Abstract
During gestation, development proceeds at a pace that is unmatched by any other stage of the lifecycle. For these reason the human fetus is particularly susceptible not only to organizing influences, but also to pathogenic disorganizing influences. Growing evidence suggests that exposure to prenatal adversity leads to neurological changes that underlie lifetime risks for mental illness. Beginning early in gestation, males and females show differential developmental trajectories and responses to stress. It is likely that sex-dependent organization of neural circuits during the fetal period influences differential vulnerability to mental health problems. We consider in this review evidence that sexually dimorphic responses to early life stress are linked to two developmental disorders: affective problems (greater female prevalence) and autism spectrum disorder (greater male prevalence). Recent prospective studies illustrating the neurodevelopmental consequences of fetal exposure to stress and stress hormones for males and females are considered here. Plausible biological mechanisms including the role of the sexually differentiated placenta are discussed. We consider in this review evidence that sexually dimorphic responses to early life stress are linked to two sets of developmental disorders: affective problems (greater female prevalence) and autism spectrum disorders (greater male prevalence).
Keywords: sex differences, prenatal, stress, early adversity, autism, anxiety, depression, fetal programming, placenta, epigenetic
There is growing recognition that there are sexually dimorphic responses to stress and adversity that contribute to sex-specific vulnerability to mental disease (Bale, 2009; Goldstein, 2006; Nugent et al., 2011). Some types of stress at specific times influence males’ behavior more than females, and other type of stress at other times influence females’ behavior more than males. Further, the same stressor may have different consequences for male and female behavior (Schulz et al., 2011). Sex-specific responses to early life stress likely contribute to sexually dimorphic vulnerability to mental disease. There is clear evidence that males are more vulnerable to developmental disorders such as autism whereas females carry an increased susceptibility to affective problems including anxiety and mood disorders. The likelihood rarely is considered that these sex differences are determined very early in fetal development.
We consider here evidence for sexually dimorphic responses to prenatal adversity as it relates to later pathology. We will then discuss the possibility that differential responses to early adversity may be a key contributing factor to sex specific risk for psychopathology focusing on development of risk for affective problems (greater female prevalence) and autism (greater male prevalence).
It is tempting to think of the two major risk categories covered in this theoretical review - - autism for males and affective problems for females - - as mirror images of each other. However, sophisticated views of the sources of sex differences in the brain (Arnold, 2012; Levine, 2014 in press) make such mirror image, “yin/yang” scenarios unlikely. Even though one of us has constructed a theory based on testosterone actions on CNS arousal systems (Pfaff et al., 2011), that view carries its own complexity, and in addition is accompanied by at least three other routes of causation that result in sexual dimorphisms and are described below. First, the testosterone-based theory must encompass the possibility that the steroid hormone actually acting on nerve cells in question may be a testosterone metabolite such as estradiol or dihydrotestosterone; and, further that the nerve cells thus affected may range widely outside of CNS arousal systems as presently understood. Second, explanations of sex differences in brain based on testosterone may be accompanied by explanations (i.) that females have more than one X-chromosome to be fully or partially inactivated; (ii.) that the actual mechanisms for X-chromosome inactivation may differ between males and females resulting in a different pattern of X-linked genes affect; and (iii.) that in females, an inactivated X-chromosome may act as a ‘sink’ that drains chromatin-modifying chemicals (such as histone acetylating or methylating enzymes) from other chromosomes. Even though data have accumulated for testosterone-dependent mechanisms, this multiplicity of potential mechanisms make it virtually impossible to argue that male vulnerabilities comprise the flip side of female vulnerabilities to neurodevelopmental disorders.
The current review considers evidence that sex-specific susceptibility to mental illness originates in the fetal period. In order to explore this possibility we consider two developmental disorders that both are sexually dimorphic and have their origin early in life. We consider the possibility that sex differences in fetal responses to adversity may contribute to our understanding of sex-specific susceptibility to developmental disorders. We selected ASD and affective disorders as illustrative examples of the links between sex-specific responses to early adversity and sexually dimorphic disease risk. Our goal is not to provide a comprehensive review of fetal programing of sex-differences in mental health outcomes, but rather to highlight the importance of evaluating sex differences as early as the fetal period in order to understand sex differences in disease risk.
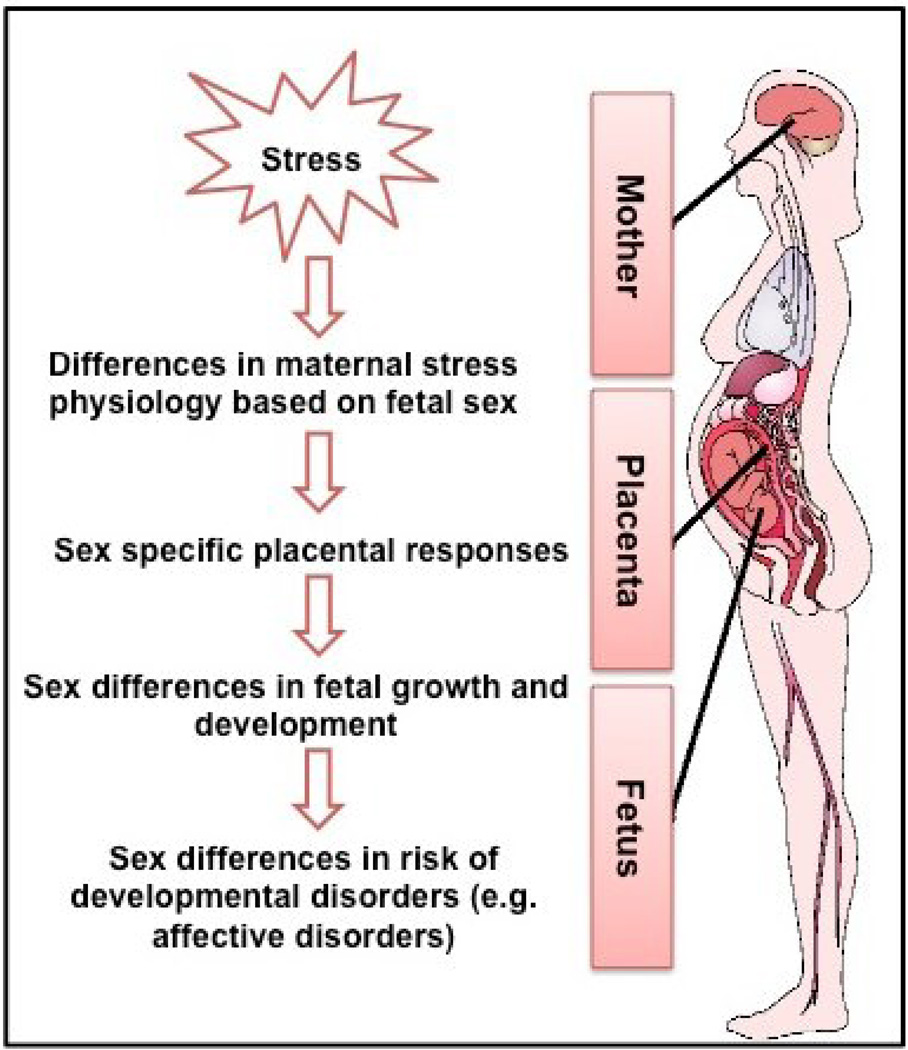
This review first will briefly review the susceptibility of the fetus to programming influences related to both fetal development and changes in maternal stress physiology. Next we will consider the placenta as a key pathway that may contribute to sex differences in neurodevelopmental disorders. We will then discuss sex differences in fetal responses to adversity. We briefly review the animal literature and then focus on prenatal factors contributing to sex differences in affective problems and ASD. An overview of our approach is illustrated in Figure 1.
Figure 1.
illustrates our hypothesis that there are sex-specific responses to prenatal stress that may contribute to sex differences in risk for developmental disorders. In humans, fetal sex has been associated with differences in maternal stress physiology, placental physiology, and fetal growth and development. These processes may affect the consequences of gestational stress exposure, and contribute to sex differences in susceptibility to mental disease
I. Background
Fetal programming
The fetal period in the life cycle is unmatched by any other in growth and development, and it is the period in the human lifespan that is most vulnerable to both organizing and disorganizing influences. The fetal programming model posits that during periods of rapid development or change the organism is susceptible to environmental influences and that these influences exert persisting consequences for health and disease risk (Barker, 1998). It is becoming increasingly evident that intrauterine conditions contribute to the development of many of the conditions that contribute to disease burden worldwide and that understanding the origins of these diseases requires a comprehensive approach that considers not only genetic risk but also the role of the early environment.
Human fetal brain development
The transformation from a zygote to the human newborn is a dramatic process involving rapid cell division and differentiation to produce all biological systems including, the brain. Human brain development begins early in gestation and follows an orchestrated and protracted series of events including processes such as proliferation, migration, and differentiation. During gestation neurogenesis proceeds at a rate of 250,000 cells per minute (Cowan, 1979). Between 8 and 16 gestational weeks, neurons migrate to form the sub plate zone and await connections from afferent neurons originating in the thalamus, basal forebrain and brainstem. Once neurons reach their final destination, they arborize and branch in an attempt to establish appropriate connections (Sidman and Rakic, 1973). Cells aggregate in the outer cerebral wall to form the cortical plate, which eventually will become the cerebral cortex. By gestational week 20, axons form synapses with the cortical plate, and cortical circuits begin to organize (Bourgeois et al., 1994; Kostovic et al., 2002). Remarkably, by gestational week 28, the number of neurons in the human fetal brain is 40% greater than that in the adult (Bourgeois, 1997; Bourgeois et al., 1994). During the last third of human pregnancy the fetal brain forms secondary and tertiary gyri, and exhibits neuronal differentiation, dendritic arborization, axonal elongation, synapse formation and collateralization, and myelination (Bourgeois, 1997; Volpe, 2008). Synaptogenesis accelerates during the third trimester to a rate of 40,000 synapse per minute (Bourgeois et al., 1994). Transcriptome studies indicate that the majority of genes are expressed in the human brain during the fetal period and that there is approximately two-fold greater gene expression in the fetal brain compared with the adult (Johnson et al., 2009). Thus, prenatal life is a time of enormous neurological change and because of that the intrauterine environment will shape the development of the fetal nervous system. The exceptionally rapid pace of the fetal brain renders it susceptible to environmental influences including exposure to stress. We consider in this paper sexually dimorphic patterns of fetal development and responses to adversity that may contribute to sex specific vulnerability to mental illness.
Changes in the maternal HPA and placental axis over the course of pregnancy
The hypothalamic pituitary adrenocortical axis is one of the major stress responsive systems that may play a critical role in fetal programing. In the non-pregnant state, stress activates hypothalamic corticotropin releasing hormone (CRH), which travels down the median eminence and stimulates the synthesis and release of adrenocorticotropic hormone (ACTH) from the anterior pituitary. The release of ACTH into the blood stream triggers the release of cortisol from the adrenal cortex. This hypothalamic–pituitary–adrenocortical (HPA) axis is controlled through negative feedback regulation and thus presence of high levels of cortisol will decrease HPA axis activity. This system is altered radically during pregnancy because the placenta expresses the genes for CRH (hCRH mRNA) and proopiomelanocortin, the precursor for ACTH and b-endorphin. All of these stress hormones increase across gestation. The increase in placental CRH is particularly dramatic. Placental CRH production increases forty-fold from the first to the third trimester (Sandman et al., 2006) with levels in maternal circulation reaching those only observed within the hypothalamic-pituitary portal system during stress. This is in contrast to the non-pregnant state in which CRH immunoreactivity in the plasma is very low or undetectable (Lowry, 1993). Placental CRH is identical to hypothalamic CRH in structure, immunoreactivity, and bioactivity. There is, however, a crucial difference in its regulation. In contrast to the negative feedback regulation of hypothalamic CRH, cortisol stimulates the expression of hCRHmRNA in the placenta, establishing a positive feedback loop that allows for the simultaneous increase of placental CRH, ACTH, and cortisol over the course of gestation. The normative increase in stress responsive hormones such as cortisol and placental CRH plays an important role in the regulation of pregnancy as well as facilitating maturation of the fetus (Davis and Sandman, 2010). However, because of the positive feedback between cortisol and placental CRH, the effects of maternal stress on the fetus may be amplified with potentially negative consequences for the developing fetus. The fetus is partially protected from the consequences of maternal cortisol by the placental enzyme 11β -HSD2 which oxidizes cortisol into its inactive form, cortisone. It is however, the case that maternal and fetal cortisol levels are correlated and thus increases in maternal cortisol may have consequences for the fetus (Gitau et al., 1998). Further, factors such as maternal Glycyrrhizin consumption (as in licorice) (Raikkonen et al., 2011) and maternal stress and anxiety (O'Donnell et al., 2012) down regulate 11β -HSD2 further exposing the fetus to maternal cortisol.
Few studies have evaluated whether fetal sex is associated with differential regulation of the HPA and placental axis during gestation. Recent evidence by DiPietro and colleagues (2011) illustrates that women carrying male fetuses have higher cortisol levels during the early third trimester. In contrast, in the later part of the third trimester women carrying female fetuses had higher cortisol levels. The authors suggest that these differences in cortisol profiles may be related to maturational differences between male and female fetuses (Buss et al., 2009; DiPietro et al., 2011). The sex of the fetus may interact with the maternal HPA axis and contribute to sex specific consequences of early adversity.
II. Sex-specific Responses of the Placenta
Sexually dimorphic patterns in response to stress may be developed, or as suggested above, genetically determined or programmed very early in gestation. The placenta, a fetal organ, produces sexually dimorphic responses to intrauterine stress exposure (Clifton, 2010; O'Connell et al., 2013). The placenta is an interface between the mother and fetus, plays a key role in the regulation of growth and development, and affects fetal programing of health and disease. Further, both animal and new human data illustrate the role of the placenta in moderating the consequences of maternal stress for the fetus (Glover et al., 2009; Jensen Pena et al., 2012). We review here evidence that the placenta plays a key role in sex-specific programing of the human fetus and raise the possibility that this may contribute to sexually dimorphic disease risk. Males are significantly more vulnerable to threats to viability throughout gestation. The accelerated growth of the male as compared to the female fetus has been proposed as one of the factors contributing to greater male susceptibility (Clifton and Murphy, 2004). The placenta is a major determinant of fetal growth and regulates growth in a sex specific fashion. The male and female placenta have different profiles of energy metabolism including glycogen storage and metabolism throughout gestation (O'Connell et al., 2013). Sex differences additionally are present in the placental response to stress or adversity. The role of the placenta in both sex specific growth trajectories and responses to stress may contribute to sex differences in disease risk across the lifespan, in addition to the well documented association with morbidity and mortality during the fetal and infant periods.
The female placenta is more responsive to stress signals and this greater sensitivity may allow the female fetus to adapt to intrauterine adversity increasing the likelihood of survival (Clifton, 2010). For example, when the intrauterine condition is not optimal, as with chronic maternal asthma, the female fetus adapts by slowing its growth trajectory. In contrast, the male fetus continues to grow normally. The male fetuses continued investment in growth increases vulnerability to subsequent adversity. When maternal asthma worsens with acute exacerbation there is an increased incidence of intrauterine growth restriction (IUGR), preterm birth and stillbirth among male fetuses (Murphy et al., 2006; Murphy et al., 2003). Clifton and colleagues have proposed that the adaptive strategy implemented by male fetuses allows them to continue to grow normally even in an adverse intrauterine environment (Clifton, 2010). This strategy has an associated cost as the male fetus is more vulnerable to subsequent stressors and may contribute to higher rates of stillbirth, preterm birth and neonatal morbidly and mortality among males. Females in contrast, adapt to a hostile intrauterine environment by slowing their growth and thus have the resources to survive subsequent adversity. The more nuanced responses of the female fetus to early adversity clearly benefits survival, but also may be associated with consequences that emerge later in development (Davis et al., 2011a).
One of the mechanisms contributing to sex specific programming of the fetus may be sex-differences in placental gene expression. Sexually dimorphic patterns of gene expression have been reported for placental genes in both rodents and humans (Gabory et al., 2013). Evidence indicates that the female placenta may induce non-random X chromosome inactivation during the early stages of implantation in an adverse intrauterine environment. This non-random X chromosome inactivation may confer an adaptive advantage to the female fetus in response to adversity especially with respect to X-linked diseases (Reik and Lewis, 2005). Sex differences in gene expression are present beyond X and Y linked genes. Interestingly, consistent with the greater intrauterine adaptation of the female fetus, evidence exists that the female placenta produced a greater epigenetic response to stress as compared to the male placenta (Osei-Kumah et al., 2011). Clifton and colleagues report that in response to stress the expression of 59 placental genes were altered in the female placenta, but only 6 in the male placenta.
Sexually dimorphic placental responses to glucocorticoid administration provide an illustrative example of the role of the placenta in sex specific programing of the fetus. Glucocorticoids routinely are administered to women in preterm labor in order to promote fetal lung maturation. Glucocorticoid administration differentially affects both glycogen storage and gene expression in the male and female mouse placenta. Glucocorticoid exposure leads to a decrease in glycogen storage in the female placenta, perhaps suggesting increased mobilization of glycogen for use by the fetus. No such alteration is seen in the male placenta (O'Connell et al., 2013). This sex difference in fetal energy availability may be one of the mechanisms that contribute to the greater female adaptability to adversity. Because the male fetus does not adapt its energy storage and metabolism in response to stress signals it may have limited ability to adjust to adversity, and is at greater risk for subsequent morbidity and mortality. Sex specific placental responses to glucocorticoid administration additionally are observed in human populations (Stark et al., 2009). Especially noteworthy is the possibility that the biochemistry of the placenta may provide early biomarkers of maternal stress, and thus provide early warning of changes in neurodevelopmental processes. For example Howerton and colleagues (2013) measured O-linked-N-acetylglucosamine transferase and found that this enzyme was lower in males and further reduced by prenatal stress (Howerton et al., 2013). We propose that sex differences in placental responses to stress play an important role in sex-specific fetal programming. The adaptive responses to intrauterine adversity displayed by the female fetus may result in both more variability among females and more subtle and specific risk for developmental impairments.
Consistent with this hypothesis is evidence that placental responses to stress are associated with neurodevelopment. Methylation patterns of stress-response genes have been associated with neurodevelopment in the neonate (Meilleur and Fombonne, 2009). New evidence suggests that associations with outcome are sex-specific. For example placental leptin promoter DNA methylation is associated with neurobehavioral impairments among male, but not female newborns (Hamdan et al., 2009). These exciting studies illustrate the importance of evaluating sex-specific fetal and placental developmental trajectories in order to understand the mechanism by which early adversity is associated with sexually dimorphic risk for mental illness.
Recent studies indicate that placental histology correlates with neurodevelopmental disorders including autism. In a sample of 117 births, Walker and colleagues discovered that pregnancies distinguished by two or more trophoblast inclusions were eightfold greater in births of children whose older siblings had been diagnosed with autism compared to control samples (Walker et al., 2013). The greater the number of trophoblast inclusions were used to distinguish the at-risk from control samples, the less the sensitivity for predicting autism, but the greater the specificity. It is plausible that evaluation of the placenta will provide clues as to which children are at risk for neurodevelopmental delay. Given compelling sex differences in placental anatomy and physiology, including in the presence of trophoblasts inclusions (Hong et al., 2013), this study further supports the necessity of considering sex specific fetal/placental development to understand which individuals will be at risk for neurodevelopmental or psychiatric disorders.
Among the mechanisms that render discussion of placental genomics and biochemistry relevant to behavior is the phenomenon that certain genes are co-regulated in placenta and hypothalamus. In the words of Broad and Keverne (2011) “such synchronized expression is regulated, in part, by the maternally imprinted gene, paternally expressed gene 3 (Peg3), which also is developmentally coexpressed in the hypothalamus and placenta at days E12–13.” Note that Peg3 is a gene that is preferentially expressed from the paternal allele in hypothalamic neurons (Keverne, 2007). Overall, Keverne’s results show how gene regulation in the placenta is linked to regulation in a specific part of the brain, thus reinforcing our argument that sex differences in placental expression of a gene related to stress responses could be relevant to the brain’s later responses to stresses.
III. Sex Specific Programming of the Human Fetus
Molecular endocrine observations make clear the possibility that sex differences in human brain development can begin very early. (i.) Arnold (2012) distinguishes four different sources of sex differences in brain and behavior: testicular hormones and their metabolites (estrogens) following expression of the SRY gene; (ii.) consequences of X-inactivation including gene dosage; (iii.) differences in X-inactivation patterns between females (with 2 X chromosomes compare to the male’s one X ); and (iv.) potential effects of the inactivated X ( in females) on genome structure. Note that estrogen receptor expression has been reported preimplantation, even in the mouse blastocyst (Hou et al., 1996).
Not only do sex differences in brain development emerge very early, but also it is clear that sex specific responses to adversity are present at the earliest stages of development (Challis et al., 2013). Our research examining the relation between early life exposures to maternal stress and stress hormones (placental CRH and cortisol) and offspring development suggests that these exposures are associated with clear sex differences in patterns of expression observed during development. We discuss here evidence for sex-specific programming of the human fetus as it might contribute to risk for affective disorders (Section V) and ASD (Section VII). We focus on these two disorders as there is sexually dimorphic risk for these outcomes and they have at least part of their origins early in life.
In response to early adversity, males are more susceptible to morbidity and mortality as compared to their female counterparts (Wells, 2000). Throughout the fetal period males are more susceptible to adversity. Males are at a greater risk for miscarriage, still birth and preterm delivery (Cooperstock and Campbell, 1996). Further, studies consistently show that males are more vulnerable to infant mortality and extreme developmental impairments (Aiken and Ozanne, 2013; Challis et al., 2013). Findings such as these have led to the tacit consensus that females are largely immune from exposure to early adversity. It is plausible, however, that under conditions of adversity the weaker males have been eliminated early in development, and this results in reduced variability among surviving males. In contrast, females adjust to early adversity with a variety of more subtle individually determined strategies resulting in a far greater range of responses later in life, increasing the probability that there will be associations between exposures and certain types of psychopathology. It is plausible that these differential responses very early in life contribute to the pervasive sex differences in child and adult outcomes (Sandman et al., 2013).
We discuss the hypothesis that the adaptive strategy implemented by female fetuses may render them susceptible to more subtle, but persisting consequences. Our data consistently show a female vulnerability that is associated with increased risk for anxiety and affective problems. These outcomes contrast to the more extreme developmental impairments, such as autism, observed when males are exposed to early adversity.
IV. Animal models of sex specific consequences of early psychobiological stress signals
It is critical to acknowledge that the differences in reproductive and stress physiology, even in very closely related species such as humans and non-human primates, limit the validity of generalizing from animal models. Further, the ability to characterize animal models of anxiety or ASD is limited. For these reasons, this review will focus primarily on studies of gestational stress in humans. Nonetheless, research with non-human animals consistently documents sex specific susceptibility to fetal adversity. We briefly review this literature here.
Review of rodent literature on prenatal stress clearly document sex specific patterns of responding. Zuena et al. (2008) reported a large increase in anxiety, measured by avoidance of light, in male mice whose mothers had been subjected to restraint stress during pregnancy. Surprisingly, the female littermates showed exactly the opposite result, decreased anxiety. Likewise, with a different kind of stress during pregnancy, social defeat, male offspring of the socially stressed animals displayed increased anxiety-like behavior compared to controls, a result that was not seen with female offspring (Brunton and Russell, 2010). Stress from repeated transports during pregnancy yielded young males who were less active but showed more signs of distress in response to novelty, than control males, again a result that did not occur with young female offspring (Roussel et al., 2005). In contrast other studies suggest that female offspring are more susceptible to the consequences of gestational stress on anxiety-like behaviors. For example, Zagron and Weinstock (2006) found that when a 30-minute restraint stress was applied prenatally, female offspring displayed an increased propensity towards anxiety-like behavior on the elevated plus maze and altered HPA axis regulation, where as males displayed learning deficits. Similarly, Richardson et al. (2006) found that in response to two different types of stressors (restraint stress and varied stress) the consequences for anxiety-like behaviors and HPA axis regulation were stronger among females. Interpretation of this literature is with respect to humans is challenging because the sex specific consequences of early life stress clearly depend on a number of parameters including the timing of exposure, the type and severity of the stressor, the type of task and the animal species (Babri et al., 2014).
It is our hypothesis that it is not that males or females are more susceptible, but rather that gestational stress has sexually dimorphic consequences. Schulz et al. (2011) evaluated the effects of prenatal exposure to unpredictable variable stress on the offspring. They observed that for males prenatal stress was associated with impaired spatial memory and object recognition and improved object context associations. Interestingly prenatally stressed males did not display increased anxiety on the elevated zero, but did display a reduction in social exploration. In contrast, prenatally stressed females displayed greater anxiety as assed with the elevated zero, but greater social investigation. This study illustrates sex-specific patterns of responding to fetal stress exposure, which may contribute to sex specific susceptibility to neurodevelopmental disorders. Research by Bale and colleagues illustrates increased susceptibility of male offspring when exposed to stress extremely early in gestation Mueller and Bale (2007, 2008) demonstrating that factors such as timing of exposure may play a critical role in determining the sex-specific consequences.
The mechanisms for sex differences in responses to early stress are not fully understood substantial evidence indicates that stress pathway dysregulation underlies the development of anxiety-like behaviors. Prenatal stress is associated with heightened corticotrophin releasing factor (CRF) expression in the central nucleus of the amygdala, markedly greater corticosterone response to restraint stress, abnormally low glucocorticoid receptor (GR) expression in Ca3 and the dentate gyrus of the hippocampus, and abnormal methylation of histones in the promoters of the CRF and GR genes (Mueller and Bale (2007, 2008). Further, adrenalectomy with corticosterone replacement eliminated the consequences of prenatal stress on anxiety behaviors (Zagron et al., 2006).
V. Effects of Early Life Stress Greater in Human Females: Affective Problems
Anxiety and mood disorders are highly prevalent disorders with pervasive sex differences. The lifetime prevalence of affective problems is alarmingly high. Recent data indicate that lifetime prevalence is approximately 29% for anxiety disorders and 21% for mood disorders (Kessler et al., 2005b). Further, females are more than twice as likely as males to experience mood disorders (Bekker and van Mens-Verhulst, 2007; Kessler et al., 2005a). Increasing evidence suggests that these disorders have part of their origin in prenatal and early postnatal experiences (Baram et al., 2012; Sandman and Davis, 2012). It has been recently estimated that early adversity may explain over 30% of mental illness (De Bellis et al., 1999; Gunnar and Quevedo, 2008; Nemeroff, 2004). Further evidence suggests that sex dependent vulnerability to affective problems may originate in the prenatal period (Bale, 2009; Buss et al., 2012).
Sex specific consequences of fetal exposure to psychobiological stress signals: Affective problems
One consistent theme in our research and others is the association between fetal exposure to stress signals and increased stress and emotional reactivity in the offspring. Fetal exposure to maternal anxiety, stress and depression as well as stress hormones is associated with stress responses (Davis et al., 2011b; O'Connor et al., 2013), fearful temperament and negative emotionality (Bergman et al., 2007; Davis et al., 2005; Davis et al., 2007; de Weerth et al., 2003; Van den Bergh, 1990) and affective problems (Buss et al., 2012; Davis and Sandman, 2012; Davis et al., 2013; O'Connor et al., 2003; Van den Bergh and Marcoen, 2004; Van den Bergh et al., 2008) during infancy, childhood and adolescence.
Perhaps surprisingly, many published studies have not evaluated sex-specific responses to fetal adversity. As illustrated in Table 1, the first author and colleagues, recently performed a reanalysis of their own published data from several studies, with sufficient sample size to assess sex differences, and demonstrate that, even as early as the fetal and infant periods, there are sex-specific responses to gestational stress (Sandman et al., 2013). Interestingly, one of the most consistent findings is a greater female vulnerability to gestational stress signals for fearful or anxious behaviors. Female fetuses exposed to higher levels of maternal psychological distress and to biological stress signals such as placental CRH and cortisol were more fearful during infancy and more anxious during childhood. Consistent with our findings, De Bruijn and colleagues have demonstrated that the relation between prenatal maternal anxiety and internalizing problems is stronger among girls1 (de Bruijn et al., 2009a). Consideration of published human literature evaluating sex-specific consequences of prenatal exposure to maternal stress suggests that although sex difference were not observed in all studies (O'Connor et al., 2003), the preponderance of the evidence indicates that girls may be more susceptible to the effects of fetal adversity on fearful temperament, emotional reactivity and internalizing problems increasing their risk for the development of affective problems. Infants who are easily aroused by varied stimulation are more likely to become behaviorally inhibited as young children (Kagan et al., 1998), exhibit social anxiety during adolescence (Schwartz et al., 1999) and show greater amygdalar activation to novelty as adults (Schwartz et al., 2003). We suggest that sexually dimorphic vulnerability to affective problems may originate during the fetal period.
Table 1.
| STUDY | PARTICIPANTS | PRIMARY SEX-SPECIFIC FINDINGS |
|---|---|---|
| Fetus | ||
| Glynn & Sandman, 2012 | 190 Mother (83 female/107 male fetuses) | Relations between maternal cortisol and fetal response to vibroacoustic stimulation were stronger for females. Among females, maternal cortisol was predictive of fetal behavior in response to stimulation at an earlier age than among males. |
| Neonate | ||
| Ellman, Dunkel-Schetter, Hobel, Chicz-Demet, Glynn, & Sandman, 2008. | 158 Mothers (80 female/78 male neonates) | The association between fetal exposure to elevated maternal cortisol and placental CRH and delayed neuromuscular development was observed only among male neonates. |
| Infant | ||
| Sandman, Glynn & Davis, 2013 and Davis & Sandman, 2010 | 165 Mothers (60 female/65 male infants) | The association between exposures to elevated maternal cortisol early in gestation and impaired cognitive performance at 1-year of age was stronger among males. |
| Sandman, Glynn & Davis, 2013 and Sandman, Davis & Glynn, 2012 | 221 Mothers (103 female/118 male infants) | Congruence between exposure to maternal depression in the pre and postnatal environments was associated with advanced maturation of motor and mental abilities in 1-year-old infants. Effects were observed at an earlier age among female infants. |
| Sandman, Glynn & Davis, 2013 and Davis, Glynn, Dunkel-Schetter, Hobel, Chicz-Demet, & Sandman, 2005 | 248 Mothers (116 female/ 132 male infants) | Elevated placental CRH at 25 gestational weeks is associated with more fearful temperament and higher levels od distress behavior among female infants, but not male infants at 2 months of age. |
| Sandman, Glynn & Davis, 2013 and Davis, Glynn, Dunkel-Schetter, Hobel, Chicz-Demet, & Sandman, 2007 | 248 Mothers (116 female/132 male infants) | Increased maternal depressive symptomatology at 25 gestational weeks predicted more fearful temperament during infancy among girls, but not boys. |
| Grey, Davis, Sandman & Glynn, 2012 | 52 Mothers (27 female/25 male infants) | The positive association between breast milk cortisol and fearful infant temperament at 3 months of age was observed only among female infants. |
| Child | ||
| Sandman, Davis, Buss, & Glynn, 2011 | 35 Mothers (17 girls/ 18 boys) | Elevated pregnancy specific anxiety early in pregnancy is associated with reduced gray matter volumes. This effect is seen primarily in girls. |
| Buss, Davis, Hobel, & Sandman, 2011 | 89 Mothers (39 girls/ 50 boys) | Pregnancy-specific anxiety predicted executive function in girls, but not boys. |
| Sandman, Glynn & Davis, 2013 | 178 Mothers (98 girls/80 boys) | The relation between prenatal maternal cortisol and child anxiety was stronger among girls. |
| Buss, Davis, Shahbaba, Pruessner, Head & Sandman, 2012 | 65 Mothers (35 girls/ 30 boys) | Reduction in brain volumes in 6–9 year-old children exposed to elevated maternal cortisol early in gestation primarily was observed in girls. |
| Sandman, Glynn & Davis, 2013 | 100 Mothers (49 girls/ 51 boys) | The association between longer gestation and increased gray matter density is stronger among girls. |
Note: This table is adapted from Sandman, Glynn and Davis, 2013 and summarizes published data from this group that has evaluated sex-specific consequences of fetal exposure to maternal stress signals..
Sex-specific programming of neurobiological pathways involved in stress regulation may contribute to greater female risk for affective problems. Girls may be more sensitive to the effects of gestational stress on child HPA axis functioning (de Bruijn et al., 2009b) and on the development of brain regions involved in stress and emotional regulation (Buss et al., 2012; Sandman et al., 2011) as well as self regulatory processes including executive function (Buss et al., 2011). It is plausible that these may be contributing mechanisms to the increased female risk for anxiety and depression. Consistent with this possibility, Van den Bergh and colleagues have reported that prenatal maternal anxiety is associated with a flattened diurnal profile in the offspring during adolescence. This cortisol profile is associated with depressive symptoms among female, but not male adolescence (Van den Bergh et al., 2008). Further, one of us recently has shown that elevated maternal cortisol at 15 gestational weeks is associated with a significantly larger right amygdala volume in girls, but not in boys (Buss et al., 2012). This effect was significant after adjusting for factors associated with pregnancy, birth outcome and current child and maternal characteristics. Moreover, we reported that the association between fetal exposure to high levels of maternal cortisol early in gestation and subsequent affective problems in children was mediated by an enlarged right amygdala. These findings support the argument that fetal exposures to maternal signals of stress and adversity have persisting effects on the female nervous system (Davis et al., 2011a) and that these effects may contribute to risk for affective problems among females. Clear evidence exists for greater female susceptibility to the intergenerational transmission of mood disorders, such as depression and the role of postnatal stressors in the emergence of disease (Gershon et al., 2011; Waugh et al., 2012). The findings presented here demonstrate that understanding the intergenerational transmission of disease requires the additional consideration of the role of the fetal environment.
Mechanisms underlying greater female vulnerability to the effects of prenatal stress on affective problems are unknown. There is ample evidence for sexual dimorphism in limbic regions, such as the amygdala, that are involved in affective problems (Goldstein, 2006). Further, sex differences in brain activity have been observed in these regions in response to emotional stimuli (Cahill et al., 2004; Kilpatrick et al., 2006). We argue that the origin of these differences may lie in the fetal period. Regions we have observed to be vulnerable to prenatal stress exposures, such as the amygdala, also are rich in receptors for sex hormones. The finding described above illustrating that influences on the development of the amygdala during the fetal period are associated with affect problems among girls (Buss et al., 2012) is consistent with the possibility that fetal exposure to stress and sex hormones contributes to sexually dimorphic susceptibility to affective problems.
VI. Effects of Early Life Stress Greater in the Human Male: Implications for ASD
Autism is now diagnosed in greater than 1 in 100 children. Because it begins so early in life and lasts into adulthood, it is occasioned by long-lasting emotional suffering and can entail enormous expenses for medical and psychological treatments. Although the hallmark of autism comprises an absence of social attention and social communication, the full range of symptoms in Autism Spectrum Disorders (ASDs) include repetitive movements and restricted interests, and language disorders. A convincing series of reports has concluded that, by far, the greater preponderance of ASD diagnoses is in young males (Auyeung et al., 2009). Social deficits associated with ASDs may be present as early as 6 months of age. Chawarska et al. (2013) reported differences in attention to social scenes as early as 6- months of age in children who were later diagnosed with ASD. We discuss the possibility that early life exposure to stress contributes to the development of ASD. Further, we propose that sexually dimorphic responses to early stress may contribute to greater male susceptibility to ASD.
In both the previous and the new versions of Diagnostic and Statistical Manual (DSM IV and V), the core diagnostic concept is the avoidance of normal social contact. Here, we focus on social anxiety of the sort that might lead to social avoidance. Sir Michael Rutter, who has participated in autism research for more than 40 years, has reviewed our current understanding of the genetic contributions to, and psychological treatments for ASDs (Rutter, 2011). Some decades ago, Folstein and Rutter (1977) reported 36% concordance of diagnoses of autism among monozygotic twins, compared with 0% in dizygotic twins, and in that paper anticipated the subsequent discussions of gene/environment interactions. Recently, in producing rigorous quantitative estimates of gene/environment contributions, Hallmayer et al. (2011) estimated ASD diagnostic concordance rates at .77 for monozygotic male twins, with a large share (58%) of the variance in liability explained by environmental factors. He has proposed novel tests of the social problems focused on by our theory, including a theory-of-mind task (Heavey et al., 2000) and has explored the relationships between the dysphasia (i.e. severe language disorders) of autism and the evident shortcomings in social communication (Cantwell et al., 1989). For this review, it is important to illustrate how early stress, especially prenatal stress, may contribute to the multiple etiologies that lead to ASDs.
Sex specific consequences of prenatal psychobiological stress signals: Risk for ASD
An authoritative metareview (Gardener et al., 2011) shows that a wide variety of early stressors clearly predispose to ASD. Particularly significant is the stress imposed by low birth weight, short gestational period, intrapartum hypoxia (Kolevzon et al., 2007) and obstetric complications (Burstyn et al., 2010). It is the cumulative exposure to multiple stressors during the perinatal period that best predicts ASD: the longer and more multivariate the stress, the greater the likelihood of autistic symptoms developing. The Gardener et al conclusions are supported by the recent study of Visser et al. (2013) in which mothers of children who would have ASD reported “more infections and more stress during pregnancy”. Interestingly, there is evidence that males have an increased risk for morbidity and mortality when exposed to these types of stressors (Aiken and Ozanne, 2013; Challis et al., 2013; Peacock et al., 2012). It is plausible, that sexually dimorphic responses to early stress contribute to greater male susceptibility to ASD.
Animal models provide overwhelming evidence that early stress contributes to the neurodevelopmental impairments associated with ASD including anxious behavior and greater stress reactivity. Our theory suggests that in some contexts this early stress may lead to increased ASD risk among males. The literature on animal research devoted to charting the behavioral consequences of early stress is more extensive than research with humans and may facilitate our understanding of the role of stress in risk for ASD. Using Rhesus monkeys for animal studies potentially relevant to human behavioral development, Suomi and his colleagues (Dettmer et al., 2012) separated certain monkeys from their mothers, stressing them through either a peer-rearing paradigm (PR) or surrogate-peer-rearing (SPR) from birth through the first eight months of their lives. Control animals were reared by their mothers and interacted with peers (MPR). Most important for the present argument about the etiology of the social anxiety of autism, PR monkeys showed the greatest amount of anxious behavior, and had high cortisol levels that remained high for month after the experiment. SPR animals also showed more anxiety than MPR animals. Subsequent work by Suomi and colleagues suggested abnormal regulation of serotonergic systems as having something to do with social withdrawal consequent to early psychosocial stress (Erickson et al., 2005). Given the centrality of social anxiety to ASD, these data are consistent with the possibility that early life stress increases risk for ASD.
Consistent with this theme, sex specific consequences to the extreme stress of orphanage rearing also have been observed. In a sample of Romanian children, Nelson and his colleagues (Bos et al., 2011) have charted how the social and material deprivation of institutional care leads to disturbed normal behavioral development, paralleled by electroencephalographic changes that may reflect a delay in maturation of the cerebral cortex (McLaughlin et al., 2010). Not only were boys more symptomatic during psychiatric interviews than girls, but also the interactions of the environmental background X sex were significant both for internalizing disorders and for “all anxiety disorders“ (Zeanah et al., 2009). This study was not designed to evaluate associations with ASD and clearly most children who develop ASDs have not experienced this type of extreme stress. Our theory, below, would suggest that such sex differences in responses to environmental adversity are linked to sex differences in autism (Pfaff et al., 2011).
There are relatively few human studies evaluating prenatal factors contributing to ASD. The majority of studies in this field indicate that prenatal stress is linked to risk for ASD. For example, Kinney et al. (2008) review data from studies with humans that indicate that prenatal stress increases the risk of developing autistic symptoms. Most existing studies are retrospective, but in some cases the differences in reported stress between mothers of autistic children and controls were large, approaching two-fold. Nevertheless, complicating factors such as reporting bias cannot be ruled out. Recent epidemiological studies address limitations related to reporting bias by using national databases to identify individuals who experienced death of a family member during pregnancy or postpartum. Using Swedish national databases Class and colleagues found that mothers who experienced bereavement stress during the prenatal or postpartum period were at an increased likelihood of having a child with ASD (Class et al., 2013). The strongest effects were observed when the stressor was experienced during the third trimester. Although these authors did not find evidence that sex moderated the consequences of prenatal stress exposure, these data provide their study provides convincing evidence for a link between prenatal and early postnatal stress exposure and ASD risk. A recent prospective study provides further evidence that fetal stress exposure is related to ASD. Ronald et al. (2011) enlisted 2900 pregnant women in a prospective study of the effects of stressful events during pregnancy. Maternal stress during pregnancy was associated with the later detection of autistic traits in boys but not in girls, whereas they were associated with ADHD in both boys and girls.
The literature with human subjects reviewed above indicates that exposure to stress during the perinatal period is linked to ASD risk. Studies that suggest an association between early life stress and ASD risk primarily reveal such an association when the stress exposure is fairly severe or when the patient has experienced an accumulation of the effects of multiple stressors. Existing data have not yet allowed us to distinguish sharply between effects of prenatal stress compared to effects of perinatal or neonatal stress.
The causal routes by which these sexually differentiated effects of prenatal stress come about remain to be discovered. Despite the variety of early stressors considered, it is likely that they involve alterations of the hypothalamic/pituitary/adrenal axis and to activation of ascending monoaminergic pathways. Both direct CNS and indirect effects must be considered. For example, as discussed, the placenta is sexually differentiated and that this fact may contribute to the sex differences in fetal growth and may be related to the vulnerability of fetal males to repeated stresses (Clifton, 2010). Because of sex differences in placental cytokine expression, it is notable that Patterson and his colleagues (see below) have reported that maternal infections can be associated with a higher prevalence of autism.
A theory of how androgenic hormones can increase the occurrence of social abnormalities of autism
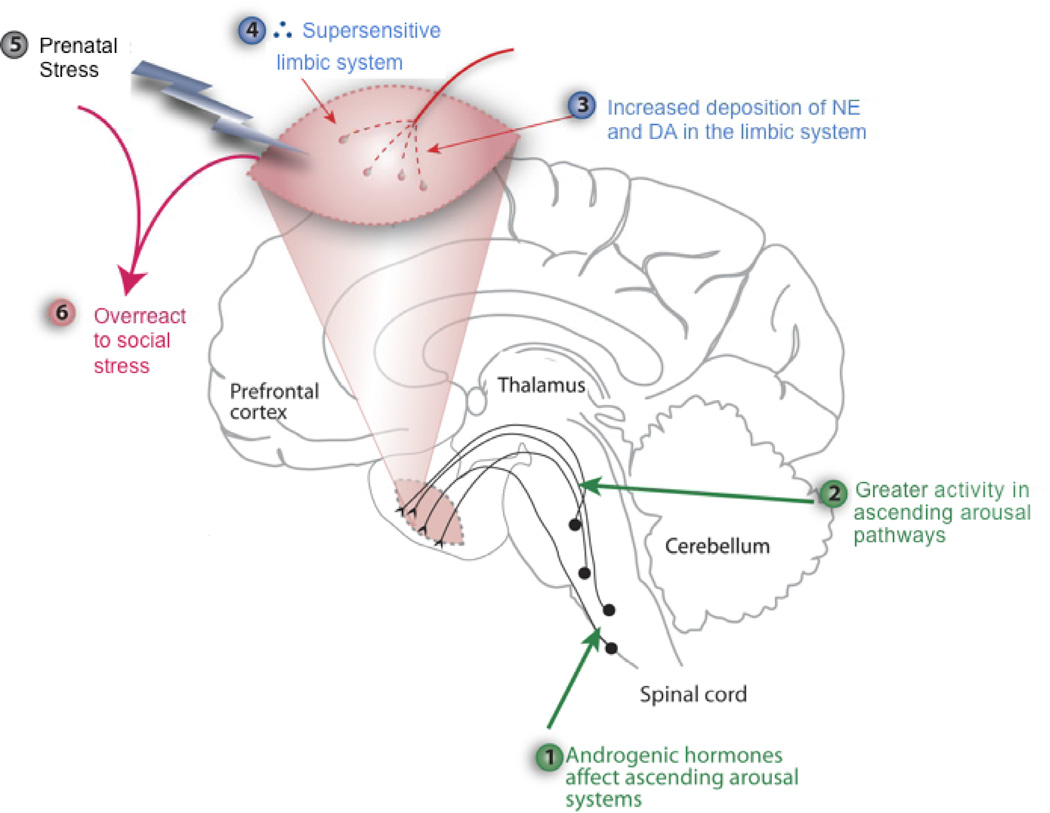
The sex difference in the prevalence of autism has been widely reported (Auyeung et al., 2009; Knickmeyer et al., 2005). In spite of the difficulties just noted, as a result of reviewing the large literatures on early stress, autism and androgen action, we have derived a theory intended to account for the large sex difference. Briefly, the theory in its initial form (Pfaff et al., 2011) hypothesizes that early stress has an inordinately disruptive effect in young boys who have had high androgenic hormone levels circulating or whose brainstem and limbic forebrain tissues are particularly sensitive to androgenic hormones. The androgen effect is disruptive because it has amplified effects of arousal pathways whose catecholaminergic transmitters, released synaptically in the amygdala as well as other limbic regions, have rendered these brain regions unusually sensitive to stress (Figure 2). Because the infant experiences this stress in a social context, and it thus constitutes a social stress the heightened limbic response leads to a level of fear inconsistent with social approach, thus predicting the social avoidance characteristic of autism.
Figure 2.
Theoretical depiction of how androgenic hormones acting on ascending arousal systems could sensitize limbic forebrain systems that regulate emotional aspects of social behaviors. Adapted from Pfaff et al (2011).
Although the theory states that contributions to the symptoms of ASDs stem from (i.) prenatal and neonatal stress, complicated by (ii.) androgenic hormone effects on CNS mechanisms for arousal and emotion; (iii.) genetic predispositions, the question arises as to how such a 3-hit theory would be tested. In this theoretical review, we have focused on (i.), early stress, partly because of the large literature on the deleterious effects of such stress on animal and human behavior. Prenatal and neonatal stress is hypothesized to affect neuronal activity in the amygdala, a brain region implicated in the recognition of emotions in facial expressions (Leppanen and Nelson, 2009). Published data indicates that prenatal stress influences amygdalar development (Buss et al., 2012). The theory postulates that as a result of early stress, the effects of which are exacerbated by androgenic hormone action and genetic predisposition, a stressed child’s amygdala has been rendered overly sensitive to subsequent social stimuli.
Recent human data indicates a role for androgenic hormone action in the development of fearful temperament. This phenomenon may be related to a significant correlation between prenatal testosterone exposure and fear reactivity (Bergman et al., 2010), at least as far as potential sex differences are concerned. Infants who later will be diagnosed with ASDs show a range of behavioral reactions including problems with eye contact and social engagement that could be interpreted as avoidance responses consequent to the development of a fearful temperament (Zwaigenbaum et al., 2005). Whether this potential connection among prenatal testosterone exposure, fear reactivity and social behavior symptoms actually can be related to the development of ASDs will require further work to determine.
Interesting support from this model comes from a twin study by Robinson and colleagues (2013). In a large sample of dizygotic twins they found that comparatively fewer familial risk factors are required to reach impairment for autistic symptoms among males as compared to females. These findings provide strong evidence that being female is protective against ASD.
Testing this complex theory will provide challenges, but some recent work in the literature on animal behavior has offered new approaches for experimentation. For example, the definition of the range of prenatal stress that can predispose to autism has been extended to immune/inflammatory stress (Patterson, 2009; Schwartzer et al., 2013). Immune/inflammatory stress leads to a set of phenomena whose causal pathways are likely to be complex and sex specific (Challis et al., 2013), but whose neurendocrine consequences are important and long lasting (Kentner and Pittman, 2010). Evidence from human subjects supports this point (Atladottir et al., 2010). Further, recent data using mice with mutations of the CNTNAP2 gene (mutations that predispose to autism) show significant effects of prenatal stress with the following three highlights: (i.) first, that the stress employed is a lipopolysaccharide (LPS) injection during early pregnancy, following on Mueller and Bale's demonstration of male-dominant effects of early gestational stress; (ii.) that such a stress should stand in for an inflammatory stress; and (iii.) that significant effects on infants' vocalizations and on young adults' social behavior came in the form of interactions with genotype - - the CNTNAP2 mutation and inflammatory stress combined to produce the behavioral effects (Schaafsma et al., unpublished observations). These recent data support the earlier contentions of Patterson et al suggesting that inflammatory pathways are related to risk for ASD (Hsiao et al., 2012).
VII. Outlook
Pervasive sex differences exist in risk for mental illness (Bale, 2009; Nugent et al., 2011; Schulz et al., 2011). In this review, we proposed that sexually dimorphic responses to early adversity are one of the key factors contributing to sex-specific risk for mental illness. We have focused on affective problems and ASDs as disorders that originate early in life and for which there are dramatic sex differences in prevalence rates. This review deals with a subject that presents difficulties in at least three ways. First, at the behavioral level, compared to many sensory stimuli and environmental conditions, stress is inherently non-specific and varies from study to study. Second, a wide variety of stressors are considered leading to difficulty comparing findings across studies. Third, there are important differences between animal and human literatures reviewed in the nature of the stress applied: In the animal studies it might be substantial, while in the human studies, natural variations from severe stress to relatively minor stresses were considered. Fourth, there is wide variability in the timing of the stress exposure during gestation further contributing to variability in associations with outcomes. Finally, our understanding of sex differences in vulnerability to mental disease will be influenced by diagnostic criteria that are not applied in the same way to boys and girls.
Both animal and human data illustrate that males are at a greater risk for morbidity and mortality in response to early life stress. Much less data exists related to sex-specific responses to early adversity and risk for mental illness. That said, tentative conclusions would indicate that (i.) that in males, early life stress is associated with ASDs. (ii.) In contrast, for females, early life stress is associated with more subtle developmental consequences including the development of affective problems. Recent animal work indicates that when the behaviors of male and female offspring are characterized across a variety of domains, both males and females are affected by early life stress, but in a sex-specific fashion (Mueller and Bale, 2007, 2008; Schulz et al., 2011). In order to understand the relation between early adversity and mental health we need to move beyond the general assumption of greater male susceptibility and consider instead the differential responses of males and females to early stress and how these distinct response patterns differentially are associated with later risk.
Speculative thinking that deserves to be followed up would envision that among females greater responsiveness to early stress signals might both increase the ability to survive and lead to greater variability among females. That is, Clifton (2010) has provided evidence that female fetuses adapt their development to more subtle stress signals. This speculation is consistent with the findings reviewed above suggesting that moderate prenatal stress is associated with increase risk for moderate developmental impairments among females. In contrast, as discussed in this review extreme stress increases neonatal morbidity and mortality and we argue risk for ASD.
Ideas for future research
Sex specific developmental trajectories and responses to adversity emerge early in gestation. The implications for mental health later in life are poorly understood. We propose here several avenues for future research. One clear theme that has emerged from review of both the animal and the human literature is the need to carefully consider timing of exposure with respect to sex differences. Rapid changes proceed throughout gestation in fetal CNS development, maternal physiology and placental physiology. Thus, it is highly likely that the consequences of stress will be dependent on the timing of exposure and that this may interact with sex-specific susceptibilities.
A second relatively unexplored direction for research is the role of stress in these sex-specific adaptations and the role of placental gene expression and subsequent placental biochemistry and physiology. Exciting new evidence suggests that sex-specific methylation patterns in the placenta are associated with neurodevelopment. Prospective studies considering sex differences in fetal and placental responses to early stress and their associations with developmental outcomes would be beneficial.
A third direction in which effort to understand the effects of early life stress on neurodevelopmental disorders includes assessment of stress-related epigenetic changes. For example, stress effects can have permanent alteration in the state of DNA methylation in the animal forebrain (Mychasiuk et al., 2011).
The literature reviewed here highlights the need to consider sex-specific responses to adversity, as early as the prenatal period in concert with genetic and epigenetic susceptibilities, in order to understand the origins of neurodevelopmental disorders. We propose that careful evaluation of sex-specific responses including maternal, placental and fetal will provide new understanding into the mechanism by which early experiences contribute to mental health.
Acknowledgments
Ideas regarding androgen-dependent gene expression influencing the development of autistic symptoms in boys were developed in collaboration with Brett Abrahams, and associated wording benefited from help by Sylvie Goldman, both at Albert Einstein School of Medicine. Hypotheses related to sex specific responses to gestational stress exposures involve collaboration with Laura M. Glynn at Chapman University and Curt A. Sandman at the University of California, Irvine. Preparation of this manuscript was supported in part by NIH awards R01 HD065823 to EPD and R01 HD005751 to DWP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note that when maternal report measures of depression and anxiety were combined to create an emotional complaint score high emotional complaints early, but not late, in gestation were associated with internalizing problems among boys.
Contributors. Professors Davis and Pfaff each contributed to the preparation of this manuscript. The idea was jointly conceived. Professor Davis bares the greater responsibility for sections related to prenatal stress and sections related to affective problems. Professor Phaff was primarily responsible for sections discussing sex differences in ASD and the role of early life stress. Both authors contributed manuscript preparation and revision.
Role of Funding Source. There are no conflicts of interest. The funding source played no role in the development of this review paper.
References
- Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145:R1–R13. doi: 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The end of gonad-centric sex determination in mammals. Trends in genetics : TIG. 2012;28:55–61. doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of autism and developmental disorders. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and autistic traits. Br J Psychol. 2009;100:1–22. doi: 10.1348/000712608X311731. [DOI] [PubMed] [Google Scholar]
- Babri S, Doosti MH, Salari AA. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain, behavior, and immunity. 2014;37:164–176. doi: 10.1016/j.bbi.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Bale TL. Neuroendocrine and immune influences on the CNS: it’s a matter of sex. Neuron. 2009;64:13–16. doi: 10.1016/j.neuron.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. The American journal of psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128. [PubMed] [Google Scholar]
- Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4(Suppl B):S178–S193. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- Bergman K, Glover V, Sarkar P, Abbott DH, O’Connor TG. In utero cortisol and testosterone exposure and fear reactivity in infancy. Hormones and behavior. 2010;57:306–312. doi: 10.1016/j.yhbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Bos K, Zeanah CH, Fox NA, Drury SS, McLaughlin KA, Nelson CA. Psychiatric outcomes in young children with a history of institutionalization. Harvard review of psychiatry. 2011;19:15–24. doi: 10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP. Synaptogenesis, heterochrony and epigenesis in the mammalian neocortex. Acta Paediatr Suppl. 1997;422:27–33. doi: 10.1111/j.1651-2227.1997.tb18340.x. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Broad KD, Keverne EB. Placental protection of the fetal brain during short-term food deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15237–15241. doi: 10.1073/pnas.1106022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. Journal of neuroendocrinology. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic diseases in Canada. 2010;30:125–134. [PubMed] [Google Scholar]
- Buss C, Davis EP, Class QA, Gierczak M, Pattillo C, Glynn LM, Sandman CA. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress (Amsterdam, Netherlands) 2011;14:665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learning & memory (Cold Spring Harbor, N.Y.) 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell DP, Baker L, Rutter M, Mawhood L. Infantile autism and developmental receptive dysphasia: a comparative follow-up into middle childhood. Journal of autism and developmental disorders. 1989;19:19–31. doi: 10.1007/BF02212715. [DOI] [PubMed] [Google Scholar]
- Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. Fetal sex and preterm birth. Placenta. 2013;34:95–99. doi: 10.1016/j.placenta.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological psychiatry. 2013;74:195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, Hultman CM, Langstrom N, Lichtenstein P, D’Onofrio BM. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychological medicine. 2013:1–14. doi: 10.1017/S0033291713000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 31 Suppl. 2010:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Murphy VE. Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta 25 Suppl A. 2004:S45–S52. doi: 10.1016/j.placenta.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Cooperstock M, Campbell J. Excess males in preterm birth: interactions with gestational age, race, and multiple birth. Obstet Gynecol. 1996;88:189–193. doi: 10.1016/0029-7844(96)00106-8. [DOI] [PubMed] [Google Scholar]
- Cowan WM. The development of the brain. Scientific American. 1979;241:113–133. [PubMed] [Google Scholar]
- Davis E, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-Releasing Hormone during Pregnancy Is Associated with Infant Temperament. Developmental neuroscience. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- Davis EP, Buss C, Muftuler LT, Head K, Hasso A, Wing DA, Hobel C, Sandman CA. Children’s Brain Development Benefits from Longer Gestation. Frontiers in psychology. 2011a;2:1. doi: 10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-DeMet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of child psychology and psychiatry, and allied disciplines. 2011b;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal Glucocorticoid Exposure Is Associated with Preadolescent Brain Development. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology Part I: Biological stress systems. Biological psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn AT, van Bakel HJ, van Baar AL. Sex differences in the relation between prenatal maternal emotional complaints and child outcome. Early Hum Dev. 2009a;85:319–324. doi: 10.1016/j.earlhumdev.2008.12.009. [DOI] [PubMed] [Google Scholar]
- de Bruijn AT, van Bakel HJ, Wijnen H, Pop VJ, van Baar AL. Prenatal maternal emotional complaints are associated with cortisol responses in toddler and preschool aged girls. Developmental psychobiology. 2009b;51:553–563. doi: 10.1002/dev.20393. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar J. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Kivlighan KT, Chen P, Laudenslager ML. Maternal salivary cortisol differs by fetal sex during the second half of pregnancy. Psychoneuroendocrinology. 2011;36:588–591. doi: 10.1016/j.psyneuen.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental psychobiology. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Gabry KE, Lindell S, Champoux M, Schulkin J, Gold P, Suomi SJ, Higley JD. Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Developmental psychobiology. 2005;46:331–339. doi: 10.1002/dev.20061. [DOI] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. Journal of child psychology and psychiatry, and allied disciplines. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4:5. doi: 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A, Hayward C, Schraedley-Desmond P, Rudolph KD, Booster GD, Gotlib IH. Life stress and first onset of psychiatric disorders in daughters of depressed mothers. Journal of psychiatric research. 2011;45:855–862. doi: 10.1016/j.jpsychires.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, O’Connor TG. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2009;34:430–435. doi: 10.1016/j.psyneuen.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Dev Sci. 2012;15:601–610. doi: 10.1111/j.1467-7687.2012.01159.x. [DOI] [PubMed] [Google Scholar]
- Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Hormones and behavior. 2006;50:612–622. doi: 10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Grey KR, Davis EP, Sandman CA, Glynn LM. Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Progress in Brain Research. 2008;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of general psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Piton A, Gauthier J, Lortie A, Dubeau F, Dobrzeniecka S, Spiegelman D, Noreau A, Pellerin S, Cote M, Henrion E, Fombonne E, Mottron L, Marineau C, Drapeau P, Lafreniere RG, Lacaille JC, Rouleau GA, Michaud JL. De novo STXBP1 mutations in mental retardation and nonsyndromic epilepsy. Annals of neurology. 2009;65:748–753. doi: 10.1002/ana.21625. [DOI] [PubMed] [Google Scholar]
- Heavey L, Phillips W, Baron-Cohen S, Rutter M. The Awkward Moments Test: a naturalistic measure of social understanding in autism. Journal of autism and developmental disorders. 2000;30:225–236. doi: 10.1023/a:1005544518785. [DOI] [PubMed] [Google Scholar]
- Hong JS, Romero R, Kusanovic JP, Kim JS, Lee J, Jin M, El Azzamy H, Lee DC, Topping V, Ahn S, Jacques S, Qureshi F, Chaiworapongsa T, Hassan SS, Korzeniewski SJ, Than NG, Kim CJ. "Trophoblast islands of the chorionic connective tissue" (TICCT): a novel placental histologic feature. Placenta. 2013;34:360–368. doi: 10.1016/j.placenta.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Paria BC, Mui C, Dey SK, Gorski J. Immunolocalization of estrogen receptor protein in the mouse blastocyst during normal and delayed implantation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2376–2381. doi: 10.1073/pnas.93.6.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5169–5174. doi: 10.1073/pnas.1300065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen Pena C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PloS one. 2012;7:e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Arcus D. Childhood derivatives of high and low reactivity in infancy. Child Development. 1998;69:1483–1493. [PubMed] [Google Scholar]
- Kentner AC, Pittman QJ. Minireview: early-life programming by inflammation of the neuroendocrine system. Endocrinology. 2010;151:4602–4606. doi: 10.1210/en.2010-0583. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005b;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB. Genomic imprinting and the evolution of sex differences in mammalian reproductive strategies. Advances in genetics. 2007;59:217–243. doi: 10.1016/S0065-2660(07)59008-5. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neuroscience and biobehavioral reviews. 2008;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. Journal of child psychology and psychiatry, and allied disciplines. 2005;46:198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Archives of pediatrics & adolescent medicine. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nature reviews. Neuroscience. 2009;10:37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. Sex differences in neuroendocrine systems. Frontiers in Neuroendocrinology. 2014 in press. [Google Scholar]
- Lowry PJ. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found Symp. 1993;172:108–115. [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, Nelson CA. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biological psychiatry. 2010;68:329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilleur AA, Fombonne E. Regression of language and non-language skills in pervasive developmental disorders. Journal of intellectual disability research : JIDR. 2009;53:115–124. doi: 10.1111/j.1365-2788.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiology & behavior. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61:169–176. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003 doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Schmold N, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R. Prenatal bystander stress alters brain, behavior, and the epigenome of developing rat offspring. Developmental neuroscience. 2011;33:159–169. doi: 10.1159/000330034. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Early-life adversity, CRF dysregulation, and vulnerability to mood and anxiety disorders. Psychopharmacol Bull. 2004;38:14–20. [PubMed] [Google Scholar]
- Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Hormones and behavior. 2011;59:338–344. doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell BA, Moritz KM, Walker DW, Dickinson H. Treatment of pregnant spiny mice at mid gestation with a synthetic glucocorticoid has sex-dependent effects on placental glycogen stores. Placenta. 2013;34:932–940. doi: 10.1016/j.placenta.2013.06.310. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental psychobiology. 2013;55:145–155. doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. Journal of Child Psychology and Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- O'Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012;37:818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Osei-Kumah A, Smith R, Jurisica I, Caniggia I, Clifton VL. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32:570–578. doi: 10.1016/j.placenta.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behavioural brain research. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatric research. 2012;71:305–310. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Rapin I, Goldman S. Male predominance in autism: neuroendocrine influences on arousal and social anxiety. Autism research : official journal of the International Society for Autism Research. 2011;4:163–176. doi: 10.1002/aur.191. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Seckl JR, Pesonen AK, Simons A, Van den Bergh BR. Stress, glucocorticoids and liquorice in human pregnancy: programmers of the offspring brain. Stress (Amsterdam, Netherlands) 2011;14:590–603. doi: 10.3109/10253890.2011.602147. [DOI] [PubMed] [Google Scholar]
- Reik W, Lewis A. Co-evolution of X-chromosome inactivation and imprinting in mammals. Nat Rev Genet. 2005;6:403–410. doi: 10.1038/nrg1602. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsater H, Happe F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJ. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Frontiers in psychology. 2011;1:223. doi: 10.3389/fpsyg.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel S, Boissy A, Montigny D, Hemsworth PH, Duvaux-Ponter C. Gender-specific effects of prenatal stress on emotional reactivity and stress physiology of goat kids. Hormones and behavior. 2005;47:256–266. doi: 10.1016/j.yhbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Rutter ML. Progress in understanding autism: 2007–2010. Journal of autism and developmental disorders. 2011;41:395–404. doi: 10.1007/s10803-011-1184-2. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert Rev Endocrinol Metab. 2012;7:445–459. doi: 10.1586/eem.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75:327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Pearson JN, Neeley EW, Berger R, Leonard S, Adams CE, Stevens KE. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiology & behavior. 2011;104:340–347. doi: 10.1016/j.physbeh.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Translational psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain research. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R510–514. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B. The influence of maternal emotion during pregnancy on fetal and neonatal behavior. Pre- and Peri-natal Psychology. 1990;5:119–130. [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Visser JC, Rommelse N, Vink L, Schrieken M, Oosterling IJ, van der Gaag RJ, Buitelaar JK. Narrowly versus broadly defined autism spectrum disorders: differences in pre- and perinatal risk factors. Journal of autism and developmental disorders. 2013;43:1505–1516. doi: 10.1007/s10803-012-1678-6. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. 5 ed. Philedelphia: Elsevier; 2008. [Google Scholar]
- Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, Hertz-Picciotto I, Kliman HJ. Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biological psychiatry. 2013;74:204–211. doi: 10.1016/j.biopsych.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Muhtadie L, Thompson RJ, Joormann J, Gotlib IH. Affective and physiological responses to stress in girls at elevated risk for depression. Development and psychopathology. 2012;24:661–675. doi: 10.1017/S0954579412000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol. 2000;202:65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behavioural brain research. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. The American journal of psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alema GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PloS one. 2008;3:e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]