Abstract
While previous research has suggested that anger and fear responses to stress are linked to distinct sympathetic nervous system (SNS) stress responses, little is known about how these emotions predict hypothalamus-pituitary-adrenal (HPA) axis reactivity. Further, earlier research primarily relied on retrospective self-report of emotion. The current study aimed at addressing both issues in male and female individuals by assessing the role of anger and fear in predicting heart rate and cortisol stress responses using both self-report and facial coding analysis to assess emotion responses.
We exposed 32 healthy students (18 female; 19.6+/−1.7 yrs.) to an acute psychosocial stress paradigm (TSST) and measured heart rate and salivary cortisol levels throughout the protocol. Anger and fear before and after stress exposure was assessed by self-report, and video recordings of the TSST were assessed by a certified facial coder to determine emotion expression (FACS).
Self-reported emotions and emotion expressions did not correlate (all p > .23). Increases in self-reported fear predicted blunted cortisol responses in men (β = 0.41, p = .04). Also for men, longer durations of anger expression predicted exaggerated cortisol responses (β = 0.67 p = .004), and more anger incidences predicted exaggerated cortisol and heart rate responses (β = 0.51, p = .033; β = 0.46, p = .066, resp.). Anger and fear did not predict SNS or HPA activity for females (all p > .23).
The current differential self-report and facial coding findings support the use of multiple modes of emotion assessment. Particularly, FACS but not self-report revealed a robust anger-stress association that could have important downstream health effects for men. For women, future research may clarify the role of other emotions, such as self-conscious expressions of shame, for physiological stress responses. A better understanding of the emotion-stress link may contribute to behavioral interventions targeting health-promoting ways of responding emotionally to stress.
Keywords: Stress, cortisol, heart rate, emotions, facial action coding system
Introduction
Stress may elicit a range of emotions, including feelings of anger and fear. Although recent studies have examined the situational and cognitive predictors and moderators of acute stress (Denson et al., 2009; Dickerson and Kemeny, 2004; Gaab et al., 2003), less is known about the role of emotion. Research is particularly lacking on how specific emotions, such as anger and fear, may predict Hypothalamic-Pituitary-Adrenal (HPA) axis activation as measured by cortisol responses to stress. Unraveling potentially specific associations between anger, fear, and cortisol stress responses could be helpful in differentiating harmful from more productive stress responses. Furthermore, it could lead to interventions that introduce better ways of coping with the emotions that stress may elicit.
Acute stress activates a coordinated set of physiological responses that prepares the body to deal with an immediate threat. A rapid “first wave” response dictated by the Sympathetic Nervous System (SNS) increases respiration and heart rate as oxygen and glucose speed to the skeletal muscles and heart (Sapolsky, 2000). A second response dictated by the Hypothalamic-Pituitary-Adrenal (HPA) axis stimulates the production and release of stress hormones including cortisol. In addition to prolonging the cardiovascular effects of the SNS, cortisol protects the body from the harmful effects of an overactive immune system by selectively suppressing those functions that are no longer needed once the stressor has ended (Besedovsky et al., 1985). Though adaptive when activated in the short term, repeated or chronic activation of the HPA axis is believed to cause wear and tear on the body, and has consistently been linked to negative health outcomes (McEwen, 1998; McEwen and Seeman, 1999; Tsigos and Chrousos, 2002).
According to appraisal theory, it is not the stressor itself, but rather what one thinks about the situation, that will determine the physiological effects that result from the experience (Lazarus and Folkman, 1984). These subjective appraisals of stressful situations are also thought to give rise to emotions. That is, if a person interprets a situation as beneficial, positive emotions result; if the situation is appraised as potentially harmful, negative emotions result (Lazarus, 1982; Smith and Lazarus, 1990). It is not yet clear, however, if and how positive or negative emotions are linked to acute biological stress responses, since prior studies failed to find such associations (Clark et al., 2001; Dickerson and Kemeny, 2004; Denson et al., 2009). Instead, some evidence suggests that within these broad valence categorizations for positive and negative emotions, individual emotions may be linked to acute stress responses. For example, a line of research has found links between self-conscious emotions such as shame and strength of cortisol stress responses (Dickerson et al., 2004; Dickerson et al., 2008).
Anger and fear, two other negative-valence emotions, have also been linked to stress responses, particularly with regards to the Sympathetic Nervous System. Both emotions have been found to be positively correlated with heart rate increases (Ekman et al., 1983; Ray et al., 2008). Compared with fear, anger seems to be more strongly associated with increased diastolic blood pressure, arterial pressure (Schwartz et al., 1981), and finger temperature (Ekman et al., 1983). Current literature also suggests possible gender differences in the emotion-SNS link. For example, in one particular study, young women showed stronger heart rate reactivity than young men in both anger- and fear-induction conditions (Labouvie-Vief et al., 2003). Taken as a whole, this research suggests that anger and fear may differentially predict specific SNS responses in the context of stress and, further, that these relationships may be moderated by gender.
With regards to the second wave of acute stress responses, studies linking emotion to HPA stress responses are few and often report conflicting results. For instance, one study found that self-reported anger after stress predicted stronger cortisol stress responses, while self-reported fear predicted decreases in cortisol levels (Moons et al., 2010). Yet, another recent study found that participants who felt more fear during a stressor actually showed higher cortisol increases (Lerner et al., 2007). The methodological differences between the studies could help explain the conflicting findings; for example, the study by Moons et al. (2010) utilized a psychosocial stress paradigm (Trier Social Stress Test: TSST) and emotions were self-reported after completion of the task, while the latter used mathematical stress tasks and facial coding analyses of emotion expressions shown throughout the stressor. It may be that the emotion-cortisol link differs based on the type of stress test given, as well as the method of emotion assessment (self-report vs. facial coding), but this has not yet been investigated. Notably, neither of the above studies reported gender differences in the emotion-HPA link. Clearly, more research is needed to tease apart the effects that anger and fear may have on the HPA axis for both men and women.
To our knowledge the study by Lerner and colleagues (2007) is the first to use facial coding to assess emotion in the context of stress. This is a notable development, since most studies rely on self-report to assess emotions. This method is not without its weaknesses; namely, due to the nature of many laboratory stress tests, questionnaires are only given before and after the stressor, and so may not capture the more immediate emotional reactions that occur during the actual stress test. According to Scherer's components processing model of emotion, emotion consists of a combination of five different elements: cognitive appraisal, bodily symptoms, action tendencies, feeling, and expression (Scherer, 1987, 2005). While self-report may address subjective experience through feelings and cognitive appraisals of a situation, facial coding may shed light on another component- that of expression. The ‘online’ way of measuring emotion versus the ‘retrospective and processed’ way of self-report could account for some of the differences currently found in the literature linking emotions and cortisol responses. Thus, for the current study in addition to self-report, we added facial coding to assess emotion expressions throughout stress exposure. The Facial Action Coding System (Ekman and Friesen, 1978, Ekman et al., 2002) is one widely-used method to categorize expression of emotion through movement of individual facial muscles. This analysis not only allows for assessment of emotion expressions as they occur, but also can detect emotions that the participant may not self-report accurately, either purposefully or unknowingly (Cronbach, 1970; Derakshan and Eysenck, 1999; Myers and Brewin, 1995; Paulhus and John, 1998). Lastly, this method allows for close examination of emotions: which type of emotion is shown, how often it is presented, how long it lasts, and how intense each occurrence may be.
In summary, stress research focused on the role of emotion on SNS reactivity often relies solely on self-report, and studies assessing HPA reactivity and emotion expressions are lacking. The current study aimed to combine self-report and facial coding to investigate whether a specific emotion expression response to an acute psychosocial stressor would differentially predict heart rate and cortisol stress responses. In parallel to earlier studies, we focused our analyses on anger and fear responses. Based on the studies described above, we predicted that 1) both self-reported anger and fear responses to stress, as well as facial expressions of these emotions, would be positively associated with heart rate increases. We further hypothesized that 2) retrospective self-report and facial expressions of anger, but not fear, would be positively associated with cortisol stress responses. Additionally, although previous studies of emotion and HPA activity do not find or report moderating effects of gender, some emotion-SNS research does suggest possible gender differences in the emotion-stress link. With this in mind, we also set out to explore gender-dependent effects of emotion stress response on heart rate and cortisol stress responses.
Methods
Participants
Forty-six participants were recruited from a pool of Brandeis undergraduate psychology students and from the general student population using fliers posted on the Brandeis campus. We excluded individuals who self-reported any cardiovascular or other chronic diseases, as well as anyone taking medication that could affect measurement of stress hormones (such as beta-blockers, statins, or oral contraceptives). Only native English speaking participants were included. From the initial 46 participants, only those participants with complete data were included in hypothesis testing. Participants were excluded due to incomplete cortisol data (n = 2), incomplete heart rate data (n = 3), incomplete questionnaire data (n = 1), and incomplete TSST video recordings, or a video that was not of high enough quality to allow for facial coding analysis (n = 7). In addition, one participant was more than 4 standard deviations above the mean in anger count and anger duration and was excluded from analysis, resulting in a final sample of 32 participants (18 female; average age: 19.6 years (range: 18-25, SD=1.7). Participants received reimbursement of $15 or 1 study credit. The study was approved by the Brandeis Institutional Review Board.
Procedure
Participants were asked to come to the laboratory on a weekday afternoon between the hours of 14:00 and 17:00. After being seated in a comfortable testing room, the study protocol was explained and the participant asked for informed consent. Once consent was given, the participant was fitted with a heart rate monitor consisting of a transmitter belt worn around the chest and a wrist watch-like receiver on the non-dominant hand. During the following 30 minutes participants were asked to answer questionnaires assessing their health and personality and otherwise were allowed to rest comfortably. Subsequently, a first saliva sample was obtained to assess baseline cortisol levels. After participants self-reported their current mood, they were led to another room where the Trier Social Stress Test (TSST) was administered according to established protocols (see below for more details). Participants were videotaped throughout the TSST. After the TSST, a second saliva sample was collected and post-stress mood was assessed. Participants spent the remainder of the experiment seated comfortably in their testing room; during this time, additional saliva samples were collected at 1, 10, 30, and 45 minutes post-TSST to capture cortisol response and recovery. At +30 minutes, participants were debriefed by the TSST panel, and after +45 minutes reimbursed for their time. Heart rate was recorded throughout the entire protocol.
Measures and Equipment
Trier Social Stress Test (TSST)
The current study utilized the Trier Social Stress Test (TSST), which combines an oral speech task and an oral quantitative task and has been shown to reliably elicit both a cardiovascular and cortisol response (Kirschbaum et al., 1993). In more detail, a panel of observers, consisting of two confederates dressed in white laboratory coats, is seated behind a large desk and a video camera. The participant is asked to imagine that (s)he has applied for a “dream job” and been invited to a job interview. In this interview, (s)he has to talk for five minutes about the characteristics of his/her personality that make him/her particularly attractive to the potential employer. The participant is then given five minutes to prepare this speech. During the actual speech, the panel of observers takes notes and assumes neutral behavior, e.g. does not respond to attempts of the participants to establish a personal relationship. If the participant stops talking, the panel waits 10 seconds and then asks the participant to continue. After five minutes of he verbal task, the participant is asked to count backwards from 2043 in increments of 17 as fast and precisely as possible. Every mistake leads to a panel member asking the participant to start over at the initial number (2043).
Facial Coding
Videotapes recorded during the TSST were used for facial coding analysis. Participants' facial behavior was coded using the Facial Action Coding System, an anatomically-based coding system (Ekman and Friesen, 1978, Ekman et al., 2002). In more detail, a specific application of this system (EMFACS-8) was utilized, which allows for expedited coding through inclusion of meaningful combinations of action units that have been shown to be associated with emotion experience (Ekman et al., 1994). For example, an AU 4 (lowering of the brow) would be meaningless and uncodeable alone, but when combined with an AU 7 (closing of the eye aperture) becomes a codeable expression for “anger.” The reliability and validity of the EMFACS system have been demonstrated in prior studies (e.g., Keltner and Bonanno, 1997). Although the EMFACS criteria were used to code expression of any emotion, only those of anger and fear were included in this study. The following AUs and combinations of AUs were used for the anger expression: 4+5, 4+7, 5+7, 23, 29, 17+23, and 17+24. The following AUs and combinations of AUs were used for fear expression: 1+2+4, 20. Coders observed the videos at normal speed, noting at about which part an expression began. From that point on, expressions were examined frame-by-frame with each frame lasting 1/30 second. Prior emotion research has utilized FACS data as a composite measure (for example, Lerner et al., 2007). Since it is still unclear whether incidence, frequency, or intensity of emotion expression may be most relevant in predicting physiological stress responses, we examined these components separately. For each emotion we assessed three separate measures: count (how many distinct times the expression occurred); total duration (how many frames the expression occurred in total over the entire stress test); and average duration (computed by the total duration divided by the count to determine the average number of frames that the expression lasted each time it occurred). In addition, emotion expression intensity was scored on a 5-point scale (1 representing minimal expression, 5 extreme). Maximum intensity denoted the strongest expression of either anger or fear throughout the stressor, and average intensity the average intensity level across all instances of that emotion. In line with procedures of similar studies, a second coder evaluated 25% of the tapes and inter-rater reliability was kappa = .83.
Questionnaires
The Positive and Negative Affect Schedule (PANAS) was used to measure the participants' emotional state before and after TSST (Watson et al., 1988). It consists of 10 items assessing positive affect (interested, excited, strong, enthusiastic, proud, alert, inspired, determined, attentive, and active) and 10 items assessing negative affect (distressed, upset, guilty, scared, hostile, irritable, ashamed, nervous, jittery, and afraid). Participants are asked to rate items on a scale from 1 to 5, based on the strength of emotion with 1 = "very slightly or not at all" and 5 = “extremely”.
The PANAS was administered five minutes before the TSST as well as immediately afterwards. For the latter, participants were instructed to answer the items focusing on how they felt during the stressor. To address the research questions of the current study, the answers to items ‘hostile’ and ‘irritable’ were averaged as a measure of self-reported levels of anger, and the responses to the items ‘scared’ and ‘afraid’ were averaged as a measure of self-reported levels of fear. This method of assessment is in line with suggested procedures by Watson, Clark & Tellegen (1988).
Heart Rate
A Polar RS800 monitor (Polar Electro, Finland) allowing beat-to-beat recording was used, and heart rate was assessed in Kubios (Tarvainen et al., 2008). Data noise due to, for example, WiFi signals, was reduced and heart rate measures were averaged across the 20 minutes prior to the TSST (baseline) and across one minute intervals throughout the TSST. Maximum heart rate increase in beats per minute (bpm) was then computed using the difference between baseline and maximum heart rate response during the TSST whenever it occurred.
Cortisol
Cortisol was measured in saliva samples collected using the Salivette collection device (Sarstedt, Newton, NC). More specifically, participants were asked to place a cotton roll into their mouth for about one minute, move it from side to side, and then put the roll back into the Salivette. Saliva samples were stored at −20C until completion of the study. Salivettes were then centrifuged and concentrations of salivary free cortisol were measured using a commercially available chemiluminescence-immuno-assay (IBL, Toronto, Canada). Intra- and inter-assay precision expressed as percent coefficient of variation were below 7%. Cortisol was measured in nanomoles per liter (nmol/l) and maximum increases were computed for each individual by subtracting maximum cortisol levels observed after TSST from pre-TSST levels (saliva sample 1).
Statistical Analysis
Preliminary Analyses
Repeated-measures ANOVAs were computed to assess changes in heart rate and cortisol stress responses over time, as well as changes in self-report from pre-TSST to post-TSST. Independent samples T-tests were computed to compare differences between male and female participants with regard to heart rate response, cortisol response, self-reported emotions, and anger and fear expressions during the TSST (females coded as 0, males coded as 1). Pearson correlations determined associations between self-report emotion change scores and emotion expression.
Testing hypotheses
To test hypothesis 1, regression analyses were performed to determine if self-report of anger or fear, or expressions of these emotions, predicted heart rate increases. Maximum heart rate increase was entered as the outcome variable. Most participants self-reported feeling both anger and fear in response to the TSST, and facial coding analysis suggested that most participants expressed both anger and fear during the TSST. We therefore included both emotions in our models at the same time to determine the effect of one emotion over and above the effect of the other. A self-report change score was computed as post-TSST levels of anger or fear minus pre-TSST (baseline) measures. To assess self-reported anger effects on heart rate, self-report of fear change as well as BMI were entered in step 1, followed by gender in step two, anger change in step 3, and gender-by-anger change interaction term in step 4. Fear change was assessed similarly, controlling for anger change in step 1.
To assess effects of emotion expression responses of anger on heart rate, fear expression and BMI were entered in step 1, and gender in step 2. Anger expression was entered in step 3, and gender-by-anger expression interaction in step 4. To assess effects of fear expressions, similar regressions were run controlling for anger emotion in step 1. Separate regressions were computed for each FACS measure, including count, total duration, average duration, maximum intensity, and average intensity.
To test hypothesis 2, regressions similar to those described above for hypothesis 1 were performed for self-reported emotion response measures as well as emotion expression measures; however, for hypothesis 2, maximum cortisol increase was entered as the dependent / outcome variable.
In the case of interaction effects, we probed these effects using simple slopes analysis according to Holmbeck (2002). Effect sizes were computed for all analyses, and partial η2 for ANOVAs, Cohen's d for independent t-tests, and in the case of regressions, R2 changes for the final model are given. For all analyses, p-values of p < .05 were considered significant.
Results
Preliminary Analyses
Physiological stress responses
Heart rate
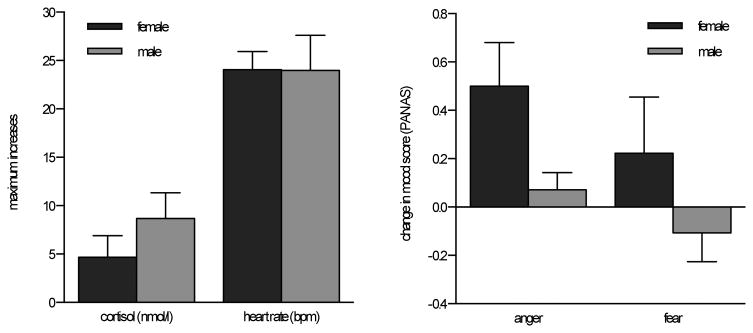
Analysis of heart rate data suggested that the TSST was successful in eliciting a significant sympathetic nervous system response (F(14, 420) = 13.60, p < .001, partial η2 = .31) in both female and male participants (gender main effect: F(1, 30) = .1.17, p = .29, partial η2 = .04; gender-by-time: F(14, 420) = 0.72, p = .76, partial η2 = .02). Women showed average peak heart rate in minute 6 of the TSST, and men an average peak in minute 7 of the TSST. Examining maximum heart rate increases confirmed comparable increases between male and female participants (t(30) = 0.01, p = .99, d = .004; see Fig 1).
Figure 1.
Gender differences in change scores (mean and standard errors) for maximum physiological responses (left); gender differences in change scores on self-report emotion measures (right).
Cortisol
Analysis of participants with full cortisol data (N = 29) revealed a significant change in cortisol levels over the course of the experiment (F(5, 135) = 3.37, p= .042, partial η2 = .11). Though there was no main effect of gender (F(1, 27)= 0.36, p= .55, partial η2 = .01), we found a trend for a gender-by-time interaction effect (F(5, 135)= 2.65, p = .081, partial η2 = .09). Men tended to show peak cortisol levels earlier than females, with men peaking on average at time 3 (10 min post-TSST), and women at time 4 (30 min post-TSST). When examining individual maximum cortisol increases, we found no significant gender differences (t(30) = −1.17, p = .25, d = .41; see Fig.1).
A Pearson correlation revealed that maximum heart rate increases and maximum cortisol increases were not associated (r(32) = .24, p = .18; controlling for gender: r(29) = .25, p = .18).
Emotion stress responses
Self-reported emotions
Descriptives for self-report data are shown in Table 1. For self-reported anger, repeated measures ANOVA revealed a main effect of time (F(1, 30) = 7.06, p = .013, partial η2 = .19) as well as a main effect of gender, with women self-reporting more anger than men (F(1, 30) = 4.18, p = .05, partial η2= .12). We also found a trend for a gender-by-time interaction effect (F(1, 30) = 3.97, p = .055, partial η2 = .12) in that females tended to report greater increases in anger than men. For self-reported fear, no significant main effect of time (F(1, 30) = 0.16, p = .69, partial η2 = .005, nor a gender-by-time interaction effect (F(1, 30) = 1.34, p = .26, partial η2 = .04) was observed. We did find a significant main effect of gender for females to report more fear than men (F(1, 30) = 4.20, p = .049, partial η2 = .12).
Table 1.
Descriptives of emotion and stress measures for men and women.
| Men | Women | ||
|---|---|---|---|
| M (SD) | M (SD) | t value | |
| Physiological Data | |||
|
|
|||
| Max Heart Rate Increase (bpm) | 23.99 (13.64) | 24.04 (8.08) | 0.01 |
| Max Cortisol Increase (nmol/L) | 8.68 (9.90) | 4.66 (9.51) | -1.17 |
| Body Mass Index (BMI) | 22.58 (2.41) | 22.57 (3.56) | -0.01 |
|
|
|||
| Self-Reported Emotions | |||
|
|
|||
| Pre-TSST Anger | 1.07 (0.18) | 1.14 (0.33) | 0.68 |
| Post-TSST Anger | 1.14 (0.23) | 1.64 (0.82) | 2.44* |
| Pre-TSST Fear | 1.29 (0.51) | 1.50 (0.69) | 0.98 |
| Post-TSST Fear | 1.18 (0.32) | 1.72 (0.88) | 2.43* |
|
|
|||
| Emotion Expression (FACS) | |||
|
|
|||
| Anger Count | 4.36 (3.20) | 7.94 (6.08) | 2.00- |
| Anger Total Duration | 180.57 (162.31) | 277.17 (229.77) | 1.33 |
| Anger Average Duration | 33.58 (30.20) | 34.13 (17.47) | 0.07 |
| Anger Maximum Intensity | 3.36 (1.91) | 3.67 (1.28) | 0.55 |
| Anger Average Intensity | 2.78 (1.57) | 2.89 (0.97) | 0.25 |
| Fear Count | 2.36 (3.32) | 2.72 (5.30) | 0.23 |
| Fear Total Duration | 104.93 (133.36) | 122.89 (253.48) | 0.24 |
| Fear Average Duration | 35.20 (44.36) | 21.73 (24.36) | -1.10 |
| Fear Maximum Intensity | 2.14 (1.99) | 2.06 (1.98) | -0.12 |
| Fear Average Intensity | 1.83 (1.65) | 1.93 (1.86) | 0.15 |
Note: Significant effects are marked with an asterisk and trends with a dash.
Emotion expression
Of 32 participants, 12 (8 females) showed anger but no fear, 3 (0 females) showed fear but no anger, and 17 (10 females) showed a both. Independent t-tests revealed a trend for females to show a higher anger count than males (t(30) = 2.00, p = .055, d = .74). No significant gender differences were observed in any other measure of anger or fear expression (all p > .19, all d < .49).
No measure of anger expression predicted change in self-reported anger in response to the TSST (all p > .23, all r < .22; controlling for gender, all p > .55, all r < .12). The same was true for fear expression predicting change in self-reported fear (all p > .45, all r < .14; controlling for gender, all p > .47, all r < .14).
Do emotions differentially predict heart rate stress responses?
Self-reported emotions and heart rate stress responses
Regression analysis revealed that stress-induced changes in self-reported anger did not appear to be associated with increases in heart rate (all p > .63, all R2 < .02). Similarly, changes in self-reported fear were not associated with maximum heart rate increases (all p > .82, all R2 < .003; see Table 2).
Table 2.
Regression analyses predicting heart rate and cortisol stress responses. Shown are betas and p-values of the final models for self-reported emotions, anger (A: left half of table) and fear (B: right half of table).
| A) | Heart Rate | Cortisol | B) | Heart Rate | Cortisol | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| beta | p | beta | p | beta | p | beta | p | ||
|
|
|
||||||||
| Fear Change | -0.02 | .92 | -0.36 | .09- | Anger Change | 0.04 | .87 | 0.21 | .28 |
| BMI | 0.06 | .77 | -0.14 | .46 | BMI | 0.06 | .78 | -0.15 | .40 |
|
|
|
||||||||
| Gender | -0.05 | .85 | 0.31 | .18 | Gender | 0.00 | .99 | 0.11 | .55 |
|
|
|
||||||||
| Anger Change | 0.09 | .70 | 0.27 | .23 | Fear Change | 0.03 | .92 | -0.21 | .31 |
|
|
|
||||||||
| Gender-by-Anger Change | -0.12 | .63 | 0.14 | .54 | Gender-by-Fear Change | -0.05 | .82 | -0.41 | .04* |
|
|
|
||||||||
Note: Significant effects are marked with an asterisk and trends with a dash.
Emotion expressions and heart rate stress responses
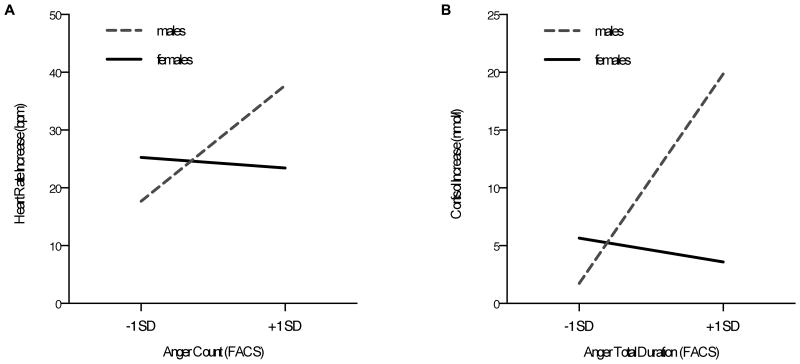
Examining facial expressions of anger or fear shown during stress exposure, we found a trend for a gender-by-expression interaction for number of anger incidences (anger count; β = 0.46, p = .066, R2 = .12; see Table 3, Fig 2A). More specifically, for women, anger expression was not associated with heart rate stress responses, while for men showing more anger during the TSST was marginally associated with a greater heart rate increase during the TSST (simple slopes analysis: females: B = −0.19, SEB = 0.46, β = −0.10,p= .67; males: B= 1.90, SEB = 0.96, β = 0.96, p = .057).
Table 3.
Regression analyses for anger predicting heart rate stress responses (top part of table) and cortisol stress responses (lower part). Shown are statistics of the final models for each of the assessed emotion expression indices.
| Count | Total Duration | Avg. Duration | Max Intensity | Avg. Intensity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| beta | p | beta | p | beta | p | beta | p | beta | p | |
| Heart rate increases: | ||||||||||
|
| ||||||||||
| Fear | -0.16 | .43 | -0.14 | .50 | 0.17 | .41 | -0.18 | .34 | -0.16 | .42 |
| BMI | -0.14 | .95 | 0.04 | .84 | 0.12 | .54 | -0.02 | .94 | 0.004 | .98 |
|
| ||||||||||
| Gender | 0.16 | .44 | 0.01 | .99 | -0.04 | .85 | 0.02 | .92 | -0.001 | .99 |
|
| ||||||||||
| Anger | -0.10 | .68 | -0.10 | .68 | -0.20 | .59 | -0.08 | .80 | -0.07 | .84 |
|
| ||||||||||
| Gender-by-Anger | 0.46 | .066- | 0.14 | .59 | 0.01 | .98 | 0.36 | .28 | 0.28 | .41 |
|
| ||||||||||
| Cortisol increases: | ||||||||||
|
| ||||||||||
| Fear | -05 | .80 | -0.03 | .86 | -0.01 | .96 | -0.30 | .09- | -0.30 | .10 |
| BMI | -0.21 | .30 | -0.29 | .13 | -0.16 | .39 | -0.16 | .36 | -0.15 | .40 |
|
| ||||||||||
| Gender | 0.38 | .06- | 0.33 | .06- | 0.21 | .23 | 0.25 | .16 | 0.21 | .23 |
|
| ||||||||||
| Anger | -0.15 | .49 | -0.22 | .29 | -0.15 | .65 | 0.04 | .88 | 0.06 | .84 |
|
| ||||||||||
| Gender-by-Anger | 0.52 | .033* | 0.67 | .004* | 0.57 | .081- | 0.38 | .20 | 0.30 | .40 |
Note: Significant effects are marked with an asterisk and trends with a dash.
Figure 2.
Anger expressions shown throughout the TSST predicted heart rate stress responses (A) and cortisol stress responses (B) for male participants only.
Number of incidences of fear (fear count) was not associated with heart rate stress responses for either gender (β = −0.17, p = .42, R2 = .002, see Table 4), nor were any of the other emotion expression indices (anger: all p > .27, all R2 < .13; fear: all p > .37, all R2 < .06).
Table 4.
Regression analyses for fear predicting heart rate stress responses (top part of table) and cortisol stress responses (lower part). Shown are statistics of the final models for each of the assessed emotion expression indices.
| Count | Total Duration | Avg. Duration | Max Intensity | Avg. Intensity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| beta | p | beta | p | beta | p | beta | p | beta | p | |
| Heart rate increases: | ||||||||||
|
| ||||||||||
| Anger | 0.13 | .56 | -.03 | .88 | -0.14 | .47 | 0.18 | .36 | 0.14 | .50 |
| BMI | 0.13 | .54 | 0.09 | .65 | 0.12 | .55 | 0.04 | .84 | 0.03 | .87 |
|
| ||||||||||
| Gender | 0.03 | .88 | -0.01 | .95 | -0.02 | .91 | 0.02 | .92 | 0.001 | .99 |
|
| ||||||||||
| Fear | -0.14 | .57 | -0.17 | .48 | -0.22 | .56 | -0.22 | .39 | -0.19 | .46 |
|
| ||||||||||
| Gender-by-Fear | -0.06 | .80 | 0.06 | .79 | 0.45 | .22 | 0.11 | .67 | 0.09 | .71 |
|
| ||||||||||
| Cortisol increases: | ||||||||||
|
| ||||||||||
| Anger | 0.11 | .60 | 0.17 | .38 | 0.36 | .07- | 0.36 | .049* | 0.33 | .08- |
| BMI | -0.06 | .78 | -0.09 | .64 | -0.18 | .34 | -0.11 | .54 | -0.13 | .47 |
|
| ||||||||||
| Gender | 0.24 | .25 | 0.24 | .22 | 0.24 | .21 | 0.25 | .16 | 0.21 | .23 |
|
| ||||||||||
| Fear | 0.04 | .86 | 0.09 | .70 | -0.25 | .48 | -0.15 | .53 | -0.17 | .46 |
|
| ||||||||||
| Gender-by-Fear | -0.20 | .37 | -0.23 | .29 | 0.21 | .56 | -0.18 | .44 | -0.17 | .45 |
Note: Significant effects are marked with an asterisk and trends with a dash.
Do emotions differentially predict cortisol stress responses?
Self-reported emotions and cortisol stress responses
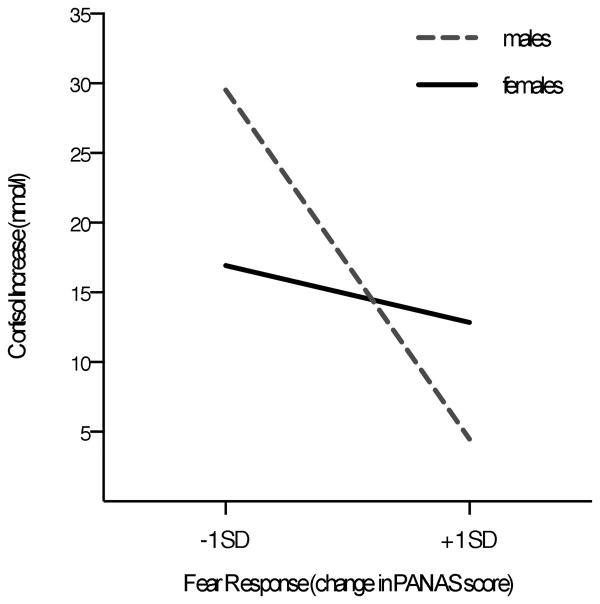
Next, we assessed the associations between self-reported emotion responses to stress and cortisol stress responses. These analyses revealed a gender-by-fear interaction effect (β = 0.41, p = .04, R2 = .12; see Fig 3), such that change in self-reported fear and cortisol responses were not associated in females (B = -2.54, SEB = 2.43, β = -.21, p = .31), while for men, increases in fear predicted attenuated cortisol stress responses (B = -15.64, SEB = 5.51, β = -1.29, p = .009). Anger change scores were not associated with cortisol stress responses (β = 0.27, p = .23, R2 = .01).
Figure 3.
Change in self-reported fear predicted cortisol decreases for male participants only.
Emotion expressions, gender, and cortisol stress responses
Another set of regressions was performed to examine whether emotion expressions shown during stress exposure would predict cortisol stress responses (anger: see Table 3; fear: see Table 4). No measure of fear expression was associated with cortisol stress responses (all p > .28, all R2 < .05). We found a significant gender-by-anger interaction for anger count (β = 0.51, p = .033, R2 = .15) and total duration (β = 0.67 p = .004, R2 = .26, see Fig 2B). This same relationship appeared as a trend for anger average duration (β = 0.57, p = .08, R2 = .10). Simple slopes analysis revealed that, for females, anger count was not associated with cortisol increases (B = -.28, SEB = 0.41, β = -0.15, p = .49), while for males, more anger predicted stronger cortisol stress responses (B = 1.90, SEB = 0.85, β = 1.03, p = .034). The same pattern held for anger total duration (women: B = -0.01, SEB = 0.01, β = -0.22, p = .29; men: B = 0.05, SEB = 0.02, β = 1.06, p = .004) as well as anger average duration (women: B = -0.06, SEB = 0.13, β = -0.15, p = .65; men: B = 0.22, SEB = 0.09, β = 0.55, p = .02). Anger average intensity was not associated with cortisol responses (β = 0.06, p = .84, R2 = .03), nor was anger maximum intensity (β = 0.04, p = .88, R2 = .05).
Exploratory/Secondary Analyses
Closer inspection of self-report data revealed little variation in both pre-and post-TSST anger and fear, as well as a negative skew. In a follow-up analysis we therefore considered self-report of emotion as a categorical variable rather than continuous variable. Change in fear (post-TSST minus pre-TSST baseline) was categorized as a decrease in fear (N = 7), no change (N = 19), or increase in fear (N = 6); anger was categorized as a decrease/no change (N = 22), or increase (N = 10). We ran an ANOVA with maximum heart rate response as the dependent variable while controlling for BMI. Controlling for anger, we found no significant effect of fear for either gender; likewise, controlling for fear, there was no significant effect of anger on heart rate stress responses (all p > .94, all partial η2 < .07). A similar ANOVA was computed using maximum cortisol increase as the dependent variable. Anger did not predict cortisol increases (F(1, 26) p = .32, partial η2 = .82). Similarly to our regression analyses, we did find that fear predicted cortisol increase in a gender-dependent manner, such that for men, increases in fear predicted attenuated cortisol stress responses (gender-by-fear interaction: F(1, 24) = 3.54, p = .045, partial η2 = .23). These analyses thus confirm the findings of the regression analyses reported earlier.
We took a similar approach with regards to facial coding variables, categorizing anger counts and durations using a median split into high (N = 16) and low (N = 16) occurrence and duration of anger expression responses, and fear count into no occurrence (N = 14) versus occurrence (N = 18), and no duration (N=14) versus any duration (N=18). Similar to the regression analyses findings, fear expression responses did not predict heart rate increases (all p > .45, all partial η2 < .79), while anger expressions predicted heart rate in a gender-dependent manner, such that men with more incidences of anger also had higher heart rate increases (gender-by-anger occurrence interaction: (F(1, 26) = 6.84, p = .015, partial η2 = .21). Anger duration did not predict heart rate increases (p = .64, partial η2 = .53). This analysis also confirms the findings from our original regressions.
With regard to cortisol stress responses, occurrence of fear did not predict strength of cortisol stress responses (F(1, 26) = 2.78, p = .41, partial η2 = .80) nor did duration (F(1, 26) = 3.36, p = .34, partial η2= .79). Anger total duration predicted cortisol responses in a gender-dependent manner, such that men who showed longer anger durations showed exaggerated cortisol stress responses (gender-by-anger interaction: F(1, 26) = 6.25, p = .019, η2 = .19). Anger count was not associated with cortisol responses (F(1, 26) = 0.50, p = .63, partial η2= .39).
Discussion
Prior research has examined links between self-reported emotions and stress, particularly with regards to sympathetic nervous system markers. We aimed at extending this research to HPA activity as measured by strength of cortisol stress responses. Further, in addition to self-report, the current study included facial coding for a more comprehensive assessment of emotion stress responses. Self-report of emotion and facial expression were not themselves related, and we found differential links for each. For males, emotion expression of anger was associated with stronger heart rate and cortisol stress responses. Also for men, change in self-reported fear was linked to blunted cortisol stress responses. For women, despite reporting more fear and anger than men, as well as showing similar amounts of fear and anger expression, neither emotion assessment method was associated with physiological measures of acute stress.
To date, the relationship between emotions elicited by acute psychosocial stress and the accompanying physiological stress response is poorly understood, in part because existing research typically relies on retrospective self-report to assess emotion responses. The current study is the first to combine self-report of emotions with facial coding of emotion expressions, an approach which allows us to examine emotions retrospectively as well as while emotion expressions occur. We were among others interested in how strongly the two measures would be related; however, we did not find significant relationships between self-reported anger and fear and emotion expressions shown during stress exposure. This finding is in line with earlier research showing that although participants may believe they are reporting their emotions very accurately, they are not very good judges of their own emotional experience (Cronbach, 1970; Derakshan and Eysenck, 1999). Because the questionnaires are retrospective, participants may also not fully remember negative emotions felt in a moment of stress, particularly if the participant has a repressive coping style (Myers and Brewin, 1995). Apart from these unconscious factors, participants may also deliberately underreport their emotions, especially negative emotions like anger and fear, in an attempt to appear more socially desirable (Paulhus and John, 1998). Lastly, facial coding may simply capture a different component of emotion – that of less processed emotion experience in the moment of stress - while retrospective self-report may reflect the perception of emotion after cognitive processing and self-reflection have occurred (Crockett et al., 1987). As such, our findings support the notion of considering emotions ‘on-line’ in the moment of stress in parallel to retrospective emotion assessment in the form of self-report questionnaires.
When examining the relationship between emotion stress responses and heart rate stress responses, we did not find that self-reported anger or fear predicted heart rate increases. Links between self-reported anger and heart rate increases have previously been reported in the literature, although these studies prompted participants to perform facial movements associated with emotions (Ekman et al., 1983), or to imagine themselves in anger- or fear-inducing situations (Labouvie-Vief et al., 2003; Ray et al., 2008). These studies find significant increases in self-reported anger and fear, likely because the tasks they employ are designed to induce those specific emotions in participants. The TSST itself is not designed to elicit any emotion specifically, but rather assumes the participants will respond with the emotions they typically feel in stressful contexts. Many of our participants did not report changes in anger or fear due to the TSST, and stressors which are not considered emotionally significant are less likely to elicit cortisol stress responses (Nejtek, 2002). It may also be that in a novel, social-evaluative situation such as the TSST, participants are more likely to feel self-conscious emotions (such as shame) than anger or fear. Links between anger and fear and SNS measures may thus be more easily seen using tasks which elicit strong feelings of anger or fear, such as emotion induction techniques. It should also be noted that our study utilized the PANAS, a questionnaire with which some prior studies have found a lack of variability (for example, Kiecolt-Glaser et al., 2010). Further, our analysis utilized only four items from this multi-item questionnaire (two for anger and two for fear), which could limit our statistical power and mask potential associations. Future studies with more detailed self-report assessment measures may therefore reveal links between anger and fear and SNS measures.
With regards to facial coding and heart rate, we found that for men who showed more incidences of anger during stress exposure tended to have higher heart rate increases, a link between emotions and the SNS that was not captured by our self-report data. This marginally-significant finding with a modest effect size should be interpreted carefully, but suggests that it was not as important for how long a participant was angry, but rather how many times he became angry. Since anger is thought to activate the ‘fight or flight’ response, displaying anger repeatedly may result in repeated and exaggerated heart rate responses for men.
We also found that anger was not associated with heart rate increases for female participants. At least one previous study has found that anger and fear conditions predicted exaggerated heart rate responses in females when compared to males (Labouvie-Vief et al., 2003). The authors suggest that females in the study may be inhibiting negative emotions, leading to exaggerated cardiovascular responses. Participants in this particular study were prompted to recall autobiographical memories pertaining to specific emotions, and therefore would be made aware of (and perhaps be more likely to suppress) negative emotions including anger and fear. In the case of the TSST, which was utilized in the current study, no specific emotion is elicited. The participant may therefore experience a wide range of emotions and may be less likely to suppress specific emotions. Indeed, there was no evidence in our study that women were suppressing negative emotions more than men, as women tended to self-report more anger than males and showed comparable levels of anger expressions as measured through FACS. Additionally, our finding that males and females did not differ with regards to strength of heart rate increases mirror other studies which utilize the TSST (Kelley et al., 2008). Overall, these findings suggest that responding to psychosocial stress with more incidences of anger, independent of their length or intensity, may have negative health consequences for men due to stronger heart rate surges.
Interestingly, we did not find any associations between heart rate stress responses and fear responses, neither for self-reported fear nor for fear expressions. Though some emotion induction research has suggested that anger and fear predict similar SNS profiles, other studies which ask participants to evoke specific emotions have found weaker associations between fear and cardiovascular measures (Prkachin et al., 1999; Roberts and Weerts, 1982; Schwartz et al. 1981). Our results mirror this finding and extend it to emotion expression as measured through FACS. The differential SNS links we find for anger and fear may reflect the different cardiovascular needs to “fight” versus “flee” in potentially threatening situations. It should be noted that fear was also not as predominant a response as anger, reducing the likelihood to find a strong effect of fear on heart rate stress responses. Further, the current study examined the most often used measure of SNS activity, i.e., heart rate, and links with emotions vary depending on which SNS measure is used. Nevertheless, our findings speak to anger playing the predominant role in driving heart rate stress responses, particularly for men.
When examining the same relationships for cortisol stress responses we found that, for men, increases in fear predicted attenuated cortisol stress responses. This finding replicates prior research in which self-reported fear predicted decreases in cortisol stress responses (Moons et al., 2010). While the study by Moons did not note any gender differences, we found that self-report of anger and fear were not predictive of cortisol stress responses in women. It is unclear if this reflects a true gender difference, since some studies do not find that stress-induced changes in anxiety are linked to cortisol stress responses in either gender (Cohen et al., 2000). It has been suggested that instead of ‘fight or flight’, women assume a stronger tendency to a ‘tend and befriend’ response geared towards attachment and maintaining social relationships (Taylor et al., 2000). Therefore, emotions associated with a loss of ego or a threatening of the social self, such as shame, embarrassment, or pride may be more predictive of a stress response in females (Dickerson and Kemeny, 2004). Links between shame and HPA activity are currently being studied, and could explain the gender-differential findings in the current study.
No associations were found between facial expressions of fear and cortisol stress responses. However, for men, showing more anger was associated with exaggerated cortisol stress responses, and this was true with regards to several anger measures. This finding mirrors similar studies which examine males exclusively, and find that participants who showed more outward anger showed the highest levels of HPA activation during a stressor (al'Absi et al., 2000), as well as a study in which high-hostility men who were harassed during a stressor showed the highest cortisol levels (Suarez et al., 1998). Our study extends our knowledge on the role of emotions for physiological stress responses by showing that the same associations are not present in females. As such, and similarly for heart rate responses discussed above, these associations emphasize the importance of understanding predictors of anger stress responses in men to determine promising gender-specific health interventions.
Besides determining predictors of physiological stress responses, another important question concerns potential gender-dependent mediators between anger and physiological stress responses, i.e., the question why anger expression drives a stress response (heart rate and cortisol) for males, but not for females. One explanation may be that females may be more emotionally expressive than men (Kring and Gordon, 1998) and that expressing rather than suppressing emotions may help in coping with stress. We considered this possibility by analyzing the presence of ‘masked anger’, or an anger expression occurring with the presence of a fake smile (AU12)1. We found no gender differences in masked anger counts or total durations, consistent with prior studies in which men and women report inhibiting anger at equal rates (Deffenbaucher et al., 1996). Further, no gender differences were found when adding masked expressions to pure anger expressions, nor when considering micro-expressions, defined in this case as expressions shorter than a second in duration. Together, these findings do not support the hypothesis that men mask emotions more than women, and so would not contribute to the observed exaggerated stress responses in the context of anger. We also directly considered emotion regulation strategy use (suppression and reappraisal), based on studies indicating that while suppression of emotions may limit facial expressions, it may not affect degree of physiological responses. Indeed, some research suggests that suppression may be linked to increased cortisol reactivity in response to a psychosocial stressor (Lam et al., 2009). Though only a subset of our sample completed an emotion regulation questionnaire (ERQ), males did not appear to differ from females in degree of emotion suppression or reappraisal.2 These are only two of a host of emotion regulation strategies, however, and more studies are needed to determine how these strategies may play into the emotion-stress link.
Taken as a whole, our study findings suggest the importance of assessing emotions via multiple means, as we found different emotion-stress links with self-report and facial coding. Our self-report results showed that men who reported more fear showed blunted cortisol responses, a finding seen in previous literature. No other associations were found with regards to self-report and physiological stress responses, perhaps unsurprising considering the methodological difficulties inherent in retrospective self-report. Our facial coding data revealed that, for men, showing more anger during the stressor was linked to exaggerated heart rate and cortisol stress responses. The addition of facial coding thus revealed a robust association that could have important downstream health effects for men who respond to stress with anger. Since we did not find significant emotion-stress links among females, more research is needed to determine which emotions (if any) may be most relevant with regards to SNS and HPA reactivity in the context of stress.
It should be noted that the current study has several limitations. Our relatively small sample was composed mostly of college students, making it difficult to generalize our findings to the general population. Additionally, our findings should be considered in light of largely marginal effect sizes, particularly with regards to self-report of emotion. Interestingly, though we lost considerable power in our exploratory analyses using emotion as a categorical variable, comparatively larger effect sizes suggest that this approach may be promising in future studies with larger sample sizes. It should also be noted that our measures of maximum physiological responses (peak-baseline) may obscure time-related differences in peak responding between men and women. For instance, we found that female participants showed peak cortisol levels later than male participants. It is unclear if these gender differences are task-related (i.e. females responding more strongly to the math task, the second part of the TSST) or somehow related to gender differences in emotional responding. Though this point is beyond the scope of the current paper, future research should determine whether these differences in time of peak physiological responding may be related to our experimental procedure and, further, whether they affect the emotion-stress link. Lastly, although we did exclude females using oral contraceptives, we were not able to control for stage of menstrual cycle.
In summary, the current study aimed at determining the relationship between emotion and HPA activation during stress utilizing both self-report questionnaires as well as facial coding to assess emotions. Interestingly, self-reported emotion responses and emotion expression responses did not themselves correlate, and each method of assessing emotions predicted different associations: self-report revealed attenuated cortisol responses in men who reported fear, while FACS analysis revealed that men high in anger showed the strongest stress responses, both with regards to heart rate increases and cortisol increases. Facial coding analysis thus revealed potentially health-relevant associations that were not seen with self-report alone. Since wear and tear on stress systems is associated with a wide range of negative health outcomes, determining emotions as predictors of biological stress responses is of great importance. A better understanding of the emotion-stress link may lead to the development of behavioral interventions targeting health-promoting ways of responding emotionally to stress. Our findings further emphasize the importance of considering for whom such interventions may be particularly successful. Specifically, for men, managing anger in times of stress may serve to curb stress responses and improve overall health. More research is needed to determine if different emotions drive a similar response in females.
Acknowledgments
The authors are extremely grateful to Thomas Arnott for his assistance in coding stress videos and thus allowing for inter-rater reliability assessment.
Funding body agreements and policies: This work was supported by the NIGMS “Brain-Body-Behavior Interface in Learning and Development Across the Lifespan” training grant T32GM084907 (SBL).
Footnotes
Gender differences in masked anger indices: all p > .12; gender differences in masked fear indices: all p > .06.
Gender differences in ERQ subscales: all p > 16.
Conflict of Interest: All authors declare they have no financial or other conflicts of interest.
Contributors: Authors J.M. Wolf and S.B. Lupis designed the study and wrote the protocol. Authors S.B. Lupis and M.H. Lerman collaborated for data collection. Authors J.M. Wolf and S.B. Lupis managed the literature searches and analyses, undertook the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al'Absi M, Bongard S, Lovallo WR. Adrenocorticotropin responses to interpersonal stress: effects of overt anger expression style and defensiveness. Int J Psychophysiol. 2000;37:257–265. doi: 10.1016/s0167-8760(00)00108-2. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Lotz W, Schwulera U. Lymphoid cells produce an immuno-regulatory glucocorticoid releasing factor (GIF) acting through the pituitary gland. Clin Exp Immunol. 1985;59:622–628. [PMC free article] [PubMed] [Google Scholar]
- Burns JW. Interactive effects of traits, states, and gender on cardiovascular reactivity during different situations. J Behav Med. 1995;18:279–303. doi: 10.1007/BF01857874. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. The influence of positive and negative mood states on risk taking, verbal fluency and salivary cortisol. J Affect Disorders. 2001;63:179–187. doi: 10.1016/s0165-0327(00)00183-x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann Behav Med. 2000;22:171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Crockett LJ, Schulenberg JE, Petersen AC. Congruence between objective and self-report data in a sample of young adolescents. J Adolescent Res. 1987;2:383–392. [Google Scholar]
- Cronbach LJ. Essentials of Psychological Testing. 3rd. Harper & Row; New York: 1970. [Google Scholar]
- Deffenbaucher JL, Oetting ER, Lynch RS, Morris CD. The expression of anger and its consequences. Behav Res Ther. 1996;34:575–590. doi: 10.1016/0005-7967(96)00018-6. [DOI] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, Normal M. Cognitive appraisals and emotions predict cortisol and immune responses: a meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Eysenck MW. Are repressors self-deceivers or other-deceivers. Cogn Emot. 1999;13:1–17. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: shame, physiology, and health. J Pers. 2004;72:1191–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychol. 2008;27:116–121. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Facial action coding system: a technique for the measurement of facial movement. Consulting Psychologists Press; Palo Alto: 1978. [Google Scholar]
- Ekman P, Friesen WV, Hager JC. Facial Action Coding System: The manual on CD ROM. A Human Face; Salt Lake City: 2002. [Google Scholar]
- Ekman P, Irwin W, Rosenberg E. EMFACS: Coders Instructions (EMFACS-8) San Francisco: University of California San Francisco Press; 1994. [Google Scholar]
- Ekman PD, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Ekman PD, Davidson RJ. The Nature of Emotion: Fundamental Questions. Oxford University Press; New York: 1994. [Google Scholar]
- Gaab J, Blättler N, Menzi T, Pabst B, Stoyer S, Ehlert U. Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrino. 2003;28:767–779. doi: 10.1016/s0306-4530(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psy. 2008;39:87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner D, Bonanno GA. A study of laughter and dissociation: distinct correlates of laughter and smiling during bereavement. J Pers Soc Psychol. 1997;73:687–702. doi: 10.1037//0022-3514.73.4.687. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:1–9. doi: 10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The Trier Social Stress Test: a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: expression, experience, and physiology. J Pers Soc Psychol. 1998;74:686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, Lumley MA, Jain E, Heinze H. Age and gender differences in cardiac reactivity and subjective emotion responses to emotional autobiographical memories. Emotion. 2003;3:115–126. doi: 10.1037/1528-3542.3.2.115. [DOI] [PubMed] [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology. 2009;34:1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Thoughts on the relation between emotion and cognition. Am Psychol. 1982;37:1019–1024. [Google Scholar]
- Lazarus RS. From psychological stress to the emotions: a history of changing outlooks. Annu Rev Psychol. 1993;44:1–21. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; New York: 1984. [Google Scholar]
- Lerner JS, Dahl RE, Hariri AR, Taylor SE. Facial expressions of emotion reveal neuroendocrine and cardiovascular stress responses. Biol Psychiat. 2007;61:253–260. doi: 10.1016/j.biopsych.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Autonomic nervous system differences among emotions. Psychol Sci. 1992;3:23–27. [Google Scholar]
- McEwen B. Protective and damaging effects of stress mediators. New Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen B, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the Hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stresshave different biological profiles. Brain Behav Immun. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Nejtek VA. High and low emotion events influence emotional stress perceptions and are associated with salivary cortisol response changes in a consecutive stress paradigm. Psychoneuroendocrinology. 2002;17:337–352. doi: 10.1016/s0306-4530(01)00055-5. [DOI] [PubMed] [Google Scholar]
- Myers LB, Brewin CR. Repressive coping and the recall of emotional material. Cogn Emot. 1995;9:637–642. [Google Scholar]
- Paulhus DL, John OP. Egoistic and moralistic biases in self-perception: The interplay of self-deceptive styles with basic traits and motives. J Pers. 1998;66:1025–1060. [Google Scholar]
- Prkachin KM, Williams-Avery RM, Zwaal C, Mills DE. Cardiovascular changes during induced emotion: An application of Lang's theory of emotional imagery. J Psychosom Res. 1999;47:255–267. doi: 10.1016/s0022-3999(99)00036-7. [DOI] [PubMed] [Google Scholar]
- Ray RD, Wilhelm FH, Gross JJ. All in the mind's eye? Anger rumination and reappraisal. J Pers Soc Psychol. 2008;94:133–145. doi: 10.1037/0022-3514.94.1.133. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Weerts TC. Cardiovascular responding during anger and fear imagery. Psychol Rep. 1982;50:219–230. doi: 10.2466/pr0.1982.50.1.219. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress hormones: good and bad. Neurobiol Dis. 2000;7:540–542. doi: 10.1006/nbdi.2000.0350. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Toward a dynamic theory of emotion: the component process model of affective states. Geneva Studies in Emotion and Communication. 1987;1:1–98. [Google Scholar]
- Scherer KR. What are emotions? And how can they be measured? Soc Sci Inform. 2005;44:693–727. [Google Scholar]
- Schwartz GE, Weinberger DA, Singer JA. Cardiovascular differentiation of happiness, sadness, anger, and fear following imagery and exercise. Psychosom Med. 1981;43:343–364. doi: 10.1097/00006842-198108000-00007. [DOI] [PubMed] [Google Scholar]
- Selye H. The Stress of Life. McGraw-Hill; New York: 1975. [Google Scholar]
- Smith CA, Lazarus RS. Emotion and Adaptation. Guilford Press; New York: 1990. [Google Scholar]
- Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Jr, Zimmerman EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosom Med. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-aho PO, Karjalainen PA. Kubios HRV – A software for advanced heart rate variability analysis. Comp Method Prog Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. J Person. 2004;72:1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP. Kubios HRV Version 20 User's Guide Department of Physics. University of Kuopio; Kuopio, Finland: 2008. [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]