Abstract
Regulatory T (Treg) cells and the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway are both critical for maintaining peripheral tolerance to self antigens. A significant subset of Treg cells constitutively expresses PD-1, which prompted an investigation into the role of PD-1/PD-L1 interactions in Treg-cell development, function and induction in vivo. The phenotype and abundance of Treg cells was not significantly altered in PD-1-deficient mice. The thymic development of polyclonal and monospecific Treg cells was not negatively impacted by PD-1 deficiency. The suppressive function of PD-1−/− Treg cells was similar to their PD-1+/+ counterparts both in vitro and in vivo. However, in three different in vivo experimental settings, PD-1−/− conventional CD4+ T cells demonstrated a strikingly diminished tendency toward differentiation into peripherally induced Treg (pTreg) cells. Our results demonstrate that PD-1 is dispensable for thymic (tTreg) Treg-cell development and suppressive function, but is critical for the extrathymic differentiation of pTreg cells in vivo. These data suggest that antibody blockade of the PD-1/PD-L1 pathway may augment T-cell responses by acting directly on conventional T cells, and also by suppressing the differentiation of pTreg cells.
Keywords: PD-1, Treg cell, Treg-cell development, Treg-cell function, Treg-cell differentiation
Introduction
Regulatory T (Treg) cells are a population of CD4+ T cells with natural suppressive capabilities, which are critical for maintaining peripheral tolerance [1-3]. Treg cells can be defined by their specific expression of the forkhead family transcription factor, FoxP3, which is necessary for their differentiation and function [4]. Mice and humans harboring a loss of function mutation at the FoxP3 locus develop lymphoproliferation and resultant severe autoimmunity affecting a wide variety of organs [5-6]. Treg cells can be categorized depending upon the location of their origin [3]. Thymic Treg (tTreg) cells develop in the thymus through high avidity peptide/MHC class II : T cell receptor (TCR) interactions, and are indispensable to prevent autoimmunity. In contrast, peripherally-induced Treg (pTreg) cells are generated from conventional CD4+ T cells in response to TCR stimulation and TGF-β [7] and are required to maintain immune tolerance to oral antigens and commensal microbes in the gut [8-10] and to suppress chronic allergic inflammation [11]. tTreg cells and pTreg cells have also been implicated in tumor immune escape [12-13].
In addition to FoxP3, Treg cells also constitutively express high levels of CD25 (the alpha chain of the IL-2 receptor), cytotoxic T lymphocyte antigen – 4 (CTLA-4) and glucocorticoid-induced TNFR-related protein (GITR), proteins that impact their suppressive capability [3]. Treg cells have also been shown to express programmed death 1 (PD-1), a coinhibitory receptor of the immunoglobulin gene superfamily, which is also expressed on activated T cells and B cells [14-15]. PD-1 has two known ligands, programmed death ligand 1 (PD-L1; B7-H1) and PD-L2 (B7-DC) [16-18]. PD-L1 demonstrates a broad tissue expression pattern on hematopoietic and non-hematopoietic cells, as well as on a wide variety of malignant cell types. Expression of PD-L2 is limited to dendritic cells (DCs), macrophages and mast cells [19]. Upon binding to its ligands, PD-1 becomes phosphorylated on intracellular tyrosine residues within its immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM). Subsequently, phosphatases, such as SHP-2, are recruited to the ITSM, become activated and inhibit proximal TCR signaling events, resulting in decreased T-cell proliferation, cytokine production and cytolytic capability [14, 20-22]. PD-1-deficient (PD-1−/−) mice develop strain-specific autoimmunity later in life, providing evidence of the negative regulatory function of this receptor and its ligands on T cells [6, 23]. Antibody-mediated blockade of PD-1/PD-L1 interactions has been shown in multiple pre-clinical cancer models and in cancer patients to promote enhanced antitumor immunity and objective tumor responses [24-31].
In addition to negatively regulating conventional T-cell function, emerging data has suggested that PD-1/PD-L1 interactions may contribute to pTreg-cell development and Treg-cell suppressive function. Using TCR transgenic CD4+ OT-II T cells, it has recently been demonstrated that conversion of OT-II T cells into pTreg cells was significantly diminished following PD-L1 blockade, and that PD-L1−/− DCs failed to support pTreg-cell generation in the presence of TGF-β in vitro [32]. Further, pTreg-cell development in a transplantable tumor model was impaired when anti-PD-L1 therapy was delivered to tumor-bearing mice [32]. Moreover, Sharpe and colleagues developed an elegant in vitro system in which they demonstrated decreased pTreg-cell generation in the presence of PD-L1−/− antigen presenting cells (APCs), while the opposite was observed when CD4+FoxP3− T cells were stimulated in the presence of a PD-L1-Ig fusion protein [33]. This group also showed that upon transfer of naïve, polyclonal CD4+FoxP3− T cells into lymphopenic Rag−/− mice also genetically deficient in PD-L1 and PD-L2, a fatal inflammatory disorder developed, which correlated with a decrease in pTreg-cell generation in these hosts [33]. Lastly, with regard to PD-1 and its possible modulation of Treg-cell function, in vitro Treg-cell suppression assays have demonstrated that PD-1−/− Treg cells were less proficient at suppressing CD8+ T-cell proliferation and cytokine production [34], arguing that PD-1 signaling in Treg cells may partially regulate their natural suppressive function. Collectively, these data argue that the PD-1/PD-L1 pathway, in addition to negatively regulating conventional T cells, may promote peripheral tolerance through positively regulating the suppressive function of Treg cells and by promoting pTreg-cell generation [33].
The observations that a subset of Treg cells constitutively expresses PD-1, and that PD-L1 appears to promote pTreg-cell differentiation, suggested that the PD-1/PD-L1 pathway may also be involved in the development and function of Treg cells, which has not been well-studied, and is highly relevant now that PD-1 and PD-L1-blocking antibodies are being utilized in the clinic. To answer these questions, a comprehensive investigation into the role of the PD-1/PD-L1 pathway in Treg-cell biology was performed. In this study, we investigated the role of PD-1 in tTreg- and pTreg-cell development and Treg-cell function in vivo. Our results demonstrate that PD-1 is not required for the development of tTreg cells and does not contribute to their natural suppressive function. However, in the periphery, conversion of CD4+ T cells into pTreg cells is highly dependent upon PD-1 in multiple in vivo experimental settings. These data provide evidence that the PD-1 receptor may promote peripheral tolerance, not only through negatively regulating the function of conventional T cells, but also by promoting pTreg-cell generation in peripheral tissues.
Results
PD-1 is expressed on a subset of Treg cells but does not regulate their phenotype
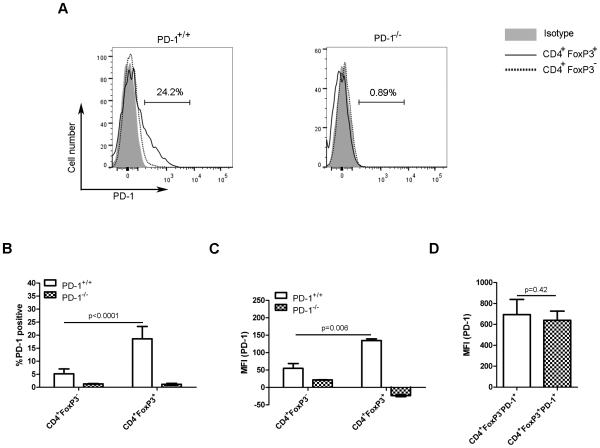
Cell surface PD-1 expression has been reported on activated, but not on naïve T cells [35]. Interestingly, PD-1 mRNA and protein expression has also been observed in Treg cells [36]. To confirm the later finding, the frequency of Treg cells expressing PD-1, as well as their relative PD-1 expression level was analyzed in 4-5 week-old PD-1+/+ and PD-1−/−FoxP3GFP mice. The frequency of Treg cells (CD4+FoxP3+ cells) expressing cell surface PD-1 protein was higher than that of conventional CD4+ T cells (CD4+FoxP3− cells) which expressed PD-1 at a level near background (Figure 1A and 1B). PD-1 expression was not observed on conventional T cells or Treg cells from PD-1−/− animals (Figure 1A and B). Similarly, the mean fluorescence intensity (MFI) of PD-1 was significantly higher on Treg cells compared with conventional CD4+ T cells (Figure 1C). However, after gating on PD-1-expressing CD4+FoxP3+ and CD4+FoxP3− cells, the MFI of PD-1 on both was similar (Figure 1D). These results confirm that PD-1 is constitutively expressed on a subset of Treg cells, which suggested that it may play a role in their development and/or function.
Figure 1. A subset of Treg cells constitutively expresses PD-1.
(A) PD-1 expression on FoxP3+ and FoxP3− splenic CD4+ T cells from 4- to 5-week old PD-1+/+Foxp3GFP (left) and PD1−/−Foxp3GFP mice (right) was assessed by flow cytometry after gating on CD4+FoxP3− and CD4+FoxP3+ cells. The horizontal bar represents the frequency of PD-1-expressing CD4+FoxP3+ T cells. Representative histograms from a single experiment representative of 3 independent experiments are shown. (B) The frequency of PD-1-expressing splenic CD4+FoxP3+ and CD4+FoxP3− T cells from mice in (A) is shown as mean + SD of 3 mice per group from a single experiment representative of 3 independent experiments. (C) The mean fluorescence intensity (MFI) of PD-1 expression on splenic CD4+FoxP3+ and CD4+FoxP3− T cells from mice in (A) are shown as mean + SD of 3 mice per group from a single experiment representative of 3 independent experiments. (D) After gating on PD-1-expressing CD4+FoxP3+ and CD4+FoxP3− cells from mice in (A), the MFI of PD-1 expression is shown as mean + SD of 3 mice per group from a single experiment representative of 3 performed experiments. p values were determined using a 2-tailed Student’s t test.
FoxP3 is a marker specific for the Treg cell lineage and is necessary for their development and function [4, 37]. In addition to FoxP3, Treg cells also constitutively express CD25, CTLA-4 and GITR [3], which are all involved in their suppressive capability. In addition, helios, a transcription factor involved in the regulation of early hematopoietic development, has also been proposed as an tTreg-cell marker [38]. To investigate whether PD-1-deficiency resulted in an altered Treg-cell phenotype, classical Treg-cell markers were analyzed on T cell subsets from PD-1+/+ and PD-1−/−animals. PD-1−/− Treg cells expressed a similar level of FoxP3 compared with PD-1+/+ Treg cells both in the thymus and spleen, which indicated that PD-1 signaling does not regulate FoxP3 expression in Treg cells (Supplementary Figure 1A-D). In the thymus, PD-1−/− and PD-1+/+ Treg cells expressed similar levels of CD25, Helios, CTLA-4 and GITR (Supplementary Figure 1A and B). In the spleen, PD-1−/− Treg cells expressed slightly higher level of helios, GITR and CTLA-4 but consistently lower levels of CD25 (Supplementary Figure 1C and D). Although there were very minor phenotypic differences observed between splenic PD-1−/− and PD-1+/+ Treg cells, the overall expression patterns in both were quite similar.
Thymic Treg-cell development is enhanced in the absence of PD-1
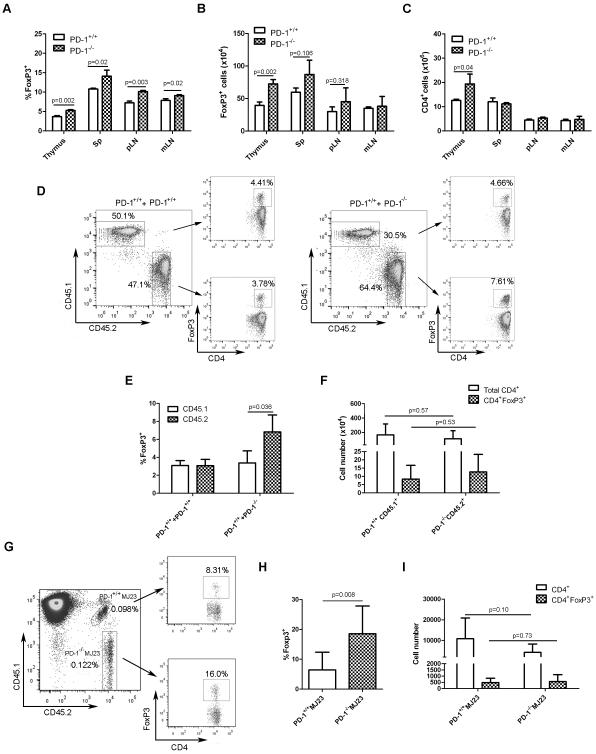
To investigate whether PD-1 was involved in Treg-cell development, the frequency and absolute numbers of Treg cells were analyzed in the thymus and peripheral lymphoid organs of 4 to 5 week-old PD-1+/+ and PD-1−/−FoxP3GFP mice. Young mice were chosen for analysis to avoid complications of autoimmunity which develops in older PD-1−/− animals. As shown in Figure 2A, slightly higher Treg-cell frequencies were observed in the thymi of PD-1−/− versus PD-1+/+ mice. The same was true for absolute numbers of thymic Treg cells (Figure 2B). Absolute numbers of total CD4+CD8− T cells were also higher in the thymi of PD-1−/− animals (Figure 2C). In the peripheral lymphoid organs there was also a mild increase in Treg-cell frequency in PD-1−/− animals (Figure 2A and B), while numbers of overall CD4+ T cells were similar (Figure 2C). These data suggest that PD-1 is not required for tTreg-cell development, and rather that Treg-cell development is actually enhanced in the thymi of PD-1−/− mice, perhaps consistent with the notion that positive selection of thymic Treg cells requires stronger TCR stimulation than for conventional T cells [39-40], such as would be expected to occur in the absence of PD-1 signaling in a developing thymocyte.
Figure 2. PD-1 is not required for thymic Treg-cell development.
(A) Frequency (mean + SD) of Treg cells in the indicated organs from 4- to 5-week-old PD-1+/+FoxP3GFP and PD-1−/− Foxp3GFP mice was determined by flow cytometry after gating on CD8−CD4+ T cells (thymus) or CD4+ T cells (spleen, pLN and mLN) . (B) Absolute numbers of Treg cells (mean + SD) present in the indicated organs from mice in (A) was determined by multiplying the Treg cell frequency by the total number of viable cells (excluding red blood cells) as measured by trypan blue exclusion. (C) Absolute numbers of total CD4+ T cells (mean + SD) present in the indicated organs from mice in (A) was determined by multiplying the CD4+ T cell frequency by the total number of viable cells as for (B). (D) T cell-depleted bone marrow cells from PD-1+/+ (CD45.1) and PD-1+/+ (CD45.2) or PD-1+/+ (CD45.1) and PD-1−/− (CD45.2) mice were mixed at a 1:1 ratio, and a total of 5 × 106 cells were injected i.v. into groups of sub-lethally irradiated (500 rad) Rag2−/− recipient mice. Five weeks later, thymi from mixed bone marrow chimeric (BMC) mice (PD-1+/+CD45.1 : PD-1+/+CD45.2 and PD-1+/+CD45.1 : PD-1−/−CD45.2) were analyzed by flow cytometry for Treg-cell frequency contributed by each compartment, after gating on CD45.1+ or CD45.2+ CD4SP cells. Representative flow plots from thymi of PD-1+/+CD45.1:PD-1+/+ CD45.2 and PD-1+/+CD45.1:PD-1−/−CD45.2 BMC mice are depicted. Numbers in plots indicate the frequency of the gated population. (E) Thymic Treg-cell frequencies (mean + SD) from mice in (D). (F) Absolute numbers (mean + SD) of total CD4+ and CD4+FoxP3+ T cells in the thymi of PD-1+/+CD45.1 : PD-1−/−CD45.2 BMC animals was determined by flow cytometry, after gating on CD4SP cells. Data are from a single experiment representative of 3 independent experiments, each with 4-5 mice per group. (G) Mixed BMC mice were generated following an i.v. inoculation of a mixture of bone marrow cells (5 × 106) isolated from female Rag1−/−PD-1+/+MJ23 mice (CD45.1.2; 5%), Rag1−/−PD1−/−MJ23 mice (CD45.2.2; 5%) and B6.SJL mice (CD45.1.1; 90%) into sub-lethally irradiated (500 rad) B6.SJL male mice. Five weeks later, the frequency of FoxP3-expressing PD-1+/+ and PD-1−/− CD4SP MJ23 T cells in the thymus was analyzed by flow cytometry. Representative plots from a single experiment (representative of 2 independent experiments) from the thymus of a mixed BMC mouse depicting the gating strategy are shown. Numbers in the plot on the left indicate the PD-1+/+MJ23 and PD-1−/−MJ23 frequency among the total gated CD4SP population. Numbers in the plots on the right indicate the percentage of Foxp3+ cells among the PD-1+/+MJ23 and PD-1−/−MJ23 CD4SP thymic populations. (H) Frequency of Treg cells (mean + SD) among the PD-1+/+MJ23 CD4SP and PD-1−/−MJ23 CD4SP thymic populations was determined by flow cytometry. (I) Absolute numbers (mean + SD) of total and FoxP3-expressing PD-1+/+MJ23 and PD-1−/−MJ23 CD4+ T cells in the thymi of mixed BMC animals was determined by multiplying the CD4SP MJ23 cell frequency or Treg-cell frequency by the total number of viable cells (excluding red blood cells) as measured by trypan blue exclusion respectively. Data from (H) and (I) were pooled from 2 independent experiments with a total of 8 mice per group. p values were determined using a 2-tailed Student’s t test.
To determine whether the effects of PD-1 deficiency on thymic Treg-cell development were cell-intrinsic, mixed bone marrow chimeras were generated using congenically-marked PD-1+/+ and PD-1−/− marrow cells (Figure 2D). Cohorts of Rag2−/− mice received sub-lethal TBI (500 rad), followed by transfer of equal numbers of T cell-depleted PD-1+/+ (CD45.2+) and PD-1−/− (CD45.1+) bone marrow cells. A control cohort of irradiated Rag2−/− mice received equal numbers of congenically-marked PD-1+/+ marrow cells. Five weeks later, the frequency of tTreg cells in the thymi of mixed bone marrow chimeric mice was analyzed (Figure 2D and 2E). As expected, equivalent frequencies of tTreg cells were observed in the thymi of the control mixed bone marrow chimeric cohort. Similar to what was observed in PD-1−/− mice, higher Treg-cell frequencies in the thymus were observed in the PD-1−/− relative to the PD-1+/+ compartment in chimeric mice (Figure 2E), while the absolute numbers of PD-1−/− and PD-1+/+ thymic Treg cells were similar (Figure 2F). Taken together, these results argue that while PD-1 is not required on developing thymocytes for their differentiation into Treg cells, PD-1 deficiency appears to enhance thymic Treg-cell development.
Thymic development of a naturally-occurring Treg-cell specificity is enhanced in the absence of PD-1
Because the analysis of bulk polyclonal Treg cells could mask effects of PD-1 deficiency on tTreg-cell development, the impact of PD-1 deficiency on the thymic development of Treg cells of a single, naturally-occurring specificity was investigated. The Treg-derived “MJ23” TCR, which was originally identified based on its recurrent expression by FoxP3+ Treg cells enriched in oncogene-driven prostate cancers in TRAMP mice. The MJ23 TCR confers reactivity to a prostate-associated self-antigen, and facilitates development of FoxP3+ MJ23 Treg cells in the thymus [41]. Thus, the development of thymocytes expressing the MJ23 TCR into FoxP3-expressing Treg cells was analyzed in chimeric mice in which bone marrow cells from MJ23 TCR transgenic Rag1−/− mice were engrafted at a low clonal frequency into sub-lethally irradiated wild-type recipients, and the fate of MJ23 T cells was assessed 5 weeks post-engraftment. Donor MJ23 TCR transgenic Rag1−/− cells consisted of a 1:1 mixture of PD-1+/+ and PD-1−/−MJ23 marrow cells which could be distinguished based on differential expression of CD45 congenic markers (Figure 2G). In the thymus, PD-1−/−MJ23 T cells developed into FoxP3+ Treg cells with enhanced efficiency compared with PD-1+/+MJ23 T cells (Figure 2H). Similar to what was observed in the polyclonal mixed bone marrow chimeric mice, increased numbers of thymic PD-1+/+MJ23 versus PD-1−/−MJ23 total CD4+ T cells were recovered, although this difference did not reach statistical significance (Figure 2I). Here again, the absolute numbers of thymic PD-1−/− and PD-1+/+MJ23 Treg cells were similar (Figure 2I). These results confirm that PD-1 is not required for the thymic development of Treg cells of this specificity. Consistent with observations in the polyclonal setting, deficiency of PD-1 on MJ23 T cells promoted their differentiation into Treg cells.
PD-1 does not regulate Treg cell-mediated suppression of conventional T cells in vitro or in vivo
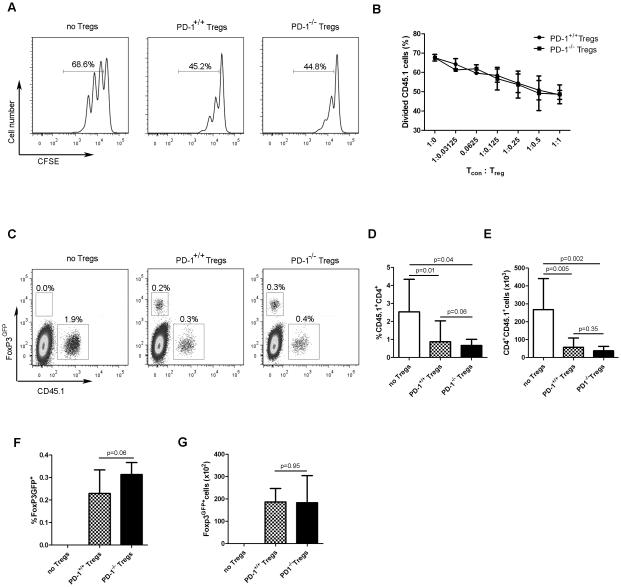
As PD-1 is expressed on a subset of Treg cells, it was formally possible that it could regulate their suppressive functions. To first investigate whether PD-1−/− Treg cells suppressed conventional T-cell responses to the same degree as PD-1+/+ Treg cells in vitro, congenically-marked, naïve CD4+ T cells were CFSE-labeled and co-cultured with PD-1+/+ or PD-1−/− Treg cells in the presence of T cell-depleted splenocytes and plate-bound anti-CD3 antibody. Proliferation of conventional CD4+ T cells was inhibited similarly by PD-1+/+ and PD-1−/− Treg cells (Figure 3A and B), indicating that PD-1 is not involved in regulating the suppressive capacity of Treg cells in vitro.
Figure 3. PD-1 does not regulate the suppressive capability of Treg cells in vitro or in vivo.
(A, B) In vitro Treg-cell suppression assay. 105 naïve CD4+ T cells (CD45.1) were cultured at the indicated ratios with Treg cells isolated from PD-1+/+FoxP3GFP or PD-1−/−FoxP3GFP mice (CD45.2), along with 5 × 105 T cell-depleted spleen cells (CD45.2) in the presence of plate-bound anti-CD3 for 3 days. (A) Representative flow cytometry plots from a single experiment (representative of 2 independent experiments) demonstrating CFSE dilution profiles of conventional CD45.1+CD4+ T cells following adoptive transfer. The percentage of proliferating conventional CD4+ T cells is shown. (B) Percentage of divided conventional CD4+ T cells (mean + SD) at the indicated Tcon:Treg cell ratios is shown. Data are pooled from 2 independent experiments with triplicates at each Tcon:Treg ratio in each experiment. (C-G) An in vivo Treg-cell suppression assay was performed, in which naïve CD4+ T cells (CD45.1) were adoptively transferred into Rag2−/− recipient mice alone, or in combination with Treg cells isolated from PD-1+/+FoxP3GFP or PD1−/−FoxP3GFP donor mice (CD45.2). (C) Representative flow cytometry plots demonstrating the frequency of transferred conventional CD4+ T cells (CD45.1) and Treg cells (CD45.2) in the spleens of recipient Rag2−/− mice 10 days following adoptive transfer. Numbers in plots indicate the frequency of the gated population among live splenic lymphocytes. (D) Frequency (mean + SD), and (E) absolute number (mean + SD) of transferred CD4+CD45.1+ T cells in the presence or absence of co-transferred PD-1+/+ or PD-1−/− Treg cells, as determined by flow cytometry. (F) Frequency (mean + SD), and (G) absolute number (mean + SD) of PD-1+/+ and PD-1−/−FoxP3GFP cells following adoptive transfer, determined by flow cytometry after gating on GFP+ cells. Data from (D) to (G) were pooled from two independent experiments with an overall total of 8 mice per group. p values were determined using a 2-tailed Student’s t test.
To determine whether PD-1−/− Treg cells suppressed normally in vivo, a model of lymphopenia-induced homeostatic proliferation (HP) was employed [42]. In this system, naïve, polyclonal CD4+ T cells undergo HP following transfer into genetically lymphopenic Rag−/− mice. However, the HP of CD4+ T cells is significantly inhibited by the co-transfer of Treg cells, serving as an in vivo read-out of their suppressive capability. Thus, 106 naïve CD4+ conventional T cells were adoptively-transferred into Rag2−/− hosts either alone, or in combination with 2.5 × 105 PD-1+/+ or PD1−/− Treg cells. Ten days later, the frequency (Figure 3C and D) and absolute numbers (Figure 3E) of the transferred conventional CD4+ T cells (CD45.1+) were enumerated. Naive CD4+ T cells proliferated vigorously following transfer into Rag2−/− hosts in the absence of Treg cells (Figure 3C-E). However, upon co-transfer of Treg cells, the numbers of recovered conventional CD4+ T cells were significantly reduced, and similar regardless of whether the co-transferred Treg cells expressed PD-1 (Figure 3C-E). This result demonstrates that PD-1 is dispensable for Treg-mediated suppression of conventional T cell proliferation. Lastly, similar frequencies and absolute numbers of PD-1+/+ and PD-1−/− Treg cells were recovered following adoptive transfer into secondary hosts (Figure 3F and G).
PD-1−/− CD4+ T cells are defective in pTreg-cell generation in a lymphopenic environment
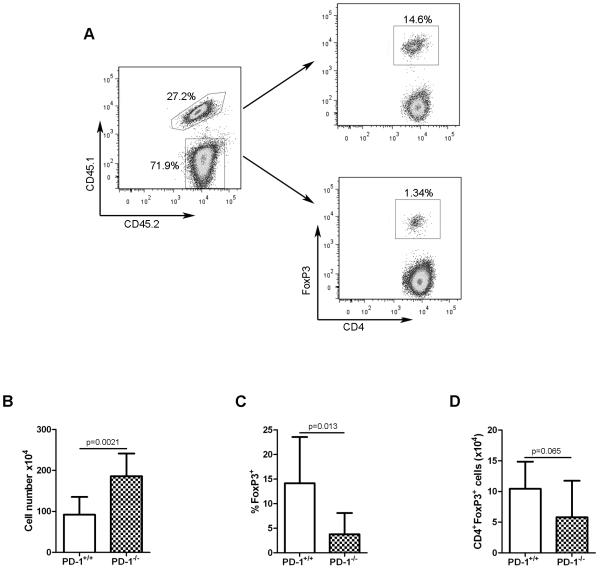
It has been demonstrated that when polyclonal CD4+FoxP3− cells are adoptively-transferred into lymphopenic recipients, a fraction of the transferred CD4+ cells are induced to upregulate FoxP3 expression and acquire suppressive capability when analyzed ex vivo (i.e. become pTreg cells) [43]. To determine whether pTreg-cell development under lymphopenic conditions was regulated by PD-1 expression on CD4+ T cells, congenically-marked naïve, polyclonal conventional CD4+ T cells were purified from PD-1+/+ or PD-1−/−FoxP3GFP mice and were co-transferred into Rag2−/− recipient mice (Figure 4A). The frequency and absolute numbers of FoxP3-expressing PD-1+/+ and PD-1−/− pTreg cells were analyzed in peripheral lymph nodes 20 days later. Although nearly two-fold more PD-1−/− versus PD-1+/+ total CD4+ T cells were recovered (Figure 4B), a significantly lower frequency of adoptively-transferred PD-1−/− CD4+ T cells was induced to express FoxP3 (Figure 4C). Non-significantly decreased absolute numbers of PD-1−/− versus PD-1+/+ pTreg cells were also observed (Figure 4D). This result demonstrated that PD-1 positively regulates pTreg-cell differentiation in this model.
Figure 4. PD-1 regulates the differentiation of conventional CD4+ T cells into Treg cells in a lymphopenic environment.
(A) CD4+CD62LhiFoxp3GFP- cells were isolated from PD-1+/+Foxp3GFP (CD45.1.2) and PD-1−/−Foxp3GFP (CD45.2.2) mice, were mixed at a 1:1 ratio, and a total of 2 × 106 cells were adoptively transferred into Rag2−/− mice. Representative flow cytometry plots from a single experiment (representative of 2 independent experiments) depicting the frequency of CD45.1.2+ (PD-1+/+) and CD45.2.2+ (PD-1−/−) cells (left) and of FoxP3-expressing CD4+ cells derived from the PD-1+/+ and PD-1−/− compartments (right) in pooled peripheral lymph nodes from a recipient Rag2−/− mouse. Numbers in plots show the frequency of the gated populations. (B) Absolute numbers (mean + SD) of total PD-1+/+ and PD-1−/− CD4+ T cells from pooled lymph nodes from Rag2−/− recipient mice in (A). (C) Frequency (mean + SD) of Foxp3GFP-expressing PD-1+/+ and PD-1−/− CD4+ T cells from mice in (A). (D) Absolute numbers (mean + SD) of Foxp3GFP-expressing PD-1+/+ and PD-1−/− CD4+ T cells from mice in (A). Data from (B) to (D) are pooled from two independent experiments with overall total of 8 mice per group. p values were determined using a 2-tailed Student’s t test.
Defective pTreg-cell generation in PD-1−/− TCR transgenic CD4+ T cells following oral antigen exposure
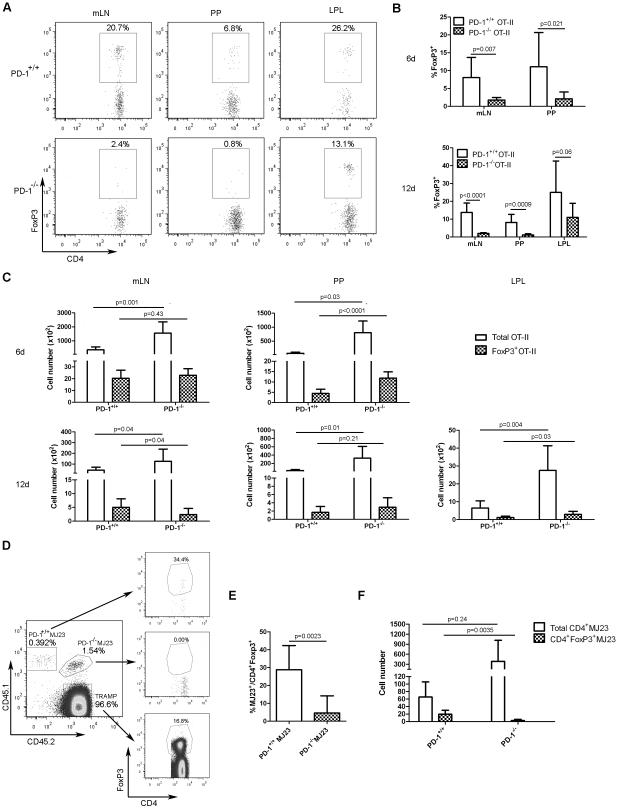
Oral antigen exposure results in rapid pTreg-cell generation within the gut and mesenteric lymph nodes (mLN) as a mechanism of tolerance to food-derived antigens [44-46]. To determine whether PD-1 signaling impacted pTreg-cell differentiation in an antigen-specific oral tolerance model, OVA-specific PD-1+/+OT-II or PD-1−/−OT-II CD4+ T cells (CD45.2+) were purified (none expressed FoxP3) and adoptively-transferred into congenic B6.SJL mice (CD45.1+). Subsequently, mice were administered untreated or 1.5% ovalbumin (OVA)-supplemented drinking water for 6 days. Peyer’s patches (PP), mLN and lamina propria lymphocytes were then isolated and analyzed for FoxP3 expression within the OT-II T-cell compartment 6 and 12 days after adoptive transfer. Oral OVA administration induced the differentiation of a significant frequency of FoxP3-expressing PD-1+/+OT-II CD4+ T cells within the mLN and PP and LPL (Figure 5A and B). In contrast, pTreg-cell differentiation of PD-1−/−OT-II CD4+ T cells was substantially impaired in the mLN and PP, and to a lesser degree, the LPL (Figure 5A and B). It has been previously shown that during the establishment of oral tolerance, induction of CD4+ T cells into pTreg cells occurs in the gut-draining lymph nodes, followed by trafficking of recently converted pTreg cells to the intestine where they undergo rapid proliferation. This observation could explain why the difference in pTreg-cell induction between PD-1+/+ and PD-1−/−OT-II cells in the LPL was less striking than that observed in the PP and mLN [10]. Significantly increased numbers of total PD-1−/−OT-II CD4+ T cells were recovered in the mLN, PP and LPL of OVA-treated animals 6 and 12 days following adoptive transfer, whereas the numbers of recovered PD-1+/+ and PD-1−/−OT-II Treg cells varied between the tissues examined (Figure 5C). Together, these data demonstrate that PD-1 promotes pTreg-cell development in an antigen-specific oral tolerance model.
Figure 5. PD-1 is critical for iTreg-cell differentiation following oral antigen exposure and in cancer-bearing hosts.
(A) CD4+ OT-II T cells (106) from Rag1−/−PD-1+/+OT-II or Rag1−/−PD1−/−OT-II mice (CD45.2+) were separately transferred i.v. into B6.SJL recipient mice (CD45.1). Mice were then given drinking water supplemented with 1.5% ovalbumin (OVA) for 6 days. On days 6 and 12, FoxP3 expression among OT-II T cells was analyzed in the mesenteric lymph node (mLNs), Peyer’s patches (PPs) and lamina propria lymphocytes (LPLs) by flow cytometry. Representative plots from a single experiment (representative of 2 independent experiments) from the indicated tissue are shown after gating on transferred CD45.2+ cells. Numbers in plots indicate the frequency of the gated populations. (B) The frequency (mean + SD) of FoxP3-expressing PD-1+/+ and PD-1−/−OT-II T cells in the indicated tissue 6 and 12 days following oral OVA exposure from mice in (A). (C) Absolute numbers (mean + SD) of total and FoxP3-expressing PD-1+/+ and PD-1−/− OT-II T cells in the indicated tissue 6 and 12 days following oral OVA exposure from mice in (A). (B-C) Data were pooled from two independent experiments with overall total of 8 mice per group. (D) CD4+ MJ23 T cells were isolated from female Rag1−/−PD-1+/+MJ23 (CD45.1.1) and Rag1−/−PD-1−/−MJ23 (CD45.1.2) donor mice. 3 × 105 PD-1+/+MJ23 and PD-1−/−MJ23 T cells were mixed at a 1:1 ratio, and a total of 6 × 105 were co-transferred into 4-5 month old male TRAMP recipients (CD45.2.2) with established prostate cancer. 30 days later, the frequencies of FoxP3-expressing PD-1+/+ or PD-1−/−MJ23 T cells among the entire PD-1+/+ or PD-1−/−MJ23 T-cell populations, respectively, were analyzed by flow cytometry. Representative plots from a single experiment (representative of 2 independent experiments) from the peri-aortic LNs of a TRAMP mouse are shown. Numbers in plots indicate frequency of the gated population. (E) The frequency (mean + SD) of FoxP3-expressing PD-1+/+ and PD-1−/−MJ23 T cells among the total respective CD4+ MJ23 T cell populations from mice in (D) (F) Absolute number (mean + SD) of total CD4+ and CD4+FoxP3+ PD-1+/+ and PD-1−/−MJ23 T cells from mice in (D). Data from (E) to (F) were pooled from 2 independent experiments each with a total of 7 mice per group. p values were determined using a 2-tailed Student’s t test.
PD-1 promotes pTreg-cell differentiation in TRAMP mice
Accumulation of Treg cells is a common phenomenon in cancer-bearing hosts, and can be due to either expansion of tTreg cells, differentiation of conventional CD4+ T cells into pTreg cells, or both [3]. To determine whether PD-1 signaling in CD4+ T cells impacted pTreg-cell differentiation in a mouse model of autochthonous prostate cancer, CD4+MJ23 T cells were purified from Rag1−/−PD-1+/+MJ23 (CD45.1.1) or Rag1−/−PD-1−/−MJ23 (CD45.1.2) female mice and co-transferred into 4-5 month old TRAMP male mice with established prostate cancer. It should be noted that mature peripheral MJ23 TCR transgenic T cells that develop in female Rag1−/− animals do not express FoxP3 [41]. Thirty days following MJ23 T-cell transfer, the fate of donor cells was assessed in the prostate-draining peri-aortic lymph nodes. Consistent with a previous study [41], a significant percentage of PD-1+/+MJ23 CD4+ T cells upregulated expression of FoxP3, indicative of pTreg-cell differentiation (Figure 5D and E). In contrast, pTreg-cell development by PD-1−/−MJ23 T cells was markedly reduced (Figure 5D and E). Not significantly higher absolute numbers of PD-1−/−MJ23 versus PD-1+/+MJ23 CD4+ T cells were observed, but with a significantly lower frequency (and absolute number) of FoxP3-expressing MJ23 T cells in the PD-1−/− versus the PD-1+/+ compartment (Figure 5F). This result indicated that in a cancer context, PD-1 contributed to pTreg-cell differentiation.
Discussion
Our data, as well as those of others have indicated that a subset of Treg cells express the PD-1 receptor on their cell surface, which led to the hypothesis that PD-1 would play a role in Treg-cell development or suppressive function, particularly given its known function in the negative regulation of conventional T cells. Cell surface phenotyping of PD-1-sufficient and -deficient Treg cells revealed similar expression of CTLA-4, GITR and CD25. Levels of the FoxP3 protein were also identical in PD-1+/+ and PD-1−/− Treg cells, indicating that FoxP3 expression occurs independently of PD-1 signaling. Helios, a controversial marker of thymic Treg cells [38, 47-49], was similarly expressed in tTreg cells from PD-1+/+ and PD-1−/− hosts, as expected. However, peripheral Treg cells in PD-1−/− hosts expressed slightly higher levels of helios, perhaps consistent with their impaired ability to generate pTreg cells in vivo.
Treg-cell frequencies and absolute numbers were higher in the thymi of PD-1−/− animals, arguing that Treg-cell development in the thymus does not depend on PD-1. A similar result was observed in mixed PD-1-sufficient and -deficient bone marrow chimeric mice. Conversely, PD-1-deficiency in developing thymocytes promoted their differentiation into tTreg cells. This might be due to an increased avidity of particular T cell receptors for their selecting antigens in the absence of inhibitory signaling through the PD-1 receptor, leading to the positive selection and differentiation of particular thymocytes into the Treg-cell lineage. It has been previously been demonstrated that the positive selection of thymocytes into Treg cells depends on strong TCR self-reactivity [50], such as likely exists in the absence of PD-1 signaling. The frequencies of CD4/CD8 double-positive and CD4 single-positive PD-1−/− and PD-1+/+ thymocytes were similar in the thymi of polyclonal mixed bone marrow chimeric mice (not shown), so a clear role for PD-1 in regulating negative selection or positive selection of CD4+ T cells into the Treg-cell lineage could not be established in this setting. However, in PD-1+/+ and PD-1−/− MJ23 mixed bone marrow chimeras, there was a tendency toward a reduced frequency of PD-1-deficient MJ23 CD4 single-positive thymocytes (not shown). Along with the increased frequency of PD-1−/− MJ23 Treg cells observed (Figure 2H), this result suggested that PD-1 may be involved in either negatively selecting CD4+ T cells or promoting their differentiation into Treg cells. However, this conclusion is speculative and the question of PD-1 and TCR signal strength received by developing thymocytes could be more thoroughly evaluated in Nur77 reporter mice bred onto a PD-1−/− background [50].
PD-1 expression on Treg cells has recently been suggested to contribute to their suppressive capability in vitro [33]. As activated conventional T cells are known to express PD-L1, it was formally possible that interactions between PD-1-expressing Treg cells and PD-L1-expressing conventional T cells may have led to the negative regulation of the later. It is also possible that PD-1 expressed on Treg cells may modulate the function of dendritic cells expressing PD-L1 which might in turn negatively regulate T cell function. Our results which employed both in vitro and in vivo Treg-cell suppression assays demonstrated identical suppressive capability of PD-1+/+ and PD-1−/− Treg cells, strongly suggesting that PD-1 plays no significant role in Treg cell-mediated suppression. Arlene Sharpe’s group, using PD-L1-Ig-coated beads, developed an in vitro culture system in which they demonstrated that not only did PD-L1 promote pTreg-cell development, but also that PD-L1-Ig stimulation enhanced the suppressive capacity of PD-1-expressing pTreg cells [33]. In the current work, we did not directly examine the suppressive function of PD-1-sufficient and -deficient pTreg cells, and so this finding is not in disagreement with our results. However, the observation that pTreg cells are partially dependent upon PD-1 for their suppressive capability suggests that non-redundant mechanisms are utilized by tTreg cells and pTreg cells to suppress conventional T-cell function.
The most striking finding in the current body of work is the critical dependence on PD-1 for the differentiation of conventional CD4+ T cells into pTreg cells. In several distinct in vivo models (CD4+FoxP3− T-cell transfer into lymphopenic hosts, oral tolerance and cancer), we have demonstrated that PD-1-deficient CD4+FoxP3− T cells were significantly impaired in their ability to differentiate into pTreg cells both in polyclonal and antigen-specific settings. PD-L1 has been reported to regulate the development and function of pTreg cells in vitro. Wang and colleagues observed diminished pTreg-cell generation by PD-L1−/− DCs in vitro [32]. Francisco et al. demonstrated in an in vitro culture system that PD-L1-Ig-coated beads (when combined with anti-CD3 and anti-CD28 stimulation) promoted FoxP3 expression in CD4+FoxP3− T cells in a TGF-β-independent manner [33]. Upon adoptive transfer of naïve CD4+FoxP3− T cells into Rag−/− or PD-L1−/−PD-L2−/−Rag−/− animals, a fatal inflammatory disease developed in the later which correlated with lower frequencies of pTreg cells [33]. We also observed significantly lower frequencies of pTreg cells in Rag−/− hosts into which naïve PD-1−/− CD4+FoxP3− T cells were transferred, although there was no evidence of lethal inflammation. Regardless, our findings in a polyclonal adoptive T cell transfer model are in agreement with previous reports. We have also extended these observations into antigen-specific models of oral tolerance and prostate cancer, in which we have further demonstrated a critical role of PD-1 for pTreg-cell differentiation.
It should be noted that several of the experiments in this manuscript involved the use of mice harboring a GFP-FoxP3 fusion protein-reporter knock-in allele (FoxP3GFP) [51]. Recently, two reports have been published demonstrating that this particular strain of FoxP3 reporter mice have relative defects in peripheral Treg-cell induction [52-53]. However, as both the PD-1+/+ and PD-1−/− mice used in our experiments expressed the same FoxP3-GFP fusion protein, and because induction of FoxP3 expression could be readily observed in CD4+ conventional T cells from both groups, we believe that the results obtained are valid. In addition, we observed a similar defect in the differentiation of PD-1−/− versus PD-1+/+ TCR transgenic OT-II and MJ23 CD4+ T cells, neither which harbor a FoxP3 reporter allele.
Regarding the mechanism of impaired pTreg-cell differentiation in the absence of PD-1 signaling, there is in vitro evidence that PD-1/PD-L1 interactions which occur during stimulation of CD4+ T cells downregulate the AKT-mTOR signaling axis, which is necessary for naïve T-cell activation and expansion, and may favor differentiation of CD4+ T cells into pTreg cells [14, 33]. Thus, in the absence of PD-1 signaling in naïve CD4+ T cells, AKT signaling would be expected to be enhanced, favoring activation and proliferation of conventional CD4+ T cells rather than their induction into Treg cells. Consistent with this hypothesis, higher numbers of FoxP3-negative PD-1−/− CD4+ T cells were recovered following their adoptive transfer compared with their PD-1+/+ counterparts in each of the three in vivo Treg-cell differentiation assays performed. Further, lack of PD-1 signaling in stimulated CD4+ T cells results in enhanced signal strength through the TCR, and strong TCR stimulation in the presence of TGF-β and IL-2 has previously been shown to inhibit pTreg-cell generation in an NF-Kβ-dependent manner [54].
Following a comprehensive analysis of the role of PD-1 in the development, function and differentiation of Treg cells, we conclude that although PD-1 signaling is dispensable for the thymic development of Treg cells and for Treg cell-mediated suppression, it is critical for pTreg-cell differentiation in a number of in vivo experimental settings, including prostate cancer. These data raise the possibility that interruption of the PD-1/PD-L1 pathway in cancer patients may enhance anti-tumor T cell-responses not only through re-activation of effector T cells but also by preventing the differentiation of pTreg cells.
Materials and methods
Mice
C57BL/6 (H-2b) mice (CD45.2), aged 6 to 12 weeks, were purchased from either the Jackson or Taconic Laboratories. PD-1−/− mice on the C57BL/6 background were a gift from Tasuku Honjo (Kyoto University, Kyoto, Japan). Mice harboring a GFP-FoxP3 fusion protein-reporter knock-in allele (FoxP3GFP) on the C57BL/6 background were a gift from Alexander Rudensky (Memorial Sloan Kettering Cancer Center, New York, NY), and have been described previously [51]. B6.SJL (CD45.1), Rag2−/−, and Rag1−/−OT-II mice were purchased from Taconic Laboratories. PD-1−/−FoxP3GFP and Rag1−/−PD-1−/−OT-II mice were generated and maintained in our animal facility. Rag1−/−MJ23 TCR transgenic mice on the C57BL/6 background have been described previously [41]. Rag1−/−PD-1−/−MJ23 mice were generated and maintained in our facility. Animals were maintained in a specific pathogen-free environment and used according to protocols approved by the Institutional Animal Care and Use Committee at the University of Chicago, according to National Institutes of Health guidelines for animal use.
Flow cytometry
Antibodies against the following proteins coupled to the indicated fluorochromes were purchased from BD PharMingen: PE-anti–PD-1 (J43), APC-anti-CD44 (1M7), PE-anti-CD62L (MEL-14), PerCP.Cy5.5-anti–CD25 (PC61), PE-anti-CD8α (53-6.7) and PerCP.Cy5.5-anti-CD4 (RM4-5). PE-Cy.7-anti-FoxP3 (FJK-16s), APC-anti-FoxP3 (FJK-16s), Alexa Fluor 647-anti-Helios (22F5) and APC-anti-CD45.1 (A20) were purchased from eBioscience. APC-anti-CTLA4 (UC10-4B9), PE-anti-GITR (YGITR 765), Pacific Blue-anti-CD4 (GK1.5), Pacific Blue-anti-CD8α (53-6.7), Pacific blue-anti-CD45.2 (104) were purchased from BioLegend. For cell surface staining, 106 cells were labeled with the indicated antibodies or appropriate isotype controls for 15 minutes at 4°C. Cells were subsequently washed twice in PBS containing 1% FCS, and re-suspended for FACS analysis. For intracellular staining (helios, FoxP3 and CTLA-4), cells were permeabilized and fixed with 0.15% saponin in PBS and BD cytofix at a 1:3 ratio at 4°C for 30 min. Flow cytometry was performed on FACS Canto or LSR II cytometers, using BD FACSDiva software. Data analysis was performed with FlowJo software.
Generation of mixed bone marrow chimeras
Bone marrow was procured under sterile conditions from femurs of PD-1+/+ (CD45.1 or CD45.2) and PD-1−/− (CD45.2) mice, and was T cell depleted using CD4 and CD8 microbeads (Miltenyi Biotech) according to the manufacturer’s protocol. Marrow cells from PD-1+/+ and PD-1−/− mice were then mixed at a 1:1 ratio, and a total of 5 × 106 cells were injected intravenously (i.v.) through the lateral tail vein into sub-lethally irradiated (500rad) Rag2−/− recipients 6 hours following total body irradiation (TBI). Five weeks later, thymi from bone marrow chimeric mice were harvested and analyzed for the frequency and absolute numbers of FoxP3-expressing CD4+ T cells within the CD45.1+ and CD45.2+ cell populations.
Generation of mixed Treg cell-specific TCR transgenic MJ23 bone marrow chimeras
BM from Rag1−/−MJ23 (CD45.1.2), Rag1−/−PD1−/−MJ23 (CD45.2.2) and B6.SJL (CD45.1.1) mice was obtained as described above. BM cells were mixed at a ratio of 5%: 5%:90% respectively (2.5 × 105 Rag1−/−PD-1+/+MJ23 cells, 2.5 × 105 Rag1−/−PD-1−/−MJ23 cells and 4.5 × 106 B6.SJL cells). A total of 5 × 106 BM cells were injected i.v. into sub-lethally irradiated B6.SJL mice (500rad) 1 day following TBI as previously described [41]. Five weeks later, thymi from bone marrow chimeric mice were harvested and analyzed for the number and frequency of FoxP3-expressing PD-1+/+MJ23 and PD-1−/−MJ23 CD4+ T cells among total PD-1+/+MJ23 and PD-1−/−MJ23 T cells, respectively.
In vitro Treg-cell suppression assay
CD4+ T cells from spleens and pooled peripheral LN of B6.SJL (CD45.1), PD-1+/+FoxP3GFP and PD-1−/−FoxP3GFP mice (CD45.2) were separately isolated using a CD4+ T-cell isolation kit (Miltenyi Biotech) according to the manufacturer’s protocol. Purified CD4+ T cells from B6.SJL mice were additionally labeled with anti-CD4, anti-CD25, anti-CD44 and anti-CD62L antibodies, and CD4+CD25−CD44loCD62Lhi cells were sorted on a FACS Aria II cytometer as naive conventional CD4+ T cells. In parallel, purified CD4+ T cells from PD-1+/+FoxP3GFP and PD-1−/−FoxP3GFP mice were labeled with an anti-CD4 antibody, and CD4+GFP+ (FoxP3+) cells were sorted as Treg cells with > 97% purity. Sorted naïve CD45.1+CD4+ T cells were further labeled with 2.5μM CFSE, and 1 × 105 were co-cultured with 5 × 105 T cell-depleted splenocytes from C57BL/6 donor mice (CD45.2), in round-bottom 96-well tissue culture plates coated with an anti-CD3 antibody (2C11; 1μg/ml). Increasing numbers of PD-1+/+ or PD-1−/− Treg cells were added to the cultures as indicated. After 72 hours, cells were harvested and labeled with anti-CD45.1, anti-CD45.2 and anti-CD4 antibodies and analyzed by flow cytometry for the CFSE dilution of CD45.1+CD4+ conventional T cells.
In vivo Treg-cell suppression assay
Naive CD4+ T cells from B6.SJL mice (CD45.1) and Treg cells from PD-1+/+FoxP3GFP and PD-1−/−FoxP3GFP mice (CD45.2) were separately prepared as described above. 1 × 106 naive CD4+ T cells were adoptively transferred i.v. into Rag2−/− recipients alone, or in combination with 2.5 × 105 PD-1+/+ or PD1−/− Treg cells. Spleens of recipient Rag2−/− mice were harvested 10 days later, and the frequency and absolute numbers of CD45.1+CD4+ T cells and FoxP3GFP+ Treg cells in each of the groups were calculated following flow cytometric analysis.
pTreg-cell development in lymphopenic hosts
CD4+ T cells from spleens of PD-1+/+FoxP3GFP mice (CD45.1.2) and PD1−/−FoxP3GFP (CD45.2.2) mice were isolated with the CD4+ T cell isolation kit (Miltenyi Biotech) and labeled with anti-CD4 and anti-CD62L antibodies in preparation for cell sorting. Purified, naïve CD4+CD62LhiFoxP3GFP- cells (purity > 99%) from PD-1+/+FoxP3GFP and PD1−/−FoxP3GFP donors were mixed at a 1:1 ratio, and a total of 2 × 106 cells were adoptively-transferred i.v. into Rag2−/− mice. Peripheral lymph nodes were harvested 20 days later and analyzed by flow cytometry for the frequency of FoxP3-expressing CD4+ T cells present in the PD-1+/+ and PD-1−/− compartments, after gating on CD45.1.2+ and CD45.2.2+ CD4+ T cells, respectively .
pTreg-cell development in an oral antigen challenge model
Ovalbumin (OVA)-specific CD4+ OT-II T cells from Rag1−/−OT-II or Rag1−/−PD1−/−OT-II mice (CD45.2) were purified from the spleen and LN using a CD4+ T cell isolation kit (Miltenyi Biotech). 1 × 106 PD-1+/+OT-II or PD-1−/−OT-II T cells were transferred i.v. into B6.SJL mice (CD45.1), which then received untreated water or 1.5% OVA-supplemented water for 6 days (Sigma Aldrich), as previously described [8, 55]. Mesenteric lymph nodes (mLN), Peyer’s patches (PP) and lamina propia lymphocytes (LPL) were isolated from recipient mice on days 6 and 12 after adoptive transfer and single cell suspensions were generated. The frequencies and absolute numbers of FoxP3-expressing CD4+ PD-1+/+OT-II or PD-1−/−OT-II T cells (CD45.2) among the entire PD-1+/+OT-II or PD-1−/−OT-II T cell populations were analyzed by flow cytometry following intracellular staining for FoxP3.
pTreg-cell development in the TRAMP prostate cancer model
MJ23 T cells from spleens and peripheral LN of female Rag1−/−PD-1+/+MJ23 (CD45.1.1) and Rag1−/−PD-1−/−MJ23 (CD45.1.2) female mice were isolated using a CD4+ T cell enrichment kit (Miltenyi Biotech). 3 × 105 PD-1+/+MJ23 and PD-1−/−MJ23 CD4+ T cells were mixed at a 1:1 ratio, and a total of 6 × 105 cells were adoptively-transferred into 4-5 month old male TRAMP mice (CD45.2.2) with established prostate cancer [56]. Four weeks later, frequencies of FoxP3-expressing PD-1+/+MJ23 or PD-1−/−MJ23 T cells among the entire PD-1+/+MJ23 or PD-1−/−MJ23 populations were analyzed following intracellular staining for FoxP3 and flow cytometry.
Statistical analysis
A 2-tailed Student’s t test was used to compare differences in frequency, absolute number and mean fluorescence intensity (MFI) between mice in different groups. A p-value < 0.05 between groups was considered to be statistically significant.
Supplementary Material
Supplementary Figure 1. PD-1+/+ and PD-1−/− Treg cells are phenotypically similar. (A) Representative flow cytometry histograms depicting expression of FoxP3, helios, CD25, GITR and CTLA-4 on thymic Treg cells (gated on CD4SPFoxP3GFP+ cells) from 4- to 5-week old PD-1+/+Foxp3GFP and PD1−/−Foxp3GFP mice. (B) MFI (+ SD) for FoxP3, helios, CD25, GITR and CTLA-4 on thymic Treg cells from mice in (A). (C) Representative flow cytometry histograms depicting expression of FoxP3, helios, CD25, GITR and CTLA-4 on splenic Treg cells (CD4+FoxP3GFP+ cells) from the same mice studied in (A). (D) MFI (+ SD) for FoxP3, helios, CD25, GITR and CTLA-4 on splenic Treg cells from mice in (C). All data are from a single experiment with 3 mice per group (representative of 3 independent experiments with a total of 9 mice per group). p values were determined using a 2-tailed Student’s t test.
Acknowledgments
This work was supported by K23 CA133196 and R01 CA16670 (to J. Kline) from the National Cancer Institute.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Cao X. Regulatory T cells and immune tolerance to tumors. Immunol Res. 2010;46:79–93. doi: 10.1007/s12026-009-8124-7. [DOI] [PubMed] [Google Scholar]
- 13.Elpek KG, Lacelle C, Singh NP, Yolcu ES, Shirwan H. CD4+CD25+ T regulatory cells dominate multiple immune evasion mechanisms in early but not late phases of tumor development in a B cell lymphoma model. J Immunol. 2007;178:6840–6848. doi: 10.4049/jimmunol.178.11.6840. [DOI] [PubMed] [Google Scholar]
- 14.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, Kurahara C, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 24.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 26.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 27.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 28.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 32.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol. 2006;176:2808–2816. doi: 10.4049/jimmunol.176.5.2808. [DOI] [PubMed] [Google Scholar]
- 37.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 38.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 40.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 41.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Workman CJ, Collison LW, Bettini M, Pillai MR, Rehg JE, Vignali DA. In vivo Treg suppression assays. Methods Mol Biol. 2011;707:119–156. doi: 10.1007/978-1-61737-979-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 45.Hauet-Broere F, Unger WW, Garssen J, Hoijer MA, Kraal G, Samsom JN. Functional CD25- and CD25+ mucosal regulatory T cells are induced in gut-draining lymphoid tissue within 48 h after oral antigen application. Eur J Immunol. 2003;33:2801–2810. doi: 10.1002/eji.200324115. [DOI] [PubMed] [Google Scholar]
- 46.Pabst O, Bernhardt G. On the road to tolerance--generation and migration of gut regulatory T cells. Eur J Immunol. 2013;43:1422–1425. doi: 10.1002/eji.201243154. [DOI] [PubMed] [Google Scholar]
- 47.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serre K, Benezech C, Desanti G, Bobat S, Toellner KM, Bird R, Chan S, et al. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One. 2011;6:e20731. doi: 10.1371/journal.pone.0020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Bettini ML, Pan F, Bettini M, Finkelstein D, Rehg JE, Floess S, Bell BD, et al. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity. 2012;36:717–730. doi: 10.1016/j.immuni.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darce J, Rudra D, Li L, Nishio J, Cipolletta D, Rudensky AY, Mathis D, et al. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity. 2012;36:731–741. doi: 10.1016/j.immuni.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-kappaB-dependent manner. J Immunol. 2011;186:4609–4617. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo X, Qiu J, Tu T, Yang X, Deng L, Anders RA, Zhou L, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40:25–39. doi: 10.1016/j.immuni.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. PD-1+/+ and PD-1−/− Treg cells are phenotypically similar. (A) Representative flow cytometry histograms depicting expression of FoxP3, helios, CD25, GITR and CTLA-4 on thymic Treg cells (gated on CD4SPFoxP3GFP+ cells) from 4- to 5-week old PD-1+/+Foxp3GFP and PD1−/−Foxp3GFP mice. (B) MFI (+ SD) for FoxP3, helios, CD25, GITR and CTLA-4 on thymic Treg cells from mice in (A). (C) Representative flow cytometry histograms depicting expression of FoxP3, helios, CD25, GITR and CTLA-4 on splenic Treg cells (CD4+FoxP3GFP+ cells) from the same mice studied in (A). (D) MFI (+ SD) for FoxP3, helios, CD25, GITR and CTLA-4 on splenic Treg cells from mice in (C). All data are from a single experiment with 3 mice per group (representative of 3 independent experiments with a total of 9 mice per group). p values were determined using a 2-tailed Student’s t test.