks pooled) for brain regions with a Recall-related signal. C: Regression analysis of brain activation vs. recall success revealed that the putamen activation correlated with recall success; one data point per participant.
Table 2.
Activation clusters related to Recall.
| % | t values | MNI Coordinates | ||||||
|---|---|---|---|---|---|---|---|---|
| Brain Regions | Volume | Mean | Max | Mean | Max | X | Y | Z |
| Cortical | ||||||||
| R ACC | 5103 | 0.31 | 0.75 | 3.99 | 6.69 | 3 | 46 | 9 |
| L Mid-Cingulate gyrus / paracentrale lobule | 4131 | 0.17 | 0.30 | 4.02 | 6.25 | -9 | -30 | 38 |
| R Insula / temporal cortex | 1701 | 0.20 | 0.44 | 3.97 | 5.22 | 42 | -8 | -29 |
| Sub-cortical | ||||||||
| R Cerebellum | 2295 | 0.38 | 0.63 | 3.99 | 5.43 | 6 | -45 | -13 |
| L Putamen | 1674 | 0.19 | 0.44 | 3.95 | 5.73 | -15 | -13 | 19 |
% represents the MRI signal of the contrast Recall vs. Learning with the corresponding t values of the t-test. ACC: anterior cingulate cortex
We also considered whether the slightly greater reaching error during Recall than that observed late in Learning (Fig. 3A) may have yielded the greater activation during Recall compared to that occurring late in Learning. To assess this possibility, we correlated the functional MRI signal in these areas with the difference in reaching error between Recall and Learning and found no area with a significant correlation between error and activation (all p > 0.29). These findings would not seem readily explained by behavioral differences or a different cognitive strategy between Learning and Recall since reaching error, variability, RT and MT remained relatively constant between late Learning and Recall. Furthermore, our procedures and analyzes limited potential confounds due to movement corrections, since we removed trials with excessive movement corrections. We also confirmed by careful visual inspection that the MRI system did not unduly distort the EPI sequences in the regions in prefrontal cortex having significant activation.
Fig. 6B shows the functional MRI signal obtained late in Learning and during Recall. For the mid-cingulate cluster, the greater activation during Recall appeared driven more by deactivation late in Learning than by increased signal during Recall. By contrast the putamen, cerebellum, ACC, and insula/temporal cortex exhibited increased activation during Recall and either deactivation or no signal change during late Learning.
We next addressed whether the greater activation during Recall might have related to successful recall of the memory developed during Learning. To assess this possibility, we first computed an index of recall success by subtracting the reaching error during Recall (pooled across all four blocks) from reaching error observed late in Learning (blocks 9 and 10 pooled; thus Learning minus Recall), as similarly done with the functional MRI signal. This index determined how well participants recalled the learned visual-motor adaptation with higher values indicating better recall ability. Note that because prior work implicated the basal ganglia in recalling memories formed following visual motor adaptation (Bédard and Sanes, 2011; Marinelli et al. 2009) and because the cluster found in the putamen also included part of the insula and thalamus, we implemented a ROI-based analysis to assess the specific role of the putamen in recall, i.e. without the influence of the insula and thalamus. We used tools in AFNI to draw a ROI that encompassed only the left putamen to assess whether the brain activation could correlate to the recall success index. We then thresholded the voxels with a p ≤ 0.005 and corrected for multiple comparisons with p = 0.05 for 6 contiguous voxels (alphasim tool in afni). This procedure yielded a cluster of 15 voxels, that is, voxels with significant activation located solely within the putamen. A regression analysis revealed that the activation in the putamen (Fig. 6C) was significantly related to recall ability (p ≤ 0.05, R = 0.55), thereby associating greater activation in the putamen with higher recall success. The remainder of the cluster, that is, the regions touching on the insula and thalamus, did not exhibit significant relationship with recall or savings (p > 0.05, R = 0.32, p > 0.05, R = 0.13, respectively). Further, we did the same analysis for the right putamen and bilateral caudate nucleus, without finding a significant cluster that survived the statistical thresholds.
Finally, we also implemented an analysis to determine whether the brain activation of the clusters identified during the Recall phase could have been explained by observed RT and would then have a possible relationship to participants implementing a cognitive strategy; we found no cluster with a significant correlation between brain activation and RT (all p > 0.29), thus, finding no evidence of an explicit cognitive strategy during recall.
Discussion
We assessed how the brain forms new motor memories upon visual-motor adaptation and how brain structures participate in recalling these memories. The most substantial findings related to an activation shift across different brain regions as participants reduced reaching error, formed and then recalled the learned visual-motor adaptation, a non-linear pattern of brain activation during learning, and finding several areas with activation correlated with after-effects and recall.
Error reduction
We used an adaptation task that initially yields large errors followed by gradual error reduction with practice, finding activation in the right cerebellum that correlated with error reduction without obvious relation to after-effects or savings. This outcome suggests that this region participated in improving performance but not in forming new motor memories. Although prior results supported cerebellar involvement in similar types of motor learning (e.g., Criscimagna-Hemminger et al. 2010; Flament et al. 1996; Galea et al. 2010; Imamizu et al. 2000; Rabe et al. 2009; Sanes et al. 1990; Seidler et al. 2006; Shadmehr and Holcomb 1997; Smith and Shadmehr 2005; Tseng et al. 2007; Werner et al. 2009), our analyses permitted identifying a cerebellar role during visual-motor adaptation related to decreasing error. This finding supports the influential idea that the cerebellum updates forward models that predict the sensory consequences of movements (Shadmehr and Krakauer 2008; Wolpert and Miall 1996). However, since we found no activation related to measures of learning new motor memory, such as savings, we propose that the activated portions of the cerebellum participate in updating but not necessarily consolidating those new sensory-motor relationships related to visual-motor adaptation.
Learning-related activation
Forming new motor memories related to visual-motor adaptation engaged a different brain network than error reduction; this network encompassed several neocortical structures without subcortical involvement (see Fig. 5). While prior work often found learning-related activation across neocortex, the evolution of brain activity across learning has not typically reflected a rising and then falling activation pattern (Della-Maggiore and McIntosh 2005; Girgenrath et al. 2008; Ghilardi et al. 2000; Grafton et al. 2008; Graydon et al. 2005; Imamizu et al. 2000; Inoue et al. 1997; 2000; Krakauer et al. 2004; Krebs et al. 1998; Seidler et al. 2006; Shadmehr and Holcomb 1997; 1999). Luauté et al. (2009) described a similar inverted U-shaped activation pattern during prism adaption in the cerebellum, but this pattern peaked when error was low. By contrast, we found that cerebellar activation corresponded to the rate of error reduction, consistent with a cerebellar role in adaptation. The difference between the two studies may correspond to task differences since Luauté et al. (2009) used prism adaptation that directly modifies visual appreciation of the world while we used a world-based rotation.
The learning-related activation in the parietal lobe (left IPS and right IPL) paralleled the magnitude of after-effects, a metric of sensory-motor memory formation (Shadmehr and Mussa-Ivaldi 1994). Prior work has already implicated parietal structures in sensory-motor memory formation (Della-Maggiore et al. 2004; Inoue et al. 1997; Mutha et al. 2011; Perfetti et al., 2011). However, there remains some controversy whether the left or the right parietal cortex has more importance for learning. Here, we found bilateral involvement of two different parietal structures while prior work highlighted a prominent role for the right (Inoue et al. 1997; Perfetti et al., 2011) or the left parietal lobe (Della-Maggiore et al. 2004; Mutha et al., 2011). More work should address this controversy especially related to rehabilitation of patients with vascular related pathology in the parietal lobe. Mechanisms within the parietal cortex likely integrate sensory information about target and hand positions to compute a movement plan (Andersen and Cui 2009), a role that relates to forming new visual-motor maps as required by adaptation to world-related distortions. Although visual-motor adaptation is visually based, proprioceptive recalibration also likely accompanies it (Cressman and Henriques 2011), an outcome that likely explains the observed S1 activation.
The non-linear activation pattern during Learning suggests specific roles of neocortical structures in sensory-motor memory formation but not to performance. First, this activation pattern had little evident relationship to reaching error or variability, MT or RT. Early in Learning, participants actively reduced error and started developing associations between the rotated world and motor commands. The slow rise of activation during Learning could reflect the initiation of the associative process, the peak in the middle of learning perhaps reflected its cessation, and the ensuing activation decline could reflect a virtual handing off of responsibility for shifting recall of sensory-motor memories to new areas. Prior work on associative motor learning reported non-linear relationships between behavioral improvements and brain responses (Mitz et al. 1991; Pasupathy and Miller 2005; Seger and Cincotta 2006). In aggregate, the CNS may treat large errors as task-irrelevant and outside its control, while viewing smaller errors as controllable. This idea may explain the observed quadratic activation pattern, since regions engaged in developing sensory-motor memory may have assigned less weight to motor commands that yield large errors, but more weight to motor commands that yield smaller errors relevant in memory formation. By contrast, other areas, including the cerebellum, would assign more weight to large errors. Thus, forming new sensory-motor memory, much like other skills, would seem to occur most actively after an initial error reduction phase and occur in a different set of areas. This notion corresponds well with results reported by Della-Maggiore et al. (2004) that showed that disrupting SPL neural processing with TMS did not reduce adaptation when large reaching error occurred, but that TMS reduced adaptation when reaching became proficient, thus with small errors (see also Orban de Xivry et al. 2011).
Shift of activation from Learning to Recall
We found a different activation pattern during Recall than Learning that involved neocortical and subcortical regions including the ACC, mid-cingulate gyrus, putamen, cerebellum, and the insula/temporal cortex. The putamen activation also correlated with recall success. This shift in the memory brain representation paralleled that observed for motor sequences and declarative memory and engaged common areas such as the ACC and putamen despite being governed by different learning rules (Donchin et al. 2002; Doyon et al. 2009). This shift could relate to different brain mechanisms involved in learning and recalling sensory-motor memory with a model-based mechanism for learning and a model-free mechanism (use-dependent plasticity and operant reinforcement) for recalling (Huang et al. 2011).
The putamen activation at Recall, but not during Learning, echoes prior reports that basal ganglia dysfunction due to Parkinson’s or Huntington’s disease does not affect sensory-motor adaptation while impairing recall (Bédard and Sanes 2011; Marinelli et al. 2009; Smith and Shadmehr 2005; see also Cavaco et al. 2010) and transfer of learning from the right to the left limb (Isaias et al., 2010). Thus, basal ganglia circuits may not have substantial involvement in forming new sensory-motor memory, but these circuits may participate more in their rehearsal and automatization. The fact that higher activation in putamen correlated with recall success suggests that it had clear involvement in selecting the correct and inhibiting the wrong motor commands, a known role of the putamen (Wichmann and DeLong 1996). From the above, one might conclude that basal ganglia circuits become engaged after forming sensory-motor memories to enable selection of motor commands that will maximize reward.
Finally, we found activation in anterior portions of the ACC, which has involvement in conflict and performance monitoring (Botvinick et al. 2004; Paus 2001) and also in retroactive switching, i.e. in switching behavior upon a contextual change (Hikosaka and Isoda 2010) that is certainly a feature of recalling motor memories. While not reported in Results, activation in the ACC, as well as that in mid-cingulate regions, did not correlate with learning, savings or recall success. Therefore, the Recall-related activation profile in ACC and mid-cingulate regions solely related to a differential activation pattern between late Learning and Recall.
In aggregate, the current results detailing brain correlates of visual-motor adaptation likely pertain to development and maintenance of internal models (IM) for motor control (Shadmehr and Wise 2005). An IM comprises an inverse and a forward model that together compute the motor commands necessary to perform voluntary movements and computes a sensory prediction of the motor commands. The commands and sensory prediction error become created and then updated during sensory-motor adaptation. Although, the current data does not readily permit disentanglement of the forward from the inverse model, nevertheless the results suggest brain areas that probably participate in development and maintenance of IMs.
Conclusions
Collectively, our findings showed that the various phases of sensory-motor adaptation recruits different brain network. Forming new sensory-motor memory following sudden environmental visual perturbations depends on the cerebellum for error reduction and on parietal and frontal regions for memory formation per se while recalling these memories depends on a different network that includes the putamen and ACC. We recognize that the short delay separating Learning and Recall may not have not permitted the sensory-motor memory to become ‘permanent’. Thus, the current results may indicate that these regions mediate a phase along the consolidation process and that the parieto-frontal motor areas and cerebellum may ultimately store these types of memory (Shadmehr and Holcomb 1997).
Highlights.
Humans adapt to world-based visual distortions
Cerebellum exhibits activation directly related to error reduction
Frontal-parietal network demonstrates learning related activation
Putamen exhibits recall related activation
Dynamic activation during motor learning and recall across brain structures
Acknowledgments
This work was supported by the Dr. Ralph and Marian Falk Medical Trust, the National Institutes of Health (IDeA P20GM103645) and a Center of Excellence grant (N9228C) from the US Department of Veterans Affairs.
Role of the funding source: The funding sources had no involvement in the study design, collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication. The NIH and Veterans Administration provided funds during manuscript preparation and review.
Footnotes
Conflict of Interest: We have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–58. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Bédard P, Proteau L. On the role of static and dynamic visual afferent information in goal-directed aiming movements. Exp Brain Res. 2001;138:419–431. doi: 10.1007/s002210100739. [DOI] [PubMed] [Google Scholar]
- Bédard P, Proteau L. On-line vs. off-line utilization of peripheral visual afferent information to ensure spatial accuracy of goal-directed movements. Exp Brain Res. 2004;158:75–85. doi: 10.1007/s00221-004-1874-5. [DOI] [PubMed] [Google Scholar]
- Bédard P, Sanes JN. On a basal ganglia role in learning and rehearsing visual-motor associations. NeuroImage. 2009;47:1701–1710. doi: 10.1016/j.neuroimage.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard P, Sanes JN. Basal ganglia-dependent processes in recalling learned visual-motor adaptations. Exp Brain Res. 2011;209(3):385–393. doi: 10.1007/s00221-011-2561-y. [DOI] [PubMed] [Google Scholar]
- Bock O, Thomas M, Grigorova V. The effect of rest breaks on human sensorimotor adaptation. Exp Brain Res. 2005;163:258–260. doi: 10.1007/s00221-005-2231-z. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sic. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci. 2004;24:8662–8671. doi: 10.1523/JNEUROSCI.2214-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Correia M, Magalhaes M, Pereira C, Tuna A, Taipa R, Pinto P, Pinto C, Cruz R, Lima AB, Castro-Caldas A, da Silva AM, Damasio H. Task-specific contribution of the human striatum to perceptual-motor skill learning. J Clin Exp Neuropsychol. 2010:1–12. doi: 10.1080/13803395.2010.493144. [DOI] [PubMed] [Google Scholar]
- Chua R, Elliott D. Visual regulation of manual aiming. Human Movement Science. 1993;12:365–401. [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Motor adaptation and proprioceptive recalibration. Prog Brain Res. 2011;191:91–99. doi: 10.1016/B978-0-444-53752-2.00011-4. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci USA. 2010;107(41):17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Malfait N, Ostry DJ, Paus T. Stimulation of the posterior parietal cortex interferes with arm trajectory adjustments during the learning of new dynamics. J Neurosci. 2004;24:9971–9976. doi: 10.1523/JNEUROSCI.2833-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, McIntosh AR. Time course of changes in brain activity and functional connectivity associated with long-term adaptation to a rotational transformation. J Neurophysiol. 2005;93:2254–2262. doi: 10.1152/jn.00984.2004. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grea H, Grethe JS, Prablanc C, Alexander GE, Grafton ST. Functional anatomy of nonvisual feedback loops during reaching: a positron emission tomography study. J Neurosci. 2001;21:2919–2928. doi: 10.1523/JNEUROSCI.21-08-02919.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Sawaki L, Madupu G, Cohen LG, Shadmehr R. Mechanisms influencing acquisition and recall of motor memories. J Neurophysiol. 2002;88:2114–2123. doi: 10.1152/jn.2002.88.4.2114. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain : Surface, Three-Dimensional Sectional Anatomy and MRI. Springer-Verlag; New York: 1991. [Google Scholar]
- Eliassen JC, Souza T, Sanes JN. Experience-dependent activation patterns in human brain during visual-motor associative learning. JNeurosci. 2003;23:10540–10547. doi: 10.1523/JNEUROSCI.23-33-10540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament D, Ellermann J, Kim SK, Ugurbi lK, Ebner TJ. Functional magnetic resonance imaging of cerebellar activation during the learning of a visuomotor dissociation task. Human Brain Mapping. 1996;4:210–226. doi: 10.1002/hbm.460040302. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Galea J, Vazquez A, Pasricha N, Orban de Xivry J-J, Celnik P. Dissociating the Roles of the Cerebellum and Motor Cortex during Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns. Cereb Cortex. 2010;21:1761–1770. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Girgenrath M, Bock O, Seitz RJ. An fMRI study of brain activation in a visual adaptation task: activation limited to sensory guidance. Exp Brain Res. 2008;184:561–569. doi: 10.1007/s00221-007-1124-8. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Schmitt P, Van Horn J, Diedrichsen J. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage. 2008;39:1383–1395. doi: 10.1016/j.neuroimage.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon FX, Friston KJ, Thomas CG, Brooks VB, Menon RS. Learning-related fMRI activation associated with a rotational visuo-motor transformation. Brain Res Cogn Brain Res. 2005;22:373–383. doi: 10.1016/j.cogbrainres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci (Regul Ed) 2010;14:154–161. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking Motor Learning and Savings in Adaptation Paradigms: Model-Free Memory for Successful Actions Combines with Internal Models. Neuron. 2011;70:787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Evolution of motor memory during the seconds after observation of motor error. J Neurophysiol. 2007;97:3976–3985. doi: 10.1152/jn.01281.2006. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Goto R, Sugiura M, Ito M, Fukuda H. Activity in the parietal area during visuomotor learning with optical rotation. Neuroreport. 1997;8:3979–3983. doi: 10.1097/00001756-199712220-00026. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Sugiura M, Goto R, Ito M, Fukuda H. A PET study of visuomotor learning under optical rotation. Neuroimage. 2000;11:505–516. doi: 10.1006/nimg.2000.0554. [DOI] [PubMed] [Google Scholar]
- Isaias IU, Moisello C, Marotta G, Schiavella M, Canesi M, Perfetti B, Cavallari P, Pezzoli G, Ghilardi MF. Dopaminergic striatal innervation predicts interlimb transfer of a visuomotor skill. J Neurosci. 2011;31:14458–14462. doi: 10.1523/JNEUROSCI.3583-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. Long-term Recall explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol. 2008;100:2948–2955. doi: 10.1152/jn.90706.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Khan MA, Franks IM, Elliott D, Lawrence GP, Chua R, Bernier PM, Hansen S, Weeks DJ. Inferring online and offline processing of visual feedback in target-directed movements from kinematic data. Neurosci Biobehav Rev. 2006;30:1106–1121. doi: 10.1016/j.neubiorev.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 2005;25:473–478. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol. 2004;91:924–933. doi: 10.1152/jn.00675.2003. [DOI] [PubMed] [Google Scholar]
- Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH, Fischman AJ, Alpert NM. Robot-aided functional imaging: application to a motor learning study. Hum Brain Mapp. 1998;6:59–72. doi: 10.1002/(SICI)1097-0193(1998)6:1<59::AID-HBM5>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, van Donkelaar P. The human dorsal premotor cortex generates on-line error corrections during sensorimotor adaptation. J Neurosci. 2006;26:3330–3334. doi: 10.1523/JNEUROSCI.3898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luauté J, Schwartz S, Rossetti Y, Spiridon M, Rode G, Boisson D, Vuilleumier P. Dynamic changes in brain activity during prism adaptation. J Neurosci. 2009;29:169–178. doi: 10.1523/JNEUROSCI.3054-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, Ghilardi MF. Learning and consolidation of visuo-motor adaptation in Parkinson’s disease. Parkinsonism Relat Discord. 2009;15:6–11. doi: 10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick MJ. Effects of overlearning on the retention of a gross motor skill. Res Q. 1971;42(1):60–9. [PubMed] [Google Scholar]
- Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JE. Optimality in human motor performance: ideal control of rapid aimed movements. Psychol Rev. 1988;95:340–370. doi: 10.1037/0033-295x.95.3.340. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M, Wise SP. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. J Neurosci. 1991;11:1855–1872. doi: 10.1523/JNEUROSCI.11-06-01855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Left parietal regions are critical for adaptive visuomotor control. J Neurosci. 2011;31:6972–6981. doi: 10.1523/JNEUROSCI.6432-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res. 2001;140:66–76. doi: 10.1007/s002210100787. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Criscimagna-Hemminger SE, Shadmehr R. Contributions of the motor cortex to adaptive control of reaching depend on the perturbation schedule. Cereb Cortex. 2011;21:1475–1484. doi: 10.1093/cercor/bhq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Perfetti B, Moisello C, Landsness EC, Kvint S, Lanzafame S, Onofrj M, Di Rocco A, Tononi G, Ghilardi MF. Modulation of Gamma and Theta Spectral Amplitude and Phase Synchronization Is Associated with the Development of Visuo-Motor Learning. J Neurosci. 2011;31:14810–14819. doi: 10.1523/JNEUROSCI.1319-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamondon R, Alimi AM. Speed/accuracy trade-offs in target-directed movements. Behav Brain Sci. 1997;20:279–303. doi: 10.1017/s0140525x97001441. [DOI] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol. 2009;101:1961–1971. doi: 10.1152/jn.91069.2008. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Dimitrov B, Hallett M. Motor learning in patients with cerebellar dysfunction. Brain. 1990;113:103–120. doi: 10.1093/brain/113.1.103. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Knill DC. Humans use continuous visual feedback from the hand to control fast reaching movements. Exp Brain Res. 2003;152:341–352. doi: 10.1007/s00221-003-1525-2. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Knill DC. Visual feedback control of hand movements. J Neurosci. 2004;24:3223–3234. doi: 10.1523/JNEUROSCI.4319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cereb Cortex. 2006;16:1546–1555. doi: 10.1093/cercor/bhj092. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175:544–555. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Inhibitory control of competing motor memories. Exp Brain Res. 1999;126:235–251. doi: 10.1007/s002210050733. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Wise SP. The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning. MIT press; Cambridge, MA: 2005. [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Franks IM. The effects of changing movement velocity and complexity on response preparation: evidence from latency, kinematic, and EMG measures. Exp Brain Res. 1991;83:618–632. doi: 10.1007/BF00229840. [DOI] [PubMed] [Google Scholar]
- Werner S, Bock O, Timmann D. The effect of cerebellar cortical degeneration on adaptive plasticity and movement control. Exp Brain Res. 2009;193:189–196. doi: 10.1007/s00221-008-1607-2. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]


