Abstract
Recombinant human mast cell chymase (rhChymase) was expressed in secreted form as an active enzyme in the SuperMan5 strain of GlycoSwitch® Pichia pastoris, which is engineered to produce proteins with (Man)5(GlcNAc)2 Asn-linked glycans. Cation exchange and heparin affinity chromatography yielded 5 mg of active rhChymase per liter of fermentation medium. Purified rhChymase migrated on SDSPAGE as a single band of 30 kDa and treatment with peptide N-glycosidase F decreased this to 25 kDa, consistent with the established properties of native human chymase (hChymase). Polyclonal antibodies against hChymase detected rhChymase by Western blot. Active site titration with Eglin C, a potent chymase inhibitor, quantified the concentration of purified active enzyme. Kinetic analyses with succinyl-Ala-Ala-Pro-Phe (suc-AAPF) p-nitroanilide and thiobenzyl ester synthetic substrates showed that heparin significantly reduced Km, whereas heparin effects on kcat were minor. Pure rhChymase with Asn-linked glycans closely resembles hChymase. This bioengineering approach avoided hyperglycosylation and provides a source of active rhChymase for other studies as well as a foundation for production of recombinant enzyme with human glycosylation patterns.
Keywords: Chymase, Serine Protease, Glycosylation, Fermentation, Pichia pastoris, Enzyme Kinetics
Introduction
Human chymase (EC 3.4.21.39), a potent serine protease with broad chymotrypsin-like substrate specificity [1], resides in the cytoplasmic granules of some mast cells [2, 3] as an active enzyme in complex with heparin [3]. Released during mast cell degranulation, active human chymase performs both pro-inflammatory and protective roles during inflammation and allergic reactions [4–8]. Human chymase recruits several types of pro-inflammatory cells in vivo [8] and promotes destruction of extracellular matrix directly by degrading fibronectin [9] and indirectly by activating matrix metalloproteinase (MMP)-9 [10] and by degrading TIMP-1, the tissue inhibitor of MMP-1 [11]. Human chymase has received much attention for its ability to generate the pro-inflammatory, pro-hypertensive peptide angiotensin II from angiotensin I [12, 13] or from angiotensin (1–12), an alternate renin independent precursor of angiotensin II [14]. Although angiotensin-converting enzyme (ACE) inhibitors are used routinely in the clinic, they do not inhibit chymase, which significantly amplifies angiotensin II production during inflammation [15]; consequently, clinically viable chymase inhibitors have potential value for treating hypertension. Many human chymase inhibitors have been made [16–27], with hopes of creating drugs to combat diseases such as hypertension, ischemia-reperfusion injury, aortic aneurism, destabilization of atherosclerotic plaques, as well as asthma- and allergy- related inflammation.
The primary natural source of human mast cell chymase is human skin tissue, making acquisition of large quantities of the enzyme difficult and biohazardous. To provide a safe and abundant alternative, recombinant human chymase (rhChymase) has been expressed previously in E. coli [28, 29], B. subtilis [30], COS cells [31], CHO cells [32], HEK cells [33, 34], baculovirus systems [20, 35–37], and the yeast Pichia pastoris [38–40]. The majority of these efforts yielded inactive zymogens of rhChymase that required enzymatic activation. Only B. subtilis [30] and P. pastoris [38–40] produced rhChymase in its active form. Baculovirus expression systems were the most productive, yielding 32 mg/L [35] and 3 mg/L [36] of enteropeptidase-activable rhChymase variants. Previously this laboratory expressed active rhChymase via secretion from P. pastoris with a yield of 2.2 mg/L of active enzyme [38]; however, some of the protein was hyperglycosylated, a well-known issue in yeast-based expression [41].
A high-yield source of active rhChymase with human-type glycosylation would facilitate chymase research by providing a better alternative to chymase from human tissues. Human chymase contains glycans at two Asn-linked glycosylation sites (Asn-72 and Asn-95) [20, 27, 42–45]; however, no studies to date have demonstrated the composition of these glycans. Some researchers indicate that human chymase may actually include sub-types that vary in tissue localization, glycan composition, and heparin binding affinity [46, 47]. Recently, researchers have engineered strains of GlycoSwitch® P. pastoris (BioGrammatics, Inc.) that produce recombinant proteins with human-type glycosylation patterns [41, 48–50]. One strain in this system, SuperMan5, has been used to generate GM-CSF with >90% product containing the (Man)5(GlcNAc)2 glycan moiety [51], which all human cells use as a foundation to form complex glycans [41, 52].
To generate a source of rhChymase with improved expression levels and the (Man)5(GlcNAc)2 glycosylation pattern, SuperMan5 P. pastoris were transformed with cDNA for active native human chymase (hChymase). The yeast expressed the protein, designated (Man)5-rhChymase, constitutively with the GAP promoter and secreted active enzyme using yeast α-mating factor and a kexin cleavage site. The biochemical properties of (Man)5-rhChymase closely resemble those of the native human enzyme and this provides a starting point for future efforts to generate rhChymase with true human-type glycosylation in P. pastoris.
Materials and Methods
Cloning and Selection
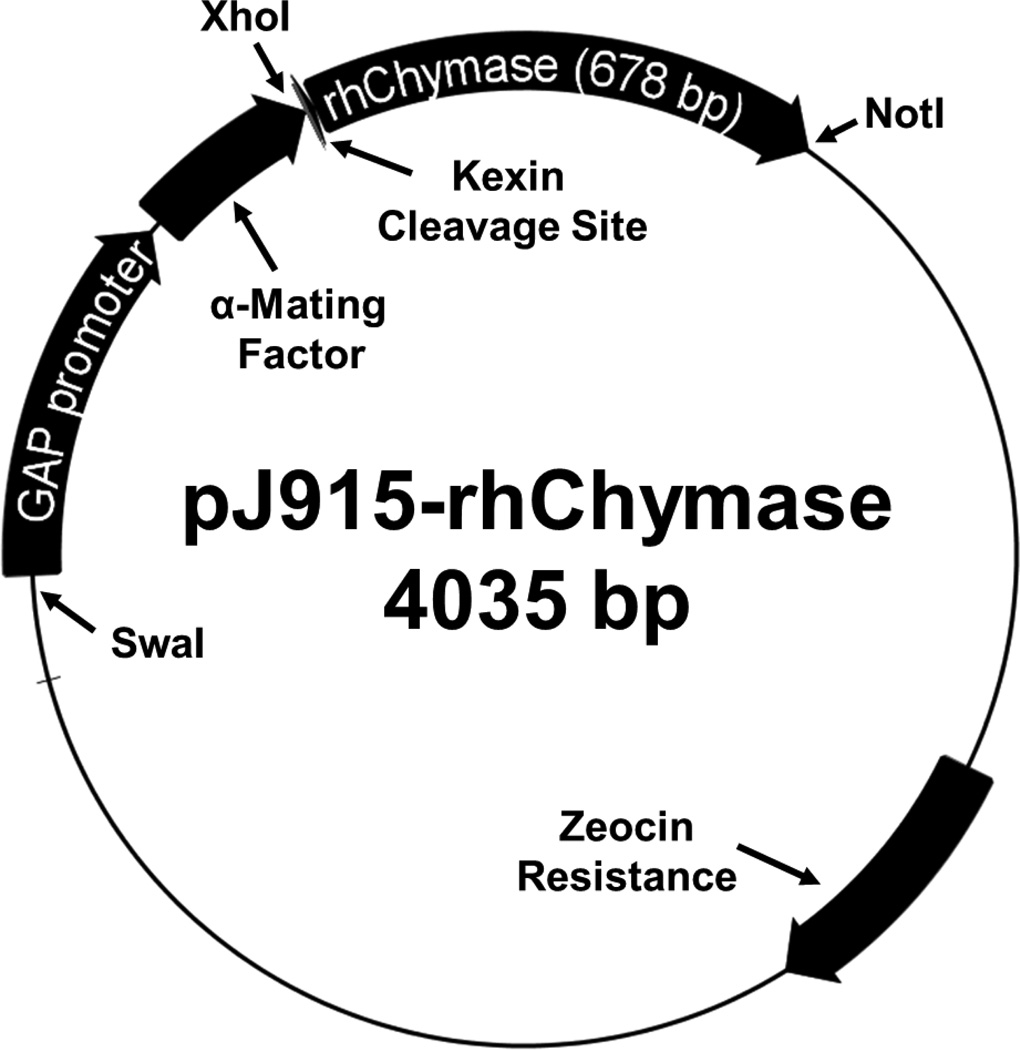
The DNA encoding human Chymase (hChymase) with an added N-terminal Kex2 protease cleavage site was isolated from the previously described plasmid pPICzα-rhChymase [38] using simultaneous digestion with XhoI and NotI restriction endonucleases (Thermo Scientific, FastDigest). P. pastoris expression plasmid pJ915 (DNA 2.0), which provides secretion directed by α-mating factor and the GAP promoter for constitutive expression, was separately treated in the same manner. The pJ915 plasmid and Kex2-hChymase insert were purified separately by electrophoresis with a 1% (w/v) LE agarose gel and subsequent isolated with a gel DNA recovery kit (Zymo Research). The plasmid and insert were ligated with Quick-Stick ligase (Bioline) and the ligation mixture was used to transform DH5α E. coli (Zymo Research). Transformants were screened for Zeocin™-resistance in low-salt LB agar, as described in the EasySelect™ Pichia Expression Kit manual (Life Technologies). Three positive E. coli clones were grown overnight in 10 mL of low salt-LB containing 25 µg/mL Zeocin™. Plasmids were recovered by miniprep (Zymo Research) and analyzed on a 1.2% agarose FlashGel™ (Lonza) following XhoI and NotI dual restriction digestion (ThermoFisher, FastDigest). All three clones contained the plasmid, designated pJ915- rhChymase (Figure 1).
Figure 1. pJ915-rhChymase vector map.
The P. pastoris expression plasmid pJ915-rhChymase (4035 bp) encodes a fusion protein that consists of rhChymase linked to yeast α-Mating Factor (a secretion signal peptide) by a kexin cleavage site. The cDNA for rhChymase with the kexin cleavage site was transferred from a previously reported plasmid, pPICzα-rhChymase [38] using 5’ XhoI and 3’ NotI restriction sites. The GAP promoter provides constitutive expression of the fusion protein and the plasmid contains a Zeocin resistance selectable marker gene. The plasmid is linearized at the SwaI restriction site prior to electroporation.
The SuperMan5 strain of GlycoSwitch® P. pastoris (BioGrammatics, Inc.) was made electrocompetent following the manufacturer’s instructions. Purified pJ915-rhChymase was linearized with SwaI (Thermo Scientific) prior to electroporation using an ECM 600 electroporation system (BTX Technologies) and the BioGrammatics protocol. Settings of 1.5 kV, 50 µF, and 186 Ω yielded a pulse duration of 7.5 ms in a 1 mm gap electroporation cuvette (Eppendorf). Positive transformants emerged after 3 days at 30°C and individually numbered clones were transferred to a fresh YPD agar plate with 100 µg/mL Zeocin™ and re-grown at 30 °C.
Numbered clones were screened as previously described [53] using 2 % starting glycerol and daily addition of 1 % glycerol. Chymase-like proteolytic activity was measured with suc-AAPF-SBzl (method below). The 5 most productive clones were then grown from equal starting cell densities of OD600 = 1.0 in 50 mL synthetic minimal medium (SMM) [53] in baffled flasks at 30 °C and 250 rpm (2.54 cm orbit) for 72 hours. Every 24 hours, cultures received 1 % glycerol and 1 mL samples were removed. Chymase activity (suc-AAPF-SBzl) and cell density (OD600) were monitored daily. The most productive clone was identified by the activity/OD600 ratio after 72 hours and named GAP-(Man)5-rhChymase.
Fermentation
Fermentation used a New Brunswick BioFlo® 110 bioreactor with 7.5 L vessel and followed the general protocol of the Life Technologies Pichia Fermentation Process Guidelines with some modification. Basal salts medium (BSM) was supplemented with PTM4 (4.35 mL/L) instead of PTM1 to reduce formation of insoluble phosphates [54]. YNB without amino acids (13.4 g/L) was also included. The pH was maintained at 4.5 by drop-wise addition of 12.5% NH4OH. The initial fermentation volume was 3.5 L after adding 300 mL of high-density inoculum that had grown overnight in SMM, as above. At the end of a 24-hour glycerol batch phase, a glycerol fed-batch phase was initiated with drop-wise addition (0.6 mL/min) of 65% (w/v) glycerol containing 4.35 mL/L PTM4. The dissolved O2 content (dO2) was maintained at 35 % of saturation by sparging with pure O2 gas (1 L/min) and computer-controlled, dO2-dependent adjustment of impeller speed. Culture density (wet cell weight in g/L) and Chymase activity (suc-AAPF-SBzl) were monitored every 24 hours post-inoculation for 96 hours, at which time the culture was harvested by centrifugation and the supernatant was clarified by filtration with 0.8 µm Supor®-800 membrane filters (Pall). Cell-free medium was stored at -20 °C.
Purification
Filtered fermentation supernatant was thawed, adjusted to pH = 6.0 with 5 M NaOH, centrifuged, and filtered as above to remove newly formed precipitate. Cation exchange chromatography used 500 mL of clarified medium diluted 1:1 with deionized water that was loaded onto a MacroPrep® High S support (Bio-Rad) column (2.5 cm diameter, 5 cm bed height, pre-equilibrated with wash buffer containing 10 mL MES, 50 mM NaCl, pH = 6.0) at 4 °C with a 0.65 mL/min flow rate. The column was rinsed with wash buffer at 5 mL/min until the A280 dropped below 0.050. Proteins eluted from the column across 150 mL of an elution gradient between 50 mM and 1 M NaCl in 10 mM MES, pH = 6.0. Fractions (2 mL) were analyzed for protein content (A280) and chymase activity. Those with suc-AAPF-SBzl activity greater than 25 units/µL (1 unit = 1 mA410/min) were pooled and concentrated with a 10 kDa molecular weight cutoff membrane (Pall) concurrent with buffer exchange into heparin column equilibration buffer (10 mM MES, 0.2 M NaCl, 10 % glycerol, 0.01 % octyl β-D-glucopyranoside, 0.02 % NaN3, pH = 6.0).
Concentrated (Man)5-rhChymase recovered by cation exchange was loaded onto a heparin HyperD® M (Pall) affinity column (1.5 cm diameter, 14 cm bed height) at 1 mL/min. The column was then washed (2 mL/min) with heparin column equilibration buffer until the A280 dropped below 0.020. An elution gradient in heparin column equilibration buffer increased the concentration of (NH4)2(SO4) from 0.0 M – 0.75 M across 150 mL. Active fractions (>20 units/µL) were pooled and analyzed for A280 and suc-AAPF-SBzl activity and concentrated with buffer exchange into the heparin column equilibration buffer without (NH4)2(SO4).
Deglycosylation
Purified (Man)5-rhChymase was treated with recombinant peptide N-glycosidase F (PNGase F) (Genzyme) per the included protocol. Briefly, 0.5 % SDS and 25 mM DTT were added to 20 µg of (Man)5-rhChymase in 10 µL of heparin column equilibration buffer and denatured by boiling (5 minutes), followed by the addition of 5 µL of 7.5 % Nonidet™ P-40 (Sigma-Aldrich), 13.8 µL deionized water, and 1.3 µL PNGase F. The 30 µL reaction mixture incubated overnight at 37 °C and was arrested by addition of 10 µL NuPAGE® 4× LDS sample loading buffer (Life Technologies) with 10 mM DTT.
Electrophoresis
SDS-PAGE used 12% Bis-Tris NuPAGE® (Life Technologies) gels, MOPS SDS running buffer, and LDS sample loading buffer containing 10 mM DTT. Spectra® multicolor broad range protein ladder (Fermentas) was used (5 µL) as a molecular weight standard. Gels ran for 45 minutes at 200 V (constant), followed by staining with NuBlu Express Coomassie Stain (NuSEP).
Western Blot
Proteins from an SDS-PAGE gel were blotted to 0.2 µm PVDF by semi-dry transfer in Western transfer buffer (50 mM Tris, 40 mM glycine, 0.04 % SDS, 20 % methanol), using 200 mA (constant) with a 20 V limit for 30 minutes (BioRad). The PVDF membrane was rinsed 3× for 5 minutes in Tris-buffered saline with Tween-20 (TBST, 50 mM Tris, 150 mM NaCl, 0.05 % Tween-20, pH = 7.6) and blocked with 1 % BSA in TBST for 1 hour. Rabbit anti-chymase polyclonal antibody (Proteintech Group) diluted 1:1000 in blocking buffer was incubated with the membrane overnight. After rinsing 5× for 5 minutes in TBST, the membrane was incubated 1 hour with HRPconjugated goat anti-rabbit IgG (Pierce) diluted 1:5000 in TBST, treated with Luminata™ Crescendo Western HRP substrate (Millipore) and visualized on a GBox (Syngene).
Activity Assays
All chymase activity assays were conducted in 100 µL reaction volumes in Costar® 96-well ½-area microtiter plates (Corning), with 410nm absorbance readings every 30 seconds for 5 minutes using a PowerWave™ XS2 plate reader with Gen5™ software (BioTek) at 25 °C. This device corrects absorbance readings for a 1 cm path length to facilitate quantification accuracy. Briefly, enzyme samples were mixed with chymase assay buffer (0.1 M HEPES, 1 M NaCl, 10 % glycerol, 0.02 % NaN3, pH = 7.5), sometimes with 0.01 mg/mL heparin, to a total volume of 50 µL. Each reaction was initiated by the addition of 50 µL of 2× substrate solution containing chymase assay buffer (with or without 0.01 mg/mL heparin), 20 % DMSO, and suc-AAPF-pNA (Sigma) [55] or suc-AAPF-SBzl (LifeTein) [56]. The 2× substrate solution for suc-AAPF-SBzl assays also included 2 mM 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB).
Active Site Titration
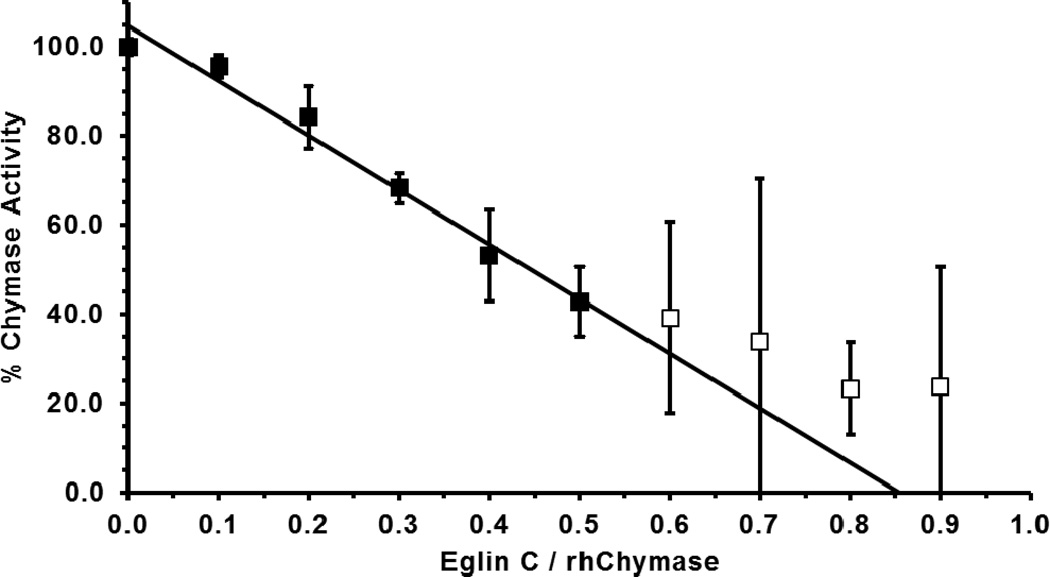
The protein concentration of purified (Man)5-rhChymase in heparin equilibration buffer was estimated with the specific absorption coefficient , as calculated by amino acid sequence [57]. A 1 mM stock of recombinant Eglin C (generously provided by Dr. H. P. Schnebi; Ciba-Geigy, Basel, Switzerland) was prepared separately in deionized water by weight and further quantified by A280 . Titration of (Man)5-rhChymase (10 pmol) was performed by adding increasing amounts of Eglin C (1–9 pmol in 1 pmol increments) [58]. After incubation at room temperature for 30 minutes in a total volume of 50 µL of chymase assay buffer containing 0.01 mg/mL heparin, activity assays were initiated by addition of 50 µL of 2× substrate solution containing 600 µM suc-AAPF-SBzl and 2 mM DTNB. Assays were repeated in triplicate and the average residual activities were expressed as percentages of the average uninhibited activity, then plotted as a function of the ratio of Eglin C/rhChymase (by A280). Regression analysis of the linear portion of the resulting curve was used to extrapolate the x-intercept, the ratio of active rhChymase/total rhChymase. This quantification of active (Man)5-rhChymase was used for subsequent Michaelis-Menten enzyme kinetic analyses.
Enzyme Kinetic Analyses
To determine Michaelis constants (KM) and catalytic rate constants (kcat), activity assays were performed with suc-AAPF-SBzl (5 – 300 µM) and suc-AAPF-pNA (0.25 – 4.0 mM) in the presence and absence of 0.01 mg/mL heparin. Assays with suc-AAPF-SBzl contained 2.5 nM active (Man)5-rhChymase and assays with suc-AAPF-pNA contained 100 nM active (Man)5-rhChymase. For these assays, 2× substrate solutions were prepared with different concentrations of substrate and the reactions were initiated by addition of (Man)5-rhChymase in chymase assay buffer. Assays were performed in triplicate and all results were used for hyperbolic regression analysis with Hyper32 (J. Easterby, © 2003).
Results
Overexpression by Fermentation
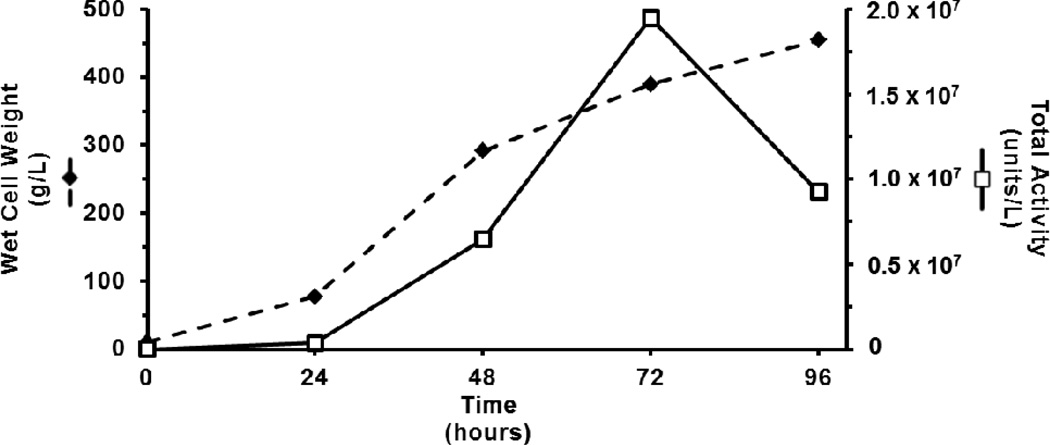
The culture biomass based on wet cell weight (g/L), expanded throughout the 96- hour fermentation with fastest growth between 24–48 hours post-inoculation, the first 24 hours of the glycerol-limited fed-batch phase (Figure 2). Chymase-like proteolytic activity, measured with the substrate suc-AAPF-SBzl, accumulated exponentially over 72 hours. After 72 hours enzyme activity decreased rapidly, despite continued accumulation of yeast biomass. Medium harvested at 96 hours contained 9.26 × 106 units of activity per liter (Figure 2).
Figure 2. Fermentation growth and productivity.
Culture density ( , WCW in g/L) and total chymase-like activity (
, WCW in g/L) and total chymase-like activity ( , units/L) for the substrate suc-AAPF-SBzl were monitored daily for 96 hours of fermentation. Although yeast biomass accumulated throughout, total activity declined rapidly after 72 hours.
, units/L) for the substrate suc-AAPF-SBzl were monitored daily for 96 hours of fermentation. Although yeast biomass accumulated throughout, total activity declined rapidly after 72 hours.
Purification
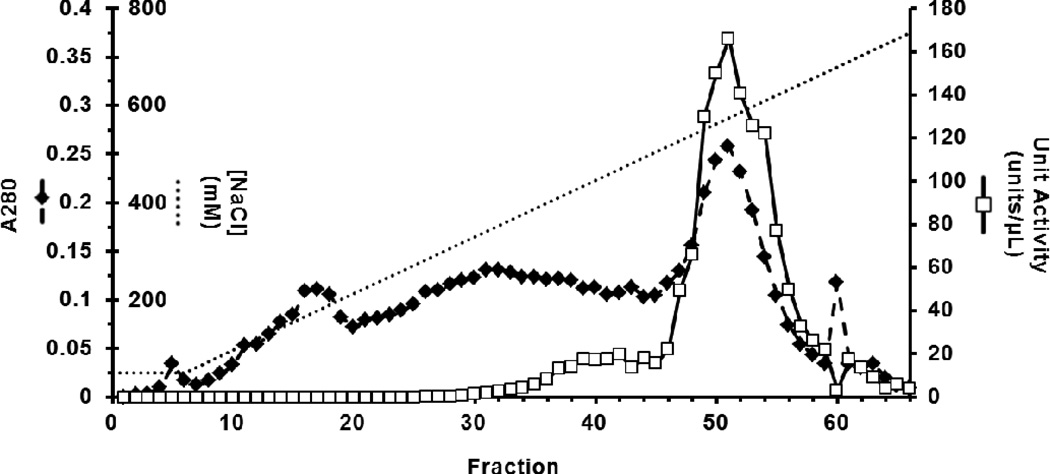
Upon thawing, the pH-adjusted and clarified medium contained 2.52 × 106 units/L, only 27% of the activity present at 96 hours (Table 1). Cation exchange chromatography yielded 5.0 mg of (Man)5-rhChymase from 500 mL of filtered supernatant that eluted at a salt concentration above 500 mM NaCl as a single, symmetrical peak in fractions 47–57 (Figure 3). Only the 11 most active fractions (22ml) were pooled to maximize purity. Some enzyme of low specific activity (units/A280) eluted between 400–500 mM NaCl in fractions 35–46 and was excluded from the pool (Figure 3). This step purified the active enzyme 35-fold from raw medium (Table 1) and resulted in nearly homogeneous protein (Figure 4a).
Table 1.
Purification of (Man)5-rhChymase.
| Step | Total Protein (A280) |
Total Activity (units) |
Specific Activity (units/A280) |
Fold- Purification |
% Yield |
|---|---|---|---|---|---|
| Diluted Medium | 7.25 | 1.26×106 | 1.74×105 | 1 | - |
| Cation Exchange | 0.243 | 1.49×106 | 6.13×106 | 35.2 | 100 |
| Heparin Affinity | 0.083 | 1.16×106 | 1.40×107 | 80.5 | 78 |
Clarified fermentation medium (500 mL) was diluted 1:1 with dH2O prior to cation exchange chromatography. Total activity (1 unit = 1 mA410/min) reports activity for the total sample volume at each step, measured using the chymase substrate suc-AAPF-SBzl. Fold-purification demonstrates increases in specific activity relative to the diluted medium. The % yield is reported as a percentage of the total activity recovered from cation exchange. Accurate quantification of yield based on fermentation medium could not be achieved due to differences in buffer conditions and potential endogenous yeast enzymes and inhibitors.
Figure 3. Elution profile for cation exchange chromatography.
Total protein ( , A280) and chymase unit activity (
, A280) and chymase unit activity ( , units/µL) eluted across a NaCl concentration gradient (······, 50–750 mM) collected in 2 mL fractions. Approximately 78 % of the total eluted enzyme activity was recovered in 22 mL as a single, symmetrical peak across fractions 47–57. Other active fractions were excluded from the sample pool to maximize purity.
, units/µL) eluted across a NaCl concentration gradient (······, 50–750 mM) collected in 2 mL fractions. Approximately 78 % of the total eluted enzyme activity was recovered in 22 mL as a single, symmetrical peak across fractions 47–57. Other active fractions were excluded from the sample pool to maximize purity.
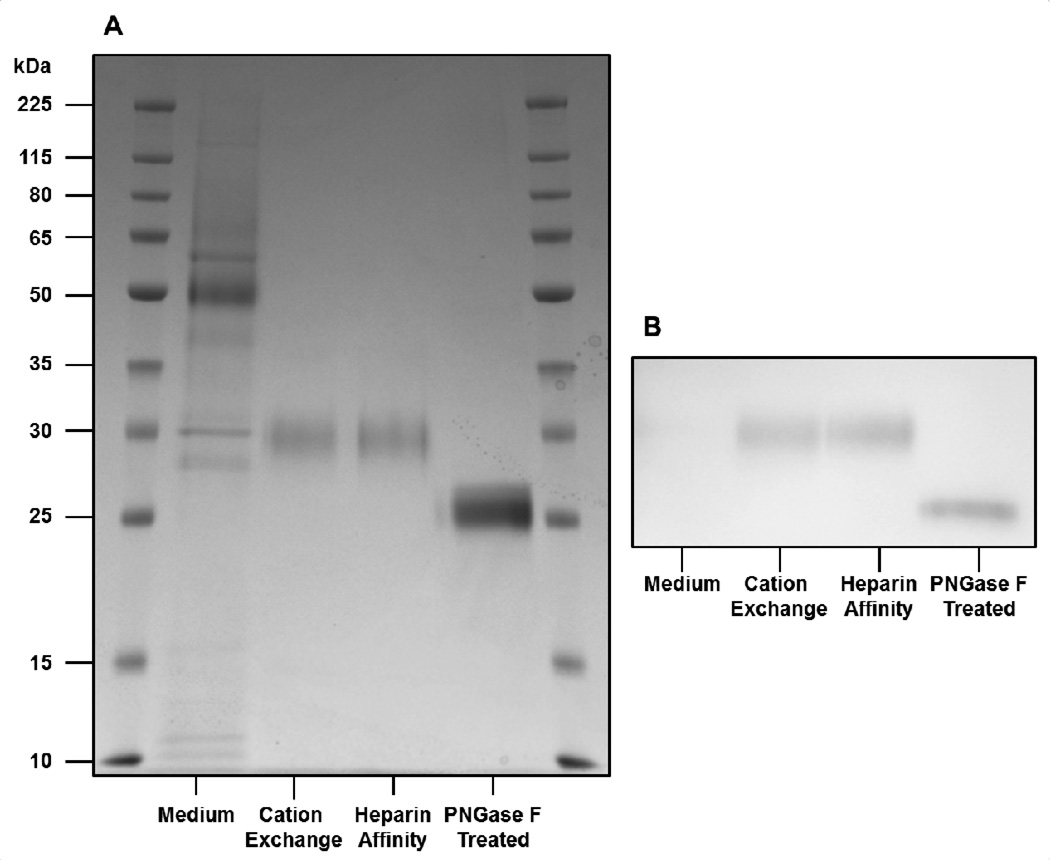
Figure 4. SDS-PAGE and Western blot demonstrate purity and identity of (Man)5-rhChymase as well as presence of Asn-linked glycosylation.
(a) SDS-PAGE shows protein content of fermentation medium as well as purification by sequential cation exchange and heparin affinity chromatography. The purified enzyme migrates at 30 kDa, as anticipated for native human chymase, and treatment with PNGase F causes a mobility shift to 25 kDa, the calculated molecular weight of deglycosylated human chymase. (b) Western blot with anti-chymase antibody labels rhChymase, with and without glycosylation.
Subsequent heparin affinity chromatography resulted in a single symmetrical peak of A280 and activity eluting above 350–400 mM (NH4)2(SO4) with slightly improved enzyme purity (Table 1; Figure 4a). To maximize purity, only fractions with activity greater than 20 units/µL were pooled, yielding 2.7 mg (by A280) of (Man)5-rhChymase for further analyses (Table 1).
Electrophoresis, Immunodetection, and Deglycosylation
Purified (Man)5-rhChymase migrated on SDS-PAGE as a single band at 30 kDa (Figure 4a, b), the migration pattern previously established for native human chymase [14, 59–61]. Antibodies specific to human chymase labeled (Man)5-rhChymase, further confirming the identity of the protein (Figure 4b). The enzyme concentration in the medium was below the detectable limit for the antibodies. Deglycosylated (Man)5-rhChymase migrated at 25 kDa, the calculated molecular weight of the human chymase amino acid sequence (Figure 4a, b). These results indicate both the purity and identity of (Man)5-rhChymase.
Enzyme Kinetic Analyses
Titration of purified (Man)5-rhChymase with Eglin C, a potent chymase inhibitor [58, 62], was used to measure the amount of active enzyme in the sample. Increasing concentrations of Eglin C inhibited (Man)5-rhChymase in a linear fashion resulting in the determination that the purified enzyme was 85% active (R2 = 0.98) (Figure 5) at the time of the assay. These data yielded precise quantification of the active enzyme for subsequent kinetic analyses with peptide p-nitroanilide and thiobenzyl ester substrates.
Figure 5. Active site titration of (Man)5-rhChymase with eglin C.
(Man)5-rhChymase (10 pmol) was incubated with 0–9 pmol eglin C for 30 minutes at room temperature prior to measuring residual activity with suc-AAPF-SBzl. Residual % activity (mean ± S.D. of three independent reactions) are shown for each eglin C/rhChymase ratio. Residual activity decreases in a linear fashion at eglin C/rhChymase ratios below 0.5 (■), becoming non-linear at ratios above 0.5 (□). Linear regression (R2 = 0.98) of data at ratios below 0.5 intercepts the xaxis at a ratio of 0.85, showing that the rhChymase sample was 85 % active.
The Michaelis constant, KM, for cleavage of suc-AAPF-pNA by (Man)5-rhChymase was 935 µM (Table 2), in close agreement with the previously reported KM of about 950 µM for native human chymase under identical buffer conditions [63]. Addition of heparin significantly (P < 0.005) reduced the KM of (Man)5-rhChymase to 488 µM (Table 2), demonstrating that heparin improves rhChymase affinity [46, 64]. The KM for the thiobenzyl ester substrate (suc-AAPF-SBzl) in the absence of heparin was 35 µM and heparin reduced the KM to 15.7 µM (Table 2).
Table 2.
Kinetic Parameters of (Man)5-rhChymase.
| KM (M) | kcat (sec−1) | kcat/KM (M−1 sec−1) | |
|---|---|---|---|
| Suc-AAPF-pNA | |||
| − Heparin | 9.35×10−4 ± 0.925×10−4 | 3.6 ± 0.1 | 3.85×103 ± 0.394×103 |
| + Heparin | 5.14×10−4 ± 0.533×10−4 | 2.5 ± 0.1 | 4.86×103 ± 0.394×103 |
| Suc-AAPF-SBzl | |||
| − Heparin | 3.55×10−5 ± 1.18×10−5 | 12.6 ± 0.9 | 3.55×105 ± 0.990×105 |
| + Heparin | 1.57×10−5 ± 0.612×10−5 | 17.8 ± 1.1 | 1.13×106 ± 0.388×106 |
Michaelis-Menten enzyme kinetics of (Man)5-rhChymase are reported for suc-AAPF-pNA and suc-AAPF-SBzl, with and without 0.01 mg/mL heparin. All reactions contained 0.1 M HEPES, 1 M NaCl, 10 % Glycerol, 0.02% NaN3, 10 % DMSO, pH = 7.5. Assays with suc-AAPF-SBzl also contained 1 mM DTNB. Values show mean ± S.D. of three independent replicates.
The catalytic rate constant, kcat, for cleavage of suc-AAPF-pNA was 3.6 s−1 and decreased to 2.5 s−1 in the presence of heparin (Table 2). Both results are much lower than the kcat of about 50 s−1 previously indicated for native human chymase under identical buffer conditions in the absence of heparin [63]. (Man)5-rhChymase cleaved suc-AAPF-SBzl with a kcat of 12.6 s−1 in the absence of heparin and the kcat increased to 17.8 s−1 with heparin.
Discussion
This work presents a robust improvement in expression of active rhChymase compared to previous work. Purification yields reported here reflect only a portion of the total quantity of active (Man)5-rhChymase produced by the fermentation. Data indicate that the medium contained much more enzyme at 72 hours. Still, 5 mg/L was recovered from a sample with approximately 1/4th the activity of the medium at 96 hours. Accurate determination of total production of active enzyme could not be achieved because the enzymatic activity is sensitive to ionic strength, as has been reported for other enzymes [65], and decreased after 72 hours, likely due to accumulation of endogenous yeast proteases. Based on the available data, the yeast may produce 30 mg/L or more of active enzyme under optimum conditions.
Biochemical characterization of purified (Man)5-rhChymase demonstrates considerable similarity to the native human enzyme. Based on apparent molecular weights, the glycosylation of (Man)5-rhChymase appears to mimic that of native human chymase, which has not been identified. Active (Man)5-rhChymase was inhibited by recombinant Eglin C in the same manner as human chymase [58].
The KM of (Man)5-rhChymase for suc-AAPF-pNA in the absence of heparin compares very closely to the KM of chymase purified from human skin under the same assay conditions. Heparin significantly improved the KM for the suc-AAPF-pNA substrate and similar effects were shown for the suc-AAPF-SBzl substrate. The kcat values for cleavage of suc-AAPF-pNA were much lower than previous findings for human chymase [63]. This difference may be due to the method used to determine the amount of active enzyme. While active site titration with recombinant Eglin C was used for our studies, prior kinetic studies were based on titration with soybean trypsin inhibitor [66]. The source of this discrepancy could not be identified.
The use of SuperMan5 P. pastoris prevented hyperglycosylation, a common issue with protein expression in yeast [41], and resulted in a homogeneous enzyme with properties similar to native human chymase. Easy production and purification avoid the biohazards associated with tissue-derived native enzyme. Further bioengineering of P. pastoris could yield fully humanized rhChymase once native glycosylation patterns have been determined; however, the (Man)5-rhChymase described provides the closest recombinant substitute currently available.
Active human chymase expressed in P. pastoris without hyperglycosylation.
Improved production levels of recombinant human chymase compared to previous work.
Structural and kinetic properties of recombinant human chymase closely resemble native enzyme.
Acknowledgments
Funding
Supported by a Student Faculty Collaborative Grant from the ETSU Honors College and ETSU Office of Research and Sponsored Programs and by NHLB grant [R15HL091770].
Abbreviations
- hChymase
Native human chymase
- rhChymase
recombinant human Chymase
- (Man)5-rhChymase
rhChymase with (Man)5(GlcNAc)2 glycans
- suc-AAPF-pNA
succinyl-Ala-Ala-Pro-Phe-p-nitroanilide
- suc-AAPF-SBzl
succinyl-Ala-Ala-Pro-Phe thiobenzyl ester
- SMM
synthetic minimal medium
- BSM
basal salts medium
- PNGase F
peptide N-glycosidase F
- DTNB
5,5’-dithiobis-(2-nitrobenzoic acid)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution
E.T.S. and D.A.J. designed the project. E.T.S., E.T.P, and M.B.S. performed the experiments. E.T.S. and D.A.J. analyzed the data and wrote the manuscript. All authors have approved the final article.
References
- 1.Andersson MK, Enoksson M, Gallwitz M, Hellman L. The extended substrate specificity of the human mast cell chymase reveals a serine protease with well-defined substrate recognition profile. Int Immunol. 2009;21:95–104. doi: 10.1093/intimm/dxn128. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611–2615. [PubMed] [Google Scholar]
- 3.Sayama S, Iozzo RV, Lazarus GS, Schechter NM. Human skin chymotrypsin-like proteinase chymase. Subcellular localization to mast cell granules and interaction with heparin and other glycosaminoglycans. J Biol Chem. 1987;262:6808–6815. [PubMed] [Google Scholar]
- 4.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 6.Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997;61:233–245. doi: 10.1002/jlb.61.3.233. [DOI] [PubMed] [Google Scholar]
- 7.Dai H, Korthuis RJ. Mast Cell Proteases and Inflammation. Drug Discov Today Dis Models. 2011;8:47–55. doi: 10.1016/j.ddmod.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998;125:1491–1500. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okumura K, Takai S, Muramatsu M, Katayama S, Sakaguchi M, Kishi K, Jin D, Miyazaki M. Human chymase degrades human fibronectin. Clin Chim Acta. 2004;347:223–225. doi: 10.1016/j.cccn.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Furubayashi K, Takai S, Jin D, Miyazaki M, Katsumata T, Inagaki S, Kimura M, Tanaka K, Nishimoto M, Fukumoto H. Chymase activates promatrix metalloproteinase-9 in human abdominal aortic aneurysm. Clin Chim Acta. 2008;388:214–216. doi: 10.1016/j.cca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Frank BT, Rossall JC, Caughey GH, Fang KC. Mast cell tissue inhibitor of metalloproteinase-1 is cleaved and inactivated extracellularly by alpha-chymase. J Immunol. 2001;166:2783–2792. doi: 10.4049/jimmunol.166.4.2783. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita A, Urata H, Bumpus FM, Husain A. Multiple determinants for the high substrate specificity of an angiotensin II-forming chymase from the human heart. J Biol Chem. 1991;266:19192–19197. [PubMed] [Google Scholar]
- 13.Komeda K, Jin D, Takai S, Hayashi M, Takeshita A, Shibayama Y, Tanigawa N, Miyazaki M. Significance of chymase-dependent angiotensin II formation in the progression of human liver fibrosis. Hepatol Res. 2008;38:501–510. doi: 10.1111/j.1872-034X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One. 2011;6:e28501. doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Company C, Piqueras L, Naim Abu Nabah Y, Escudero P, Blanes JI, Jose PJ, Morcillo EJ, Sanz MJ. Contributions of ACE and mast cell chymase to endogenous angiotensin II generation and leucocyte recruitment in vivo. Cardiovasc Res. 2011;92:48–56. doi: 10.1093/cvr/cvr147. [DOI] [PubMed] [Google Scholar]
- 16.Arooj M, Kim S, Sakkiah S, Cao GP, Lee Y, Lee KW. Molecular modeling study for inhibition mechanism of human chymase and its application in inhibitor design. PLoS One. 2013;8:e62740. doi: 10.1371/journal.pone.0062740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arooj M, Sakkiah S, Kim S, Arulalapperumal V, Lee KW. A combination of receptor-based pharmacophore modeling & QM techniques for identification of human chymase inhibitors. PLoS One. 2013;8:e63030. doi: 10.1371/journal.pone.0063030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka T, Sugawara H, Maruoka H, Imajo S, Muto T. Discovery of novel series of 6-benzyl substituted 4-aminocarbonyl-1,4-diazepane-2,5-diones as human chymase inhibitors using structure-based drug design. Bioorg Med Chem. 2013;21:4233–4249. doi: 10.1016/j.bmc.2013.04.079. [DOI] [PubMed] [Google Scholar]
- 19.Arooj M, Thangapandian S, John S, Hwang S, Park JK, Lee KW. 3D QSAR pharmacophore modeling, in silico screening, and density functional theory (DFT) approaches for identification of human chymase inhibitors. Int J Mol Sci. 2011;12:9236–9264. doi: 10.3390/ijms12129236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kervinen J, Crysler C, Bayoumy S, Abad MC, Spurlino J, Deckman I, Greco MN, Maryanoff BE, de Garavilla L. Potency variation of small-molecule chymase inhibitors across species. Biochem Pharmacol. 2010;80:1033–1041. doi: 10.1016/j.bcp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Greco MN, Hawkins MJ, Powell ET, Almond HR, de Garavilla L, Hall J, Minor LK, Wang Y, Corcoran TW, Di Cera E, Cantwell AM, Savvides SN, Damiano BP, Maryanoff BE. Discovery of potent, selective, orally active, nonpeptide inhibitors of human mast cell chymase. J Med Chem. 2007;50:1727–1730. doi: 10.1021/jm0700619. [DOI] [PubMed] [Google Scholar]
- 22.Masaki H, Mizuno Y, Tatui A, Murakami A, Koide Y, Satoh S, Takahashi A. Structure-activity relationship of benzo[b]thiophene-2-sulfonamide derivatives as novel human chymase inhibitors. Bioorg Med Chem Lett. 2003;13:4085–4088. doi: 10.1016/j.bmcl.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 23.Akahoshi F, Ashimori A, Sakashita H, Yoshimura T, Eda M, Imada T, Nakajima M, Mitsutomi N, Kuwahara S, Ohtsuka T, Fukaya C, Miyazaki M, Nakamura N. Synthesis, structure-activity relationships, and pharmacokinetic profiles of nonpeptidic difluoromethylene ketones as novel inhibitors of human chymase. J Med Chem. 2001;44:1297–1304. doi: 10.1021/jm000497n. [DOI] [PubMed] [Google Scholar]
- 24.Akahoshi F, Ashimori A, Sakashita H, Yoshimura T, Imada T, Nakajima M, Mitsutomi N, Kuwahara S, Ohtsuka T, Fukaya C, Miyazaki M, Nakamura N. Synthesis, structure-activity relationships, and pharmacokinetic profiles of nonpeptidic alpha-keto heterocycles as novel inhibitors of human chymase. J Med Chem. 2001;44:1286–1296. doi: 10.1021/jm000496v. [DOI] [PubMed] [Google Scholar]
- 25.Akahoshi F, Ashimori A, Yoshimura T, Imada T, Nakajima M, Mitsutomi N, Kuwahara S, Ohtsuka T, Fukaya C, Miyazaki M, Nakamura N. Non-peptidic inhibitors of human chymase. Synthesis, structure-activity relationships, and pharmacokinetic profiles of a series of 5-amino-6-oxo-1,6-dihydropyrimidine-containing trifluoromethyl ketones. Bioorg Med Chem. 2001;9:301–315. doi: 10.1016/s0968-0896(00)00244-3. [DOI] [PubMed] [Google Scholar]
- 26.Iijima K, Katada J, Hayashi Y. Symmetrical anhydride-type serine protease inhibitors: structureactivity relationship studies of human chymase inhibitors. Bioorg Med Chem Lett. 1999;9:413–418. doi: 10.1016/s0960-894x(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 27.de Garavilla L, Greco MN, Sukumar N, Chen ZW, Pineda AO, Mathews FS, Di Cera E, Giardino EC, Wells GI, Haertlein BJ, Kauffman JA, Corcoran TW, Derian CK, Eckardt AJ, Damiano BP, Andrade-Gordon P, Maryanoff BE. A novel, potent dual inhibitor of the leukocyte proteases cathepsin G and chymase: molecular mechanisms and anti-inflammatory activity in vivo. J Biol Chem. 2005;280:18001–18007. doi: 10.1074/jbc.M501302200. [DOI] [PubMed] [Google Scholar]
- 28.Takai S, Sumi S, Aoike M, Sakaguchi M, Itoh Y, Jin D, Matsumura E, Miyazaki M. Characterization of recombinant human chymase expressed in Escherichia coli. Jpn J Pharmacol. 2000;82:144–149. doi: 10.1254/jjp.82.144. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZM, Rubin H, Schechter NM. Production of active recombinant human chymase from a construct containing the enterokinase cleavage site of trypsinogen in place of the native propeptide sequence. Biol Chem Hoppe Seyler. 1995;376:681–684. doi: 10.1515/bchm3.1995.376.11.681. [DOI] [PubMed] [Google Scholar]
- 30.McGrath ME, Osawa AE, Barnes MG, Clark JM, Mortara KD, Schmidt BF. Production of crystallizable human chymase from a Bacillus subtilis system. FEBS Lett. 1997;413:486–488. doi: 10.1016/s0014-5793(97)00962-9. [DOI] [PubMed] [Google Scholar]
- 31.Urata H, Karnik SS, Graham RM, Husain A. Dipeptide processing activates recombinant human prochymase. J Biol Chem. 1993;268:24318–24322. [PubMed] [Google Scholar]
- 32.Ferry G, Gillet L, Bruneau V, Banales JM, Beauverger P, Cogé F, Galizzi JP, Scalbert E, Okamoto T, Urata H, Boutin JA. Development of new assays and improved procedures for the purification of recombinant human chymase. Eur J Biochem. 2001;268:5885–5893. doi: 10.1046/j.0014-2956.2001.02544.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahooghalandari P, Hanke N, Thorpe M, Witte A, Messinger J, Hellman L. Mutations in Arg143 and Lys192 of the Human Mast Cell Chymase Markedly Affect the Activity of Five Potent Human Chymase Inhibitors. PLoS One. 2013;8:e65988. doi: 10.1371/journal.pone.0065988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson MK, Thorpe M, Hellman L. Arg143 and Lys192 of the human mast cell chymase mediate the preference for acidic amino acids in position P2' of substrates. FEBS J. 2010;277:2255–2267. doi: 10.1111/j.1742-4658.2010.07642.x. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Kaki H, Naya S, Murayama S, Tatsui A, Nagai A, Takai S, Miyazaki M. Recombinant human chymase produced by silkworm-baculovirus expression system: its application for a chymase detection kit. Jpn J Pharmacol. 2002;90:210–213. doi: 10.1254/jjp.90.210. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Walter M, Selwood T, Rubin H, Schechter NM. Recombinant expression of human mast cell proteases chymase and tryptase. Biol Chem. 1998;379:167–174. doi: 10.1515/bchm.1998.379.2.167. [DOI] [PubMed] [Google Scholar]
- 37.McEuen AR, Ashworth DM, Walls AF. The conversion of recombinant human mast cell prochymase to enzymatically active chymase by dipeptidyl peptidase I is inhibited by heparin and histamine. Eur J Biochem. 1998;253:300–308. doi: 10.1046/j.1432-1327.1998.2530300.x. [DOI] [PubMed] [Google Scholar]
- 38.Lockhart BE, Vencill JR, Felix CM, Johnson DA. Recombinant human mast-cell chymase: an improved procedure for expression in Pichia pastoris and purification of the highly active enzyme. Biotechnol Appl Biochem. 2005;41:89–95. doi: 10.1042/BA20040074. [DOI] [PubMed] [Google Scholar]
- 39.Nakakubo H, Fukuyama H, Nakajima M, Imada T, Uno S, Shiota N, Takai S, Miyazaki M, Nakamura N. Secretory production of recombinant human chymase as an active form in Pichia pastoris. Yeast. 2000;16:315–323. doi: 10.1002/1097-0061(20000315)16:4<315::AID-YEA527>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Nakakubo H, Morita M, Imada T, Takai S, Shiota N, Miyazaki M, Nakamura N. Functional reconstitution of an active recombinant human chymase from Pichia pastoris cell lysate. Yeast. 2000;16:1387–1396. doi: 10.1002/1097-0061(200011)16:15<1387::AID-YEA634>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Vervecken W, Kaigorodov V, Callewaert N, Geysens S, De Vusser K, Contreras R. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl Environ Microbiol. 2004;70:2639–2646. doi: 10.1128/AEM.70.5.2639-2646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen R, Jiang X, Sun D, Han G, Wang F, Ye M, Wang L, Zou H. Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J Proteome Res. 2009;8:651–661. doi: 10.1021/pr8008012. [DOI] [PubMed] [Google Scholar]
- 43.Lo HY, Nemoto PA, Kim JM, Hao MH, Qian KC, Farrow NA, Albaugh DR, Fowler DM, Schneiderman RD, Michael August E, Martin L, Hill-Drzewi M, Pullen SS, Takahashi H, De Lombaert S. Benzimidazolone as potent chymase inhibitor: modulation of reactive metabolite formation in the hydrophobic (P1) region. Bioorg Med Chem Lett. 2011;21:4533–4539. doi: 10.1016/j.bmcl.2011.05.126. [DOI] [PubMed] [Google Scholar]
- 44.Reiling KK, Krucinski J, Miercke LJ, Raymond WW, Caughey GH, Stroud RM. Structure of human pro-chymase: a model for the activating transition of granule-associated proteases. Biochemistry. 2003;42:2616–2624. doi: 10.1021/bi020594d. [DOI] [PubMed] [Google Scholar]
- 45.Pereira PJ, Wang ZM, Rubin H, Huber R, Bode W, Schechter NM, Strobl S. The 2.2 A crystal structure of human chymase in complex with succinyl-ala-ala-pro-phe-chloromethylketone: structural explanation for its dipeptidyl carboxypeptidase specificity. J Mol Biol. 1999;287:817. doi: 10.1006/jmbi.1999.2691. [DOI] [PubMed] [Google Scholar]
- 46.McEuen AR, Gaça MD, Buckley MG, He S, Gore MG, Walls AF. Two distinct forms of human mast cell chymase--differences in affinity for heparin and in distribution in skin, heart, and other tissues. Eur J Biochem. 1998;256:461–470. doi: 10.1046/j.1432-1327.1998.2560461.x. [DOI] [PubMed] [Google Scholar]
- 47.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 48.Jacobs PP, Geysens S, Vervecken W, Contreras R, Callewaert N. Engineering complex-type Nglycosylation in Pichia pastoris using GlycoSwitch technology. Nat Protoc. 2009;4:58–70. doi: 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- 49.Vervecken W, Callewaert N, Kaigorodov V, Geysens S, Contreras R. Modification of the Nglycosylation pathway to produce homogeneous, human-like glycans using GlycoSwitch plasmids. Methods Mol Biol. 2007;389:119–138. doi: 10.1007/978-1-59745-456-8_9. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs PP, Callewaert N. N-glycosylation engineering of biopharmaceutical expression systems. Curr Mol Med. 2009;9:774–800. doi: 10.2174/156652409789105552. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs PP, Inan M, Festjens N, Haustraete J, Van Hecke A, Contreras R, Meagher MM, Callewaert N. Fed-batch fermentation of GM-CSF-producing glycoengineered Pichia pastoris under controlled specific growth rate. Microb Cell Fact. 2010;9:93. doi: 10.1186/1475-2859-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 53.Smith ET, Johnson DA. Human enteropeptidase light chain: bioengineering of recombinants and kinetic investigations of structure and function. Protein Sci. 2013;22:577–585. doi: 10.1002/pro.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stratton J, Chiruvolu V, Meagher M. High cell-density fermentation. Methods Mol Biol. 1998;103:107–120. doi: 10.1385/0-89603-421-6:107. [DOI] [PubMed] [Google Scholar]
- 55.DelMar EG, Largman C, Brodrick JW, Geokas MC. A sensitive new substrate for chymotrypsin. Anal Biochem. 1979;99:316–320. doi: 10.1016/s0003-2697(79)80013-5. [DOI] [PubMed] [Google Scholar]
- 56.Johnson DA. Human mast cell proteases: activity assays using thiobenzyl ester substrates. Methods Mol Biol. 2006;315:193–202. doi: 10.1385/1-59259-967-2:193. [DOI] [PubMed] [Google Scholar]
- 57.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fink E, Nettelbeck R, Fritz H. Inhibition of mast cell chymase by eglin c and antileukoprotease (HUSI-I). Indications for potential biological functions of these inhibitors. Biol Chem Hoppe Seyler. 1986;367:567–571. doi: 10.1515/bchm3.1986.367.2.567. [DOI] [PubMed] [Google Scholar]
- 59.Takai S, Shiota N, Sakaguchi M, Muraguchi H, Matsumura E, Miyazaki M. Characterization of chymase from human vascular tissues. Clin Chim Acta. 1997;265:13–20. doi: 10.1016/s0009-8981(97)00114-9. [DOI] [PubMed] [Google Scholar]
- 60.Takao K, Takai S, Shiota N, Song K, Nishimura K, Ishihara T, Miyazaki M. Lack of effect of carbohydrate depletion on some properties of human mast cell chymase. Biochim Biophys Acta. 1999;1427:74–81. doi: 10.1016/s0304-4165(99)00002-1. [DOI] [PubMed] [Google Scholar]
- 61.Schechter NM, Fräki JE, Geesin JC, Lazarus GS. Human skin chymotryptic proteinase. Isolation and relation to cathepsin g and rat mast cell proteinase I. J Biol Chem. 1983;258:2973–2978. [PubMed] [Google Scholar]
- 62.Fukusen N, Kato Y, Kido H, Katunuma N. Kinetic studies on the inhibitions of mast cell chymase by natural serine protease inhibitors: indications for potential biological functions of these inhibitors. Biochem Med Metab Biol. 1987;38:165–169. doi: 10.1016/0885-4505(87)90076-4. [DOI] [PubMed] [Google Scholar]
- 63.Schechter NM, Plotnick M, Selwood T, Walter M, Rubin H. Diverse effects of pH on the inhibition of human chymase by serpins. J Biol Chem. 1997;272:24499–24507. doi: 10.1074/jbc.272.39.24499. [DOI] [PubMed] [Google Scholar]
- 64.Pejler G. Mast cell chymase in complex with heparin proteoglycan is regulated by protamine. FEBS Lett. 1996;383:170–174. doi: 10.1016/0014-5793(96)00239-6. [DOI] [PubMed] [Google Scholar]
- 65.Rao L, Zhao X, Pan F, Li Y, Xue Y, Ma Y, Lu JR. Solution behavior and activity of a halophilic esterase under high salt concentration. PLoS One. 2009;4:e6980. doi: 10.1371/journal.pone.0006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schechter NM, Sprows JL, Schoenberger OL, Lazarus GS, Cooperman BS, Rubin H. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989;264:21308–21315. [PubMed] [Google Scholar]