Summary
NK cells are important for the control of vaccinia virus (VV) in vivo. Recent studies have shown that multiple pathways are required for effective activation of NK cells. These include both TLR-dependent and -independent pathways, as well as the NKG2D activating receptor that recognizes host stress-induced NKG2D ligands. However, it remains largely unknown what controls the upregulation of NKG2D ligands in response to VV infection. In this study, we first showed that IL-18 is critical for NK cell activation and viral clearance. We then demonstrated that IL-18 signaling on both NK cells and DCs is required for efficient NK cell activation upon VV infection in vitro. We further showed in vivo that efficient NK cell activation to VV is dependent on DCs and IL-18 signaling in non-NK cells, suggesting an essential role for NK cell-extrinsic IL-18 signaling in NK cell activation. Mechanistically, IL-18 signaling in DCs promotes expression of Rae-1, an NKG2D ligand. Collectively, our data reveal a previously unrecognized role for NK cell-extrinsic IL-18 signaling in NK cell activation through upregulation of NKG2D ligands. These observations may provide insights into the design of effective NK cell-based therapies for viral infections and cancer.
Keywords: NK cells, Vaccinia virus, IL-18
Introduction
NK cells play an important role in antiviral responses [1, 2]. NK cells are particularly critical for the control of poxviruses. Previous studies have shown that NK cells are activated in response to poxviral infections and mobilized to the site of infection, leading to effective viral control [3-6]. Vaccinia virus (VV) is the most studied member of the poxvirus family and is the live vaccine responsible for the successful elimination of smallpox [7]. Efficient NK cell activation represents the initial step in the control of VV infection. We have recently shown that efficient activation of NK cells and subsequent control of VV infection in vivo requires both TLR-dependent and -independent pathways, as well as the NKG2D activating receptor that recognizes host stress-induced NKG2D ligands [5, 6, 8]. However, while we recently showed how STAT1 could influence NKG2D ligand expression [9], it remains largely unknown what controls the upregulation of NKG2D ligands in response to VV infection.
IL-18, originally called IFN-γ-inducing factor [10], is a pro-inflammatory cytokine that plays an important role in innate and adaptive immune responses [11]. Studies have shown that IL-18 is critical for NK cell activation and function both in vitro and in vivo [12, 13]. This is accomplished by enhancing IFN-γ production by NK cells [14-16], as well as by stimulating NK cell proliferation and cytotoxicity [12, 13, 17]. IL-18 is also required for the activation of NK cells in response to infections with VV and MCMV and plays a critical role in antiviral defense [14, 15, 18]. But, how IL-18 contributes to the regulation of NK cell activation and function remains incompletely defined.
In a model of VV infection, we first showed that NK cell activation and function are severely compromised in IL-18 receptor-deficient (IL-18R−/−) mice, leading to impaired viral clearance. We then demonstrated that IL-18 signaling on both NK cells and accessory cells such as DCs, is critical for efficient NK cell activation in vitro. We further showed in vivo that efficient NK cell activation to VV is dependent on DCs and IL-18 signaling in non-NK cells, suggesting an essential role for NK cell-extrinsic IL-18 signaling in NK cell activation. Furthermore, IL-18R−/− DCs failed to upregulate the expression of Rae-1, an NKG2D ligand, which is required for efficient NK cell activation upon VV infection via the NKG2D pathway. Collectively, the data presented here suggest a critical role for NK cell-extrinsic IL-18 signaling in NK cell activation through upregulation of NKG2D ligands.
Results
IL-18 is required for efficient NK cell activation and VV clearance
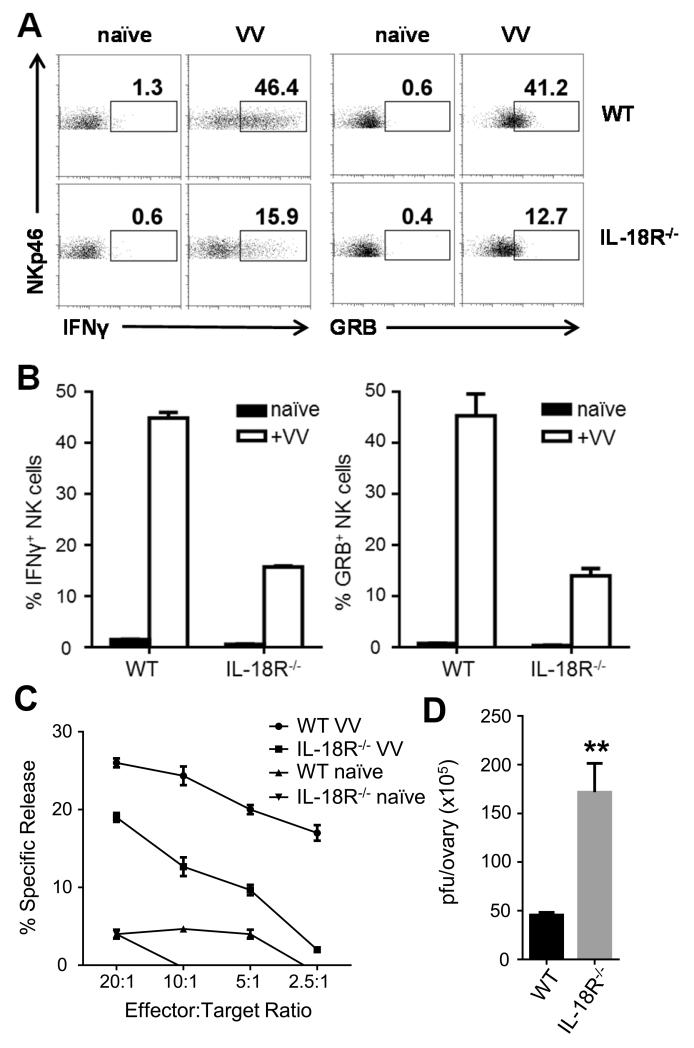
Previous studies have shown that recombinant IL-18 or IL-18-expressing VV promotes NK cell activation and VV clearance [14, 18]. However, it remains to be defined whether IL-18 signaling is necessary for NK cell activation and VV clearance. Here, we used IL-18R−/− to address this question. We first examined whether IL-18 signaling is critical for NK cell activation upon VV infection in vivo. WT or IL-18R−/− C57BL/6 mice were infected with VV intraperitoneally and splenic NK cells were analyzed 24 h later. WT NK cells produced significant amounts of GRB and IFN-γ (Fig. 1A, B), compared to the naïve control. However, the production of GRB and IFN-γ by IL-18R−/−NK cells was significantly (p < 0.01) reduced compared to the WT controls (Fig. 1A, B). For the measurement of IFN-γ production NK cells intracellularly, we used low doses of PMA and ionomycin (25ng/ml each) to re-stimulate the NK cells ex vivo in order to increase the sensitivity of intracellular IFN-γ detection. Without PMA/ionomycin restimulation, the production of IFN-γ – and the sensitivity of our assay - is reduced (Fig. S1B, C) compared to that with PMA/ionomycin (Fig 1A, B). However, the addition of PMA/ionomycin at these low doses (25ng/ml) did not stimulate naïve NK cells to produce IFN-γ, suggesting the specificity of the assay. We also found that VV-infected WT NK cells had enhanced lytic activity on YAC-1 target cells, compared to naïve control (Fig. 1C), whereas NK cells from VV-infected IL-18R−/− mice showed a significantly (p < 0.05) reduced lytic activity on YAC-1 cells (Fig. 1C). We further observed that the compromised NK cell activation in IL-18R−/− mice was associated with a significantly (p < 0.01) higher viral load compared to WT mice (Fig. 1D). Viral loads were measured in the ovaries where the virus has been known to accumulate early in the infection [6]. These results indicate that IL-18 signaling is crucial for NK cell activation and the innate immune control of VV infection in vivo.
FIGURE 1.
IL-18 is required for efficient NK cell activation and VV clearance. WT and IL-18R−/− C57BL/6 mice were infected by i.p. injection of 5 × 106 pfu VV or left uninfected (naïve). (A) 24 h after infection, splenocytes were assayed for IFN-γ and GRB production. Representative FACS plots showing the percentage of IFN-γ and GRB-positive NKp46+CD3-NK cells are shown. (B) The mean percentages ±s.e.m. of IFN-γ and GRB-positive NK cells (n=3 mice per group) are shown. Interaction term for two-way ANOVA is p<0.01 for IFN-γ and GRB. Data is representative of three independent experiments. (C) 48 h after infection, splenocytes were enriched for DX5+ cells and NK cell lytic activity was assayed on YAC-1 target cells by a standard 4-hour chromium release assay at different effector:target ratios. The mean percentages ±s.e.m. of specific lysis are indicated (n=3 per group). ANCOVA comparing infected WT and IL-18R−/− mice shows p<0.05. Data is representative of two independent experiments. (D) 48 h after infection, ovaries of female mice were harvested for measurement of viral load by plaque assay using TK-143B cells. Data represents the mean viral titer ±s.e.m. as pfu per ovary (n=4 per group). Data is representative of two independent experiments. ** signifies a p-value <0.01 on an unpaired student’s t test.
IL-18 signaling on both NK cells and DCs is required for NK cell activation to VV in vitro
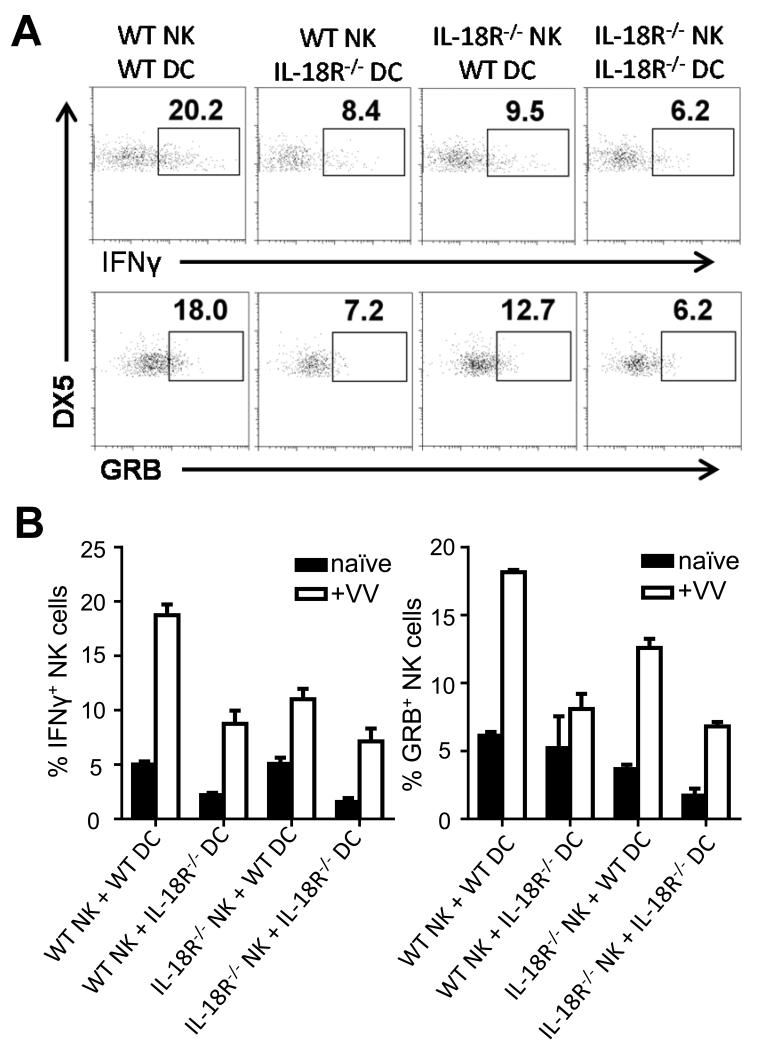
How does IL-18 signaling promote the activation of NK cells in response to VV infection? Previous studies in other models have suggested that IL-18 can act directly on NK cells for their activation [16, 19, 20]. To address this question, we utilized an in vitro DC-NK cell co-culture system [5]. Purified WT or IL-18R−/− NK cells were co-cultured with WT or IL-18R−/− bone marrow-derived CD11c+ DCs, followed by infection with VV. Under these conditions, IL-18 was produced in the culture in response to the virus (Fig. S2A) and both DCs and NK cells express the IL-18R (Fig. S2B). NK cells were analyzed for the production of IFN-γ and GRB 18 h post-infection. Our results showed that NK cell activation was compromised (p < 0.01) when IL-18R−/− NK cells were used for stimulation (Fig. 2A, B), suggesting that direct IL-18 signaling on NK cells is important for their activation upon VV infection. This is consistent with previous reports in other settings [16, 19, 20]. We further observed that when IL-18R−/− DCs were used for stimulation, the production of IFN-γ and GRB by WT NK cells in response to VV is also significantly (p < 0.01) reduced (2A, B), indicating IL-18 signaling on accessory DCs is also critical for NK cell activation. These results indicate that IL-18 signaling on both NK and accessory cells is required for the activation of NK cells by VV in vitro.
FIGURE 2.
IL-18 signaling on both NK cells and DCs is required for NK cell activation to VV in vitro. WT or IL-18R−/− NK cells were co-cultured with WT or IL-18R−/− bone-marrow derived CD11c+ DCs and stimulated with VV or left uninfected (naïve) for 18 h. (A) The percentage of IFN-γ and GRB-positive DX5+CD3− NK cells are shown on representative FACS plots. (B) The mean percentages ±s.e.m. of IFN-γ and GRB-positive DX5+CD3− NK cells are shown (n=3 wells per condition). Data is representative of three independent experiments. Interaction term for two-way ANOVA is p<0.01 for both IFN-γ and GRB.
NK cell-extrinsic IL-18 signaling is critical for NK cell activation to VV infection in vivo
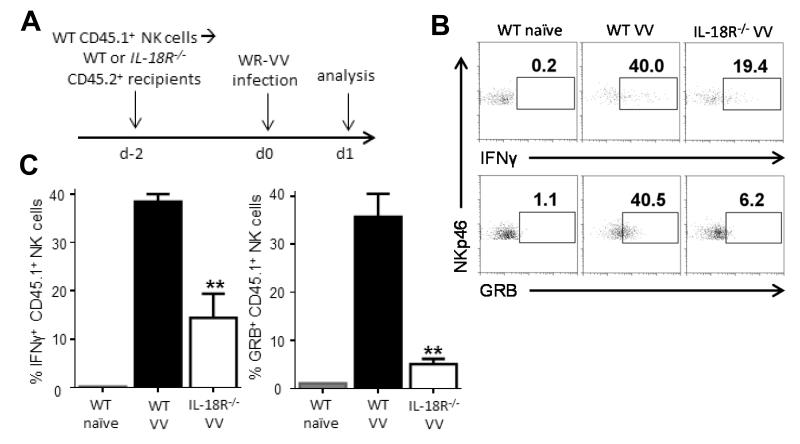
The observation that NK cell-extrinsic IL-18 signaling is also critical for NK cell activation in vitro led us to examine the physiological relevance of this finding in response to VV infection in vivo. NK cells were purified from CD45.1+ C57BL/6 mice by FACS sorting and adoptively transferred into CD45.2+ WT or IL-18R−/− recipient mice, followed by VV infection intraperitoneally 2 days later (Fig. 3A). The production of IFN-γ and GRB was assessed on the transferred CD45.1+ WT NK cells 24 h after infection. WT NK cells transferred into WT recipients were able to mount an adequate response after VV infection (Fig. 3B, C). However, the production of IFN-γ and GRB was significantly (p < 0.05) reduced when WT NK cells were transferred into IL-18R−/− recipients (Fig. 3B, C), suggesting that IL-18 signaling on non-NK cells is required for NK cell activation. Thus, our results demonstrate that NK cell-extrinsic IL-18 signaling is required for NK cell responses during VV infection in vivo.
FIGURE 3.
NK cell-extrinsic IL-18 signaling is critical for NK cell activation in vivo. (A) Five to 10 × 105 DX5+CD3− NK cells from the spleens of CD45.1+ WT C57BL/6 mice were purified by cell sorting and injected intravenously into WT or IL-18R−/− recipients, which are both CD45.2+. Two days after cell transfer, recipient mice were infected with 5 × 106pfu VV i.p. or left uninfected (naïve). 24 h after infection, spleens were assayed for IFN-γ and GRB production by donor (CD45.1+) NK cells. (B) The percentages of IFN-γ and GRB-positive NKp46+CD3− NK cells from the WT CD45.1+ donor NK cells are shown on representative FACS plots with recipient genotype and stimulation indicated above. (C) The mean percentages ±s.e.m. of IFN-γ and GRB-positive WT donor NKp46+CD3− NK cells are shown (n=2 infected mice). Interaction term for one-way ANOVA is p<0.01 for IFN-γ and GRB production. Data is representative of four independent experiments. ** signifies a p-value <0.05 on post-hoc unpaired student’s t test comparing WT donor NK cell activation between infected WT and IL-18R−/− recipients.
Dendritic cells are required for NK cell activation to VV in vivo
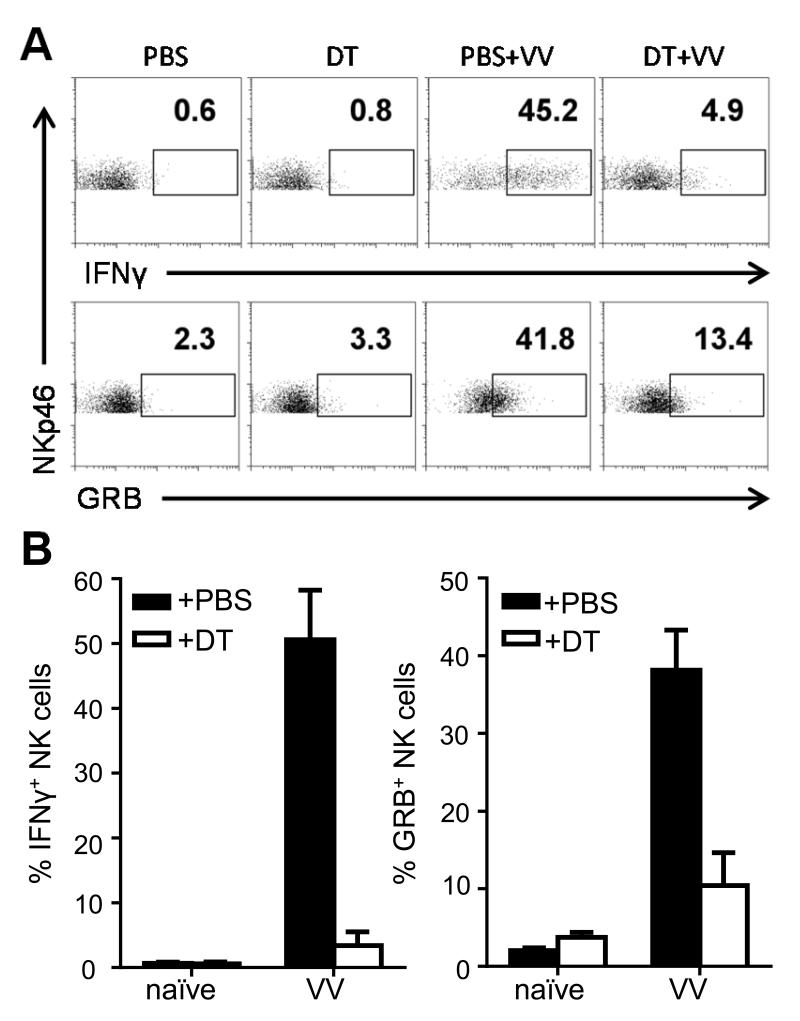
We next investigated which non-NK cell component is responsible for NK cell activation in response to VV infection in vivo. Conventional CD11c+ DCs have been shown to play a critical role in NK cell activation [21, 22]. Furthermore, our data in vitro also suggest that IL-18 signaling on DCs is critical for NK cell activation. Based on these findings, we hypothesized that DCs are required for the NK cell response to VV infection in vivo. To test this, we used mice carrying the CD11c-DTR transgene where the diphtheria toxin (DT) receptor is driven by the CD11c promoter so that it is expressed in CD11c+ conventional DCs [23]. This allows administration of DT to ablate DCs in vivo. While a few confounding cell populations do weakly express CD11c, upon administration of DT, we found DCs were efficiently depleted. The activation of NK cells assessed by the production of IFN-γ and GRB was significantly (p < 0.01) impaired in response to VV upon DC ablation (Fig. 4A, B). Relative and absolute numbers of NK cells were unaffected by DT treatment (data not shown). These results successfully replicate what others have shown [22] and suggest, in the context of our work, that DCs do play an important role in NK cell activation in response to VV infection in vivo.
FIGURE 4.
Dendritic cells are required for NK cell activation to VV in vivo. CD11c-DTR+ transgenic mice were treated with diphtheria toxin (DT) and the next day were infected i.p. with 5 × 106pfu VV or left uninfected (naïve). Control mice were injected with PBS. 24 h after infection, splenocytes were assayed for IFN-γ and GRB production. In the naïve mice, CD11c+ DCs, but not NK cells or total splenocytes, were depleted in mice carrying the transgene (data not shown). (A) Representative FACS plots with the percentages of IFN-γ and GRB-positive NKp46+CD3− NK cells are shown. (B) The mean percentages ± s.e.m. of IFN-γ and GRB-positive NKp46+CD3− NK cells (n=2 mice per group). Interaction term for two-way ANOVA is p<0.01 for IFN-γ and GRB. Data is representative of two independent experiments.
IL-18 signaling on DCs is required for Rae-1 upregulation to VV in vivo
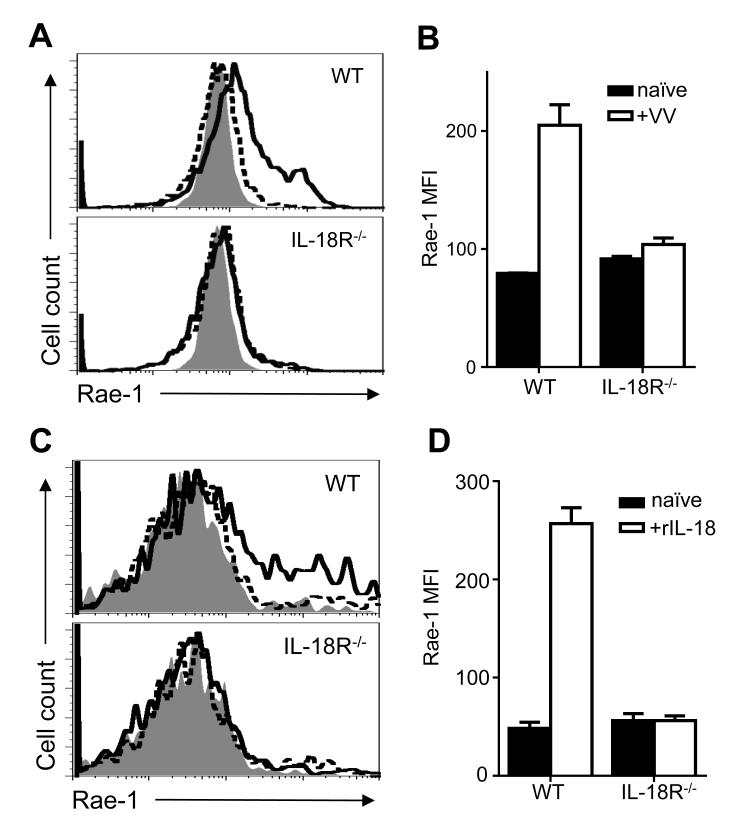
The requirement of IL-18 signaling on DCs for NK cell activation prompted us to investigate the underlying mechanism. We have previously shown that the interaction between NKG2D ligand expressed on DCs and NKG2D on NK cells is critical for NK cell activation to VV infection [6]. NKG2D recognizes a diverse class of stress-induced host ligands that are all poorly expressed on healthy adult cells, but become upregulated during instances of cellular stress, such as viral infection [21, 24]. We hypothesized that upregulation of NKG2D ligands upon VV infection is dependent on IL-18 signaling on DCs. To test this, we examined the expression of the NKG2D ligand, Rae-1, on splenic CD11c+B220−CD11b+ conventional DCs in WT and IL-18R−/− mice 24 h after VV infection. We found a significant (p < 0.01) upregulation of Rae-1 expression in infected WT DCs compared to the uninfected naïve control (Fig. 5A, B). However, the upregulation of Rae-1 expression is significantly (p < 0.01) reduced in DCs from IL-18R−/− mice (Fig. 5A, B). To further demonstrate that IL-18 alone can directly act on DCs to upregulate Rae-1, we found that rIL-18 can be sufficient to upregulate Rae-1 expression on WT, but not IL-18R−/− DCs (p < 0.0001) in vitro (Fig. 5C). Thus, while IL-18 signaling on another cell-type may be contributing NKG2D ligand upregulation on the DC, DC-intrinsic IL-18 signaling can upregulate Rae-1 expression. These results suggest that IL-18 signaling on DCs is required for upregulation of NKG2D ligand expression, which in turn promotes NK cell activation via the NKG2D pathway.
FIGURE 5.
IL-18 signaling on DCs is required for Rae-1 upregulation to VV in vivo. WT or IL-18R−/− mice were infected with 5 × 106pfu VV i.p. or left uninfected (naïve). 24 h after infection, splenocytes were assayed for Rae-1 surface expression on CD11c+CD11b+B220− cDCs. (A) Histograms compare Rae-1 expression on cDCs from infected (thick line) and naïve (dotted line) WT (top panel) and IL-18R−/− (bottom panel) mice. Shaded histogram indicates isotype control. (B) The mean fluorescent intensity ±s.e.m. of Rae-1 on cDCs is shown (n=3 infected mice). Data is representative of three independent experiments. Interaction term for two-way ANOVA is p<0.01. (C) Histograms compare Rae-1 expression on WT (top panel) and IL-18R−/− (bottom panel) bone marrow-derived DCs cultured with 5 ng/mL rIL-18 (thick line) or left untreated (dotted line) for four days. Shaded histogram indicates isotype control (D) The mean fluorescent intensity ±s.e.m. of Rae-1 on bone-marrow derived DCs is shown (n=3 wells). Data is representative of three independent experiments. Interaction term for two-way ANOVA is p<0.0001.
Discussion
In this report, we showed that IL-18 is critical for NK cell activation upon VV infection and the subsequent viral clearance in vivo. In addition to the requirement of NK cell-intrinsic IL-18 signaling for NK cell activation, we revealed a previously unknown mechanism by which IL-18 activates NK cells through DCs by promoting the expression of NKG2D ligands, which is required for NK cell activation via the NKG2D pathway. We showed that this NK cell-extrinsic IL-18 signaling is critical for NK cell response to VV infection.
Although IL-18 had been shown to play an important role in NK cell activation [12, 13] and antiviral defense [14, 15, 18], how IL-18 promotes NK cell activation and function during viral infections remained incompletely defined. This is the first report showing that IL-18 is critical for NK cell activation and the clearance of VV infection in vivo. Furthermore, we provided evidence that IL-18 signaling on both NK cells and DCs are important for NK cell activation. The NK cell-intrinsic role of IL-18 is consistent with previous reports in other models that IL-18 acts directly on NK cells for their activation [16, 19, 20]. However, the NK cell-extrinsic role of IL-18 in NK activation upon viral infection represents a novel finding. This observation not only advances our understanding for how IL-18 works in promoting NK cell activation, but more importantly, may inform clinical applications of IL-18 in NK cell-based therapies. Since we have shown that IL-18 action on DCs is required to fully activate NK cells, new treatments should exploit the same mechanism in order to achieve a maximal therapeutic effect.
NK cell activation in response to viral infection is regulated by engagement of NK activating receptors such as Ly49H, NKp46 and NKG2D [24-26]. We have previously shown that the interaction between NKG2D ligands on accessory cells such as DCs, and NKG2D on NK cells is critical for NK cell activation and function in the setting of VV infection [6]. However, it remained largely unclear how the upregulation of NKG2D ligands on DCs in response to VV infection is regulated. In this study, we provided evidence that IL-18 signaling on DCs is critical for upregulating NKG2D ligand expression upon VV infection. Recent studies have shown that TLR stimulation on macrophages can upregulate NKG2D ligand expression [27, 28]. Interestingly, both TLR and IL-18R signaling are mediated by the common adaptor, MyD88 [29, 30]. In addition, we have recently shown that STAT1 signaling in DCs is also critical for the upregulation of NKG2D ligands [9], although this finding was in the 129 background. How IL-18R/TLR signaling promotes the upregulation of NKG2D ligands remains unknown. Thus, future studies are needed to define mechanisms responsible for IL-18R/TLR-mediated upregulation of NKG2D ligands. Similarly, a potentially synergistic role of STAT1 activation and IL-18/TLR signaling in NKG2D ligand upregulation requires further investigation.
It is important to note, however, that other accessory cells may also play roles in receiving IL-18 signals to activate NK cells via NKG2D ligand upregulation. Our data shows a clear role for DCs in receiving IL-18 signals to activate NK cells via NKG2D and, using the DTR ablation model, we have shown that NK cell activation depends on the presence of DCs in vivo. However, we have not entirely excluded a role for other accessory cells, such as macrophages, in contributing to NK cell activation by upregulating ligands to NKG2D in response to IL-18.
This work should also not exclude other mechanisms for cell-extrinsic IL-18 signaling in NK cell activation. We have demonstrated one route for how IL-18, acting on DCs, can boost NK cell activation by control of NKG2D ligand expression. However, this does not completely rule out other putative mechanisms, including IL-18-induced production of other pro-inflammatory cytokines known also known to play roles in NK cell activation and proliferation such as IL-12 and IL-15. Furthermore, while IL-18 can upregulate Rae-1 expression on DCs in vitro, whether direct IL-18 signaling on DCs causes Rae-1 upregulation in vivo has not been conclusively demonstrated and IL-18 could conceivably act through another cell to induce the upregulation of NKG2D ligands on DCs.
In conclusion, we have shown that IL-18 is required for the innate immune control of VV in vivo. This is mediated by promoting the activation and effector function of NK cells. This is dependent on IL-18 signaling on both NK cells and DCs in response to VV in vitro. Furthermore, we showed in vivo that efficient NK cell activation to VV is dependent on DCs and IL-18 signaling in non-NK cells, suggesting an essential role for NK cell-extrinsic IL-18 signaling in NK cell activation. In addition, IL-18 signaling on DCs promotes expression of Rae-1, an NKG2D ligand. Taken together, our results reveal a previously unknown role for NK cell-extrinsic IL-18 signaling in NK cell activation through upregulation of NKG2D ligands and may provide insights into the design of effective NK cell-based therapies for viral infections and cancer.
Materials and Methods
Mice
CD45.1+ and CD45.2+ C57BL/6 mice were obtained from the Frederick National Laboratory for Cancer Research at the National Cancer Institute (Frederick, MD). IL-18R−/− and CD11c-DTR mice on the C57BL/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME). Groups of 8- to 12-week-old mice were selected for this study. All experiments involving the use of mice were done in accordance with protocols approved by the Institutional Animal Care and Use Committee of Duke University (Durham, NC).
Vaccinia virus
The Western Reserve (WR) strain of VV was purchased from American Type Culture Collection (ATCC, Manassas, VA). VV was grown in TK-143B cells (ATCC) and purified by a 35% sucrose cushion as described [8]. The titer was determined by plaque assay on TK-143B cells and VV was stored at −80°C until use. For in vitro studies, VV was used at an MOI of 1. For in vivo studies, 5 × 106 pfu of live VV in 0.05 mL of 1 mM Tris pH 9.0 was injected intraperitoneally.
Antibodies and flow cytometry
PE-conjugated anti-CD49b (clone DX5), PE-Cy5-conjugated anti-CD3ε (clone 145-2C11), FITC-conjugated IFN-γ (clone XMG1.2), FITC-conjugated CD11c (clone HL3), APC-conjugated IFN-γ (clone XMG1.2), APC-conjugated B220 (clone RA3-6B2), and Streptavidinconjugated APC were purchased from BD Biosciences. PE-conjugated anti-NKp46 (clone 29A1.4), Biotin-conjugated CD45.1 (clone A20), FITC-conjugated GRB (clone NGZB), PECy5-conjugated CD11b (clone M1/10), PE-conjugated Rae-1 (clone CX1), PE-conjugated rat IgG2b (clone eB149/10H5), PE-conjugated hamster IgG (clone eBio299Arm), and PE-conjugated rat IgG2a (clone eBR2a) were purchased from eBioscience.
To assess intracellular production of IFN-γ, splenocytes were incubated with 25ng/mL PMA, 25ng/mL ionomycin, and 5μg/mL Brefeldin A containing Golgi-Plug (BD Biosciences) for 4 hours at 37°C. To assess intracellular production of GRB, splenocytes were cultured with 5μg/mL Brefeldin A containing Golgi-Plug alone for 4 hours at 37°C. Following incubation, cells were stained for surface molecules, permeablized using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions and stained for intracellular molecules. FACS Canto (BD Biosciences) was used for flow cytometry event collection, which was analyzed using FlowJo software 9.5.3 (Tree Star, Ashland, OR).
NK cell cytotoxicity assay
NK cell cytotoxicity was performed by a standard 4-hour chromium-51 release assay as described [5]. Splenocytes were enriched for DX5+ NK cells by positive selection with PE-conjugated anti-DX5 and anti-PE microbeads (Miltenyi Biotec). YAC-1 target cells (ATCC), which are susceptible to NK cell-mediated cytotoxicity, were labeled with chromium-51 and then incubated with DX5+ cells at different effector:target ratios for 4 hours at 37°C. The specific release of chromium-51 into the supernatant, measured by counts per minute, assessed target cell lysis with no effector (spontaneous lysis) wells and 1% SDS (maximum lysis) wells used as controls. Specific release was calculated as (experimental – spontaneous)/(maximum-spontaneous) x100.
Measurement of viral load
Viral load in the ovaries was measured by plaque-forming assay as described [5]. Two days post-infection with 5 × 106 pfu VV, ovaries were harvested into 10 mM Tris pH9.0 and stored at −80°C until use. Ovarian viral load was then measured by plaque-forming assay. Ovaries were first homogenized and freeze-thawed three times. Serial dilutions of the lysate were added to confluent TK-143B cells and the number of plaques was counted using crystal violet staining after two days of culture at 37°C.
NK cell-DC co-culture
NK-DC co-culture was performed as described [6]. Briefly, splenocytes were enriched for NK cells by positive selection with PE-conjugated anti-DX5 and anti-PE microbeads (Miltenyi Biotec). DCs were generated from bone-marrow cells as described after five days of culture with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) (R&D Systems) [6]. DX5+ NK cells (2 × 105) were then cultured with the bone-marrow-derived DCs (105) in a ratio of 2:1 in the presence or absence of VV at an MOI of 1 overnight at 37°C. IL-18 production was assayed by harvesting the supernatant for analysis by ELISA (eBioscience). IL-18R expression was determined by sort-purifying NK cells or DCs into TRI Reagent (Sigma) for RNA purification and semi-quantitative RT-PCR (Promega) PCR for IL-18R. Forward primer 5′-AGAGCTTCGTCTTGGTGAGAA-3′ and reverse primer 5′-TACCTGTTAGTGTCTCGTCTCTT-3′ were purchased from Integrated DNA Technologies to assess IL-18R expression. Recombinant mouse IL-18 protein was purchased from Life Technologies.
NK cell transfer experiment
DX5+CD3− NK cells were first enriched from the naïve splenocytes of CD45.1+mice by positive selection with PE-conjugated anti-DX5 and anti-PE microbeads (Miltenyi Biotec) and then purified via flow cytometry sorting on a FACS DiVA with a purity >95%. 5 to 10 × 105 NK cells were transferred by tail-vein intravenous injection into recipient CD45.2+ C57BL/6 or IL-18R−/−mice. After two days, mice were infected by injection of 5 × 106 pfu VV, i.p. or left naïve and sacrificed 24 hours post-infection for analysis.
DC Ablation
CD11c-DTR+ mice were ablated of their CD11c+ DCs by a single i.p. injection of 4 ng diphtheria toxin (DT, Sigma) per gram of body weight (~100 ng/mouse) in 100ul PBS as described [23]. Control mice received PBS injections. One day after DT administration, mice were infected by i.p. injection of 5 × 106 pfu VV or left naïve and sacrificed 24 hours post-infection for analysis. We observed ~90% reduction in CD11c+ DCs upon DT administration and could not appreciate a concomitant effect on the relative or absolute number of NK cells.
Statistical Analysis
All data are presented as means and standard errors. All indicated statistical tests, including unpaired student’s t test, ANOVA, and ANCOVA, were performed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA). Significance was assumed at p<0.05.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health grants AI083000, CA136934, and CA047741 (to Y.Y.). Additional support was from the National Institutes of Health grants T32GM00717 and T32CA009111 (to J.D.B.).
Non-standard abbreviations
- VV
Vaccinia virus
- GRB
Granzyme B
- DT
diphtheria toxin
- IL-18R−/−
IL-18R-deficient
Footnotes
Conflicts of Interest The authors have no conflicts of interest to declare.
References
- 1.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 4.Natuk RJ, Welsh RM. Accumulation and chemotaxis of natural killer/large granular lymphocytes at sites of virus replication. J Immunol. 1987;138:877–883. [PubMed] [Google Scholar]
- 5.Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- 6.Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenner F HD, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- 8.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-{beta} Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin C, Huang X, Yang Y. Both NK Cell-Intrinsic and -Extrinsic STAT1 Signaling Are Required for NK Cell Response against Vaccinia Virus. J Immunol. 2013;191:363–368. doi: 10.4049/jimmunol.1202714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 11.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 13.Dao T, Mehal WZ, Crispe IN. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J Immunol. 1998;161:2217–2222. [PubMed] [Google Scholar]
- 14.Tanaka-Kataoka M, Kunikata T, Takayama S, Iwaki K, Ohashi K, Ikeda M, Kurimoto M. In vivo antiviral effect of interleukin 18 in a mouse model of vaccinia virus infection. Cytokine. 1999;11:593–599. doi: 10.1006/cyto.1998.0453. [DOI] [PubMed] [Google Scholar]
- 15.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 16.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French AR, Holroyd EB, Yang L, Kim S, Yokoyama WM. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine. 2006;35:229–234. doi: 10.1016/j.cyto.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Gherardi MM, Ramirez JC, Esteban M. IL-12 and IL-18 act in synergy to clear vaccinia virus infection: involvement of innate and adaptive components of the immune system. J Gen Virol. 2003;84:1961–1972. doi: 10.1099/vir.0.19120-0. [DOI] [PubMed] [Google Scholar]
- 19.Humann J, Lenz LL. Activation of naive NK cells in response to Listeria monocytogenes requires IL-18 and contact with infected dendritic cells. J Immunol. 2010;184:5172–5178. doi: 10.4049/jimmunol.0903759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 22.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guma M, Angulo A, Lopez-Botet M. NK cell receptors involved in the response to human cytomegalovirus infection. Curr Top Microbiol Immunol. 2006;298:207–223. doi: 10.1007/3-540-27743-9_11. [DOI] [PubMed] [Google Scholar]
- 25.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 26.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 27.Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 28.Eissmann P, Evans JH, Mehrabi M, Rose EL, Nedvetzki S, Davis DM. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J Immunol. 2010;184:6901–6909. doi: 10.4049/jimmunol.0903985. [DOI] [PubMed] [Google Scholar]
- 29.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.