Abstract
Background
We previously described the construction of an HIV-1 envelope glycoprotein complex (Env) that is stabilized by an engineered intermolecular disulfide bond (SOS) between gp120 and gp41. The modified Env protein antigenically mimics the functional wild-type Env complex. Here, we explore the effects of the covalent gp120 – gp41 interaction on virus replication and evolution.
Results
An HIV-1 molecular clone containing the SOS Env gene was only minimally replication competent, suggesting that the engineered disulfide bond substantially impaired Env function. However, virus evolution occurred in cell culture infections, and it eventually always led to elimination of the intermolecular disulfide bond. In the course of these evolution studies, we identified additional and unusual second-site reversions within gp41.
Conclusions
These evolution paths highlight residues that play an important role in the interaction between gp120 and gp41. Furthermore, our results suggest that a covalent gp120 – gp41 interaction is incompatible with HIV-1 Env function, probably because this impedes conformational changes that are necessary for fusion to occur, which may involve the complete dissociation of gp120 from gp41.
Background
The trimeric HIV-1 envelope glycoprotein complex (Env) mediates viral entry into susceptible target cells. The surface subunit (SU; gp120) attaches to the receptor (CD4) and the coreceptor (CCR5 or CXCR4) on the cell surface, and subsequent conformational changes within the Env complex lead to membrane fusion mediated by the transmembrane subunit (TM; gp41) [1-4]. After intracellular cleavage of the precursor gp160 protein, three gp120 subunits stay non-covalently associated with three gp41 subunits. However, these non-covalent interactions are weak and gp120 dissociates easily from gp41, a process that, if it occurs spontaneously and prematurely, inactivates the Env complex and leads to the exposure of non-neutralizing, immune-decoy epitopes on both gp120 and gp41 [5-7]. HIV-1 vaccine strategies aimed at generating neutralizing antibodies have yielded various Env immunogens that have gp120 stably attached to gp41, usually by elimination of the natural cleavage site between gp120 and gp41. Uncleaved Env proteins, however, like the dissociated subunits, expose non-neutralizing epitopes [5-9].
We previously described the construction of a soluble Env variant that is stabilized by the introduction of an intermolecular disulfide bond between gp120 and the gp41 ectodomain (gp41e) [9,10]. This SOS gp140 protein is cleaved and it is antigenically similar to native Env. Thus, neutralizing epitopes are exposed while several non-neutralizing epitopes, which are also not accessible on the functional Env complex, are occluded. The SOS gp140 protein is conformationally flexible in that CD4 can induce conformational changes that expose the coreceptor binding site. Moreover, SOS Env can be rendered fully functional by reduction of the intermolecular disulfide bond upon the engagement of CD4 and a coreceptor [11,12]. Extensive mutagenesis revealed that the appropriate positioning of the intermolecular disulfide bond is essential. Thus, only the introduction of cysteines at position 501 in gp120 and 605 in gp41 yielded a stable, properly folded gp120/gp41 complex [9]. The extra disulfide is indeed formed, and there is no evidence that the native intramolecular disulfide bonds are affected.
Stabilization of the native Env complex by disulfide bond linkage is likely to impose constraints on Env function because a certain degree of flexibility is probably essential for Env to undergo the conformational changes that eventually lead to fusion of the viral and cellular membranes. The gp120 – gp41 interface is considered to be structurally flexible, so constraining its motion might have adverse effects [13]. For example, the conformational changes in gp120 that are induced by receptor and coreceptor binding might not be transduced to the gp41 fusion machinery because of the engineered disulfide bond between the two subunits. In addition, appropriately timed gp120 shedding may be necessary for receptor-mediated fusion, and this step is blocked by the SOS disulfide bond bridge. We have investigated whether HIV-1 would be able to accept the engineered disulfide bond by spontaneous adaptation and optimization during evolution in cell culture. This exercise could learn us more about the interaction between gp120 and gp41. Identifying compensatory mutations that would accommodate the SOS disulfide bond in a replicating virus might also be useful for the design of improved Env immunogens.
Results and Discussion
Replication of HIV-1 mutants with cysteine substitutions in gp120 and gp41
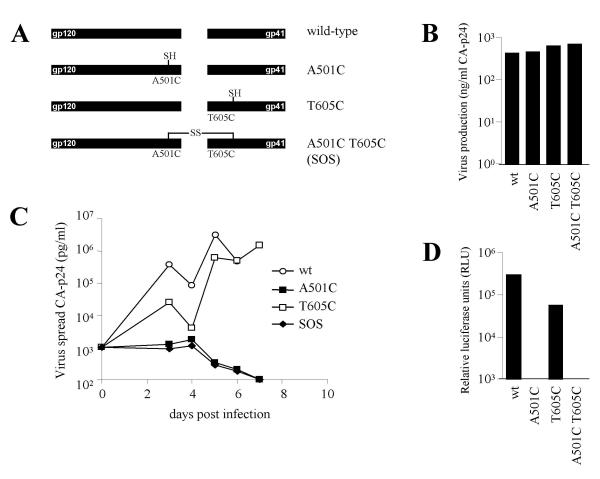
We investigated the replication potential of HIV-1 containing cysteine substitutions that are able to form an intersubunit disulfide bond between gp120 and gp41. The A501C and T605C substitutions alone or in combination (SOS Env) were introduced into the molecular clone of the CXCR4-using strain HIV-1LAI (fig. 1A). Virus stocks were generated in non-susceptible C33A producer cells by transient transfection. The three mutant viruses and the wild-type (wt) parent all produced comparable amounts of CA-p24 antigen (fig. 1B). The virus stocks were then used to infect MT-2 T cells (fig. 1C). The SOS virus was not able to initiate a spreading infection and the A501C single mutant was also replication-defective. In contrast and perhaps surprisingly, the T605C single mutant replicated efficiently, albeit with delayed kinetics compared to the wt control. Similar results were obtained using the SupT1 T cell line (results not shown). When we studied virus entry into a reporter cell line, we measured efficient entry of the wt and T605C viruses, while the A501C and SOS viruses were not able to enter the target cells (fig. 1D). We conclude that the SOS Env protein does not support virus replication, consistent with previous studies using a cell-cell fusion assay or Env-pseudotyped viruses in a single-cycle infection protocol [11,12].
Figure 1.
HIV-1LAI with an SOS-linked Env is replication-defective. A. Schematic representation of the A501C and T605C single and double (SOS) mutants used in this study. Free cysteines with a sulfhydryl group are indicated by SH and an intermolecular disulfide bond between gp120 and gp41 is indicated by SS. B. 375 × 103 MT-2 T cells were infected with 1.5 ng CA-p24 of C33A-produced virus and virus spread was monitored for 7 days by CA-p24 ELISA.
Evolution of HIV-1 with a disulfide bond between gp120 and gp41
To investigate the structural constraints imposed upon the SOS Env protein by the engineered disulfide bond and to identify viruses with potentially interesting second-site reversions, we passaged several virus cultures for a prolonged period (table 1, cultures A-C). One culture containing the A501C virus was also maintained for many weeks (table 1, culture D). Despite these efforts, we were unable to obtain any revertants of the two replication-impaired mutant viruses, underlining the deleterious effect of the intermolecular disulfide bond and the A501C single substitution on Env function. We therefore revised our experimental design by varying the cell type and increasing the amount of the transfected plasmid DNA. We also added low concentrations of β-mercaptoethanol (BME) to some of the cultures, reasoning that this reducing agent may reduce the SOS disulfide bond, thereby increasing the fusion capacity of SOS Env and virus evolution [11,12]. We first determined the concentrations of BME that are toxic for MT-2 and SupT1 cells. At 0.3 mM, BME marginally impaired the growth of both cell types, so we did not exceed this concentration. The various cultures are listed in table 1. The evolution experiments were started by transfecting 5 × 106 cells with 10 or 40 μg of the SOS Env molecular clone. The cells were cultured in small (T25) flasks for 7 days and subsequently transferred to large (T75) flasks to increase the probability of detecting a rare evolution event.
Table 1.
SOS evolution cultures
| culture | mutant | cell line | pLAI (μg) | BME (mM) | Reversiona |
| A | SOS | SupT1 | 10 | - | - |
| B | SOS | SupT1 | 10 | - | - |
| C | SOS | SupT1 | 10 | - | - |
| D | A501C | SupT1 | 10 | - | - |
| E | SOS | SupT1 | 10 | - | - |
| F1 | SOS | SupT1 | 10 | 0.1 | - |
| F3 | SOS | SupT1 | 10 | 0.3 | - |
| I | SOS | SupT1 | 40 | - | - |
| J1 | SOS | SupT1 | 40 | 0.1 | - |
| J3 | SOS | SupT1 | 40 | 0.3 | - |
| Q | SOS | MT-2 | 10 | - | - |
| R | SOS | MT-2 | 10 | 0.1 | - |
| T | SOS | MT-2 | 10 | 0.3 | - |
| U | SOS | MT-2 | 40 | - | - |
| V | SOS | MT-2 | 40 | 0.1 | - |
| X | SOS | MT-2 | 40 | 0.3 | + |
a after 7 weeks (12 weeks for cultures A-D)
The SOS Env virus acquires compensatory second-site reversions
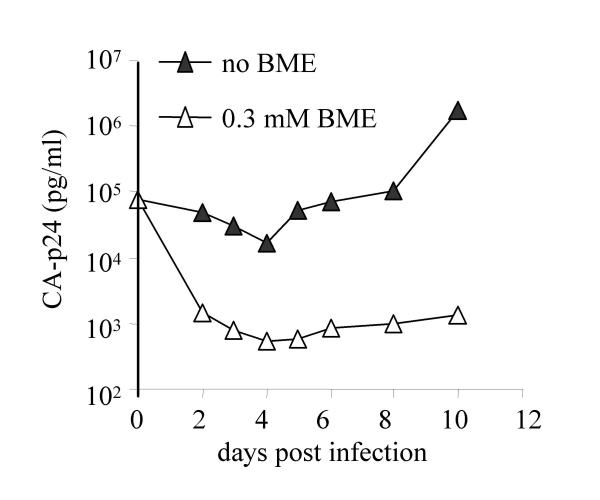
After 7 weeks of culture, we detected virus spread, as measured by CA-p24 production, in one of the 15 cultures (culture X in table 1). This culture contained MT-2 cells grown with 0.3 mM BME. To investigate whether replication of the evolved virus was triggered by or even dependent on the reducing agent, we passaged the variant onto fresh MT-2 cells in the absence or presence of BME (fig. 2). The evolved virus replicated poorly, but spread more efficiently without BME. This suggests that BME was not required for Env function and the toxicity of this compound may actually have hindered virus replication. Nevertheless, it remains possible that the initial evolution event itself was facilitated by BME, for instance by triggering entry of the original input SOS virus into cells.
Figure 2.
Replication of the evolved SOS revertant virus in the absence and presence of reducing agent. 100 μl (78 ng CA-p24) of the cell-free culture supernatant of culture X (see the text) was passaged onto 5 × 106 fresh MT-2 T cells in the presence or absence of 0.3 mM BME and virus spread was measured for 10 days.
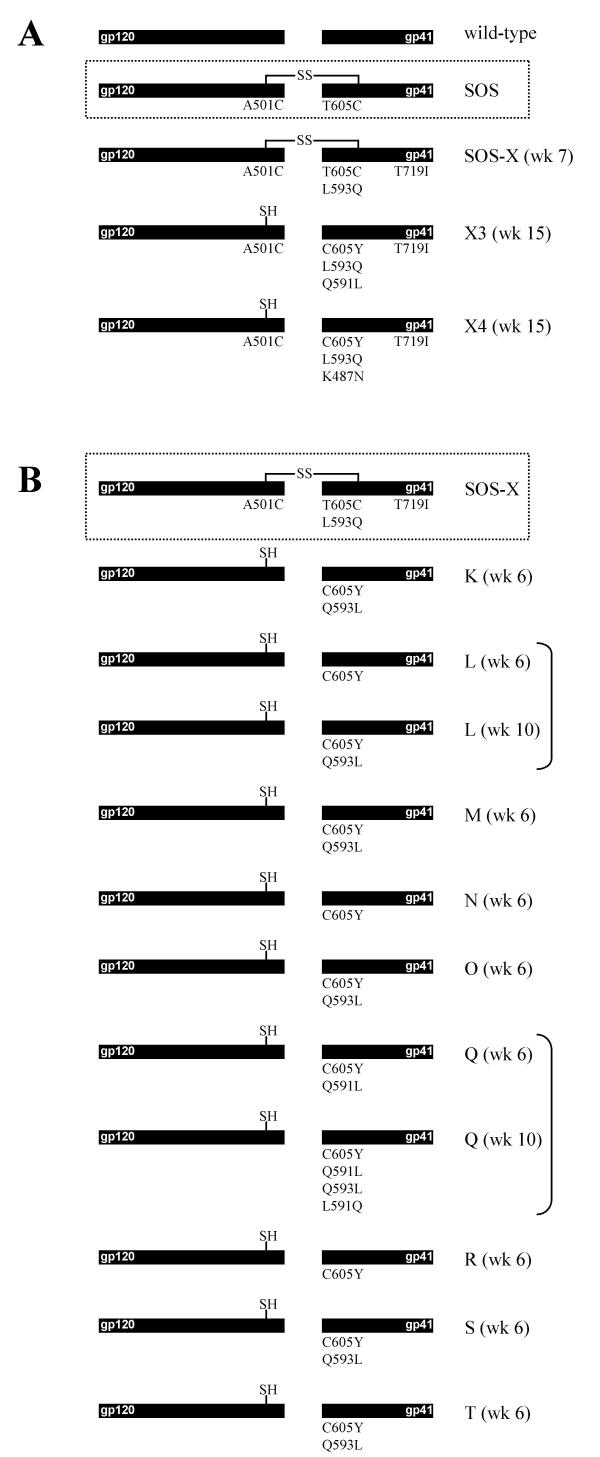
Proviral DNA was isolated from the positive culture X after 7 weeks and the env gene was PCR-amplified. Sequencing of the viral quasispecies revealed that the original SOS cysteine substitutions were still present. Two additional reversions were found: L593Q in the gp41 loop 12 residues upstream of the introduced A605C SOS cysteine, and T719I in the gp41 intracytoplasmic tail (fig. 3A).
Figure 3.
Schematic of SOS virus evolution. A. The wt Env protein and the SOS mutant are shown. SOS Env formed the starting point for evolution of the revertant virus in culture X at week 7, and this culture was split in two and cultured up to week 15 (X3 and X4; see the text). B. Virus evolution starting with the SOS-X molecular clone (A501C T605C L593Q T719I). Nine independent cultures were followed over time.
Prolonged evolution leads to elimination of the SOS disulfide bond
The slowly replicating virus present in culture X (SOS-X) was used to initiate two new infections that were continued for another two months to monitor additional evolution events (cultures X3 and X4). Consistent with a further improvement of their fitness, the resulting viruses replicated faster than the original SOS-X virus, as monitored by the rate of appearance of syncytia and CA-p24 antigen production. The env genes were PCR-amplified from proviral DNA and sequenced (fig. 3A). In both cultures, the SOS cysteine at position 605 had been replaced by a tyrosine, thus eliminating the intersubunit disulfide bond. Note that the wt amino-acid at position 605 is a threonine, but reversion to the wt codon is unlikely because it requires two nucleotide changes; a change to tyrosine requires only a single G-to-A transition. An additional reversion event was observed in each culture: Q591L in culture X3 and K487N in culture X4 (fig. 3A).
In an attempt to study the properties of a replication-competent, clonal virus that maintained the SOS disulfide bond, we cloned the env gene from the original escape virus in culture X and inserted it into the HIV-1LAI molecular clone. The variant molecular clone contained the L593Q and T719I changes, but retained the SOS disulfide bond and is designated SOS-X (A501C T605C L593Q T719I). We used this molecular clone to initiate multiple new and independent evolution experiments, hoping that escape routes might be identified that would not result in elimination of the intersubunit disulfide bond. MT2 cells were transfected with 40 μg of pLAI-SOS-X and cultured for 6–10 weeks in the absence of BME. We eventually observed faster replicating viruses in most cultures, as indicated by the appearance of syncytia and the production of CA-p24. The proviral env genes were PCR-amplified and sequenced (fig. 3B). Strikingly, the viruses in all 9 independent cultures eliminated the intersubunit disulfide bond via the C605Y first-site pseudo-reversion that we previously observed in the X3 and X4 cultures. In three cultures, no mutations other than this C605Y change occurred. Surprisingly, the L593Q substitution, which was selected in the initial SOS-X evolution, was eventually lost in 6 cultures by a de novo first-site reversion (Q593L). Two cultures exemplified that the Q593L reversion occurred after the loss of the cysteine at position 605 (cultures L and Q, compare sequences from weeks 6 and 10). The idea that the C605Y change has to precede Q593L reversion is supported by the fact that three cultures contain exclusively the C605Y reversion, but no cultures have Q593L as an individual substitution. In one culture, we detected a very similar amino-acid substitution nearby: Q591L (culture Q at week 6), which was already observed in culture X3. The Q culture evolved further in a surprising way: both the 593 and 591 residues eventually reverted to the wt residues (culture Q at week 10).
Oscillation and co-variation of the L593Q and Q591L substitutions in gp41
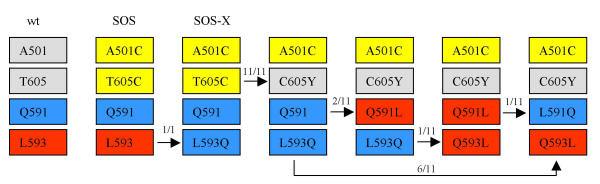
The various virus evolution pathways are depicted in figure 4. This scheme combines the results of the original cultures (X3 and X4) and the subsequent experiments (K through T), yielding 11 evolution events that started with SOS-X (A501C T605C L593Q T719I). The T719I substitution in the gp41 intracytoplasmic domain (in culture X) and the K487N substitution (in culture X4) were not tested further and are omitted from the scheme. It is possible that these reversions contributed to the gain of replication capacity by the SOS-X and X4 variants, respectively, but we chose to focus on residues in the gp41 ectodomain (residues 591 and 593). These residues are located near the SOS 605 cysteine in a region that is important for interaction with gp120 [9,13-17].
Figure 4.
SOS evolution pathways. The SOS-escape routes are summarized by focusing on four key amino-acid positions. The two SOS cysteines are marked in yellow, and loss of a cysteine changes the colour to grey. The oscillating 591 and 593 residues are also color-coded: red is L and, blue is Q. The observed frequencies of various reversions are indicated above the arrows. Both the original cultures (X3 and X4 in fig. 3A) and the subsequent cultures (K through T in fig. 3B) are included. The K487N reversion is left out of the scheme since it was only observed once (in X4) and the T719I reversion is not indicated since it was unchanged after its appearance in culture X.
The selection of the L593Q substitution in the SOS to SOS-X evolution strongly suggests that it is advantageous for viral replication in the presence of the SOS disulfide bond. However, it appears to be disadvantageous and is eliminated once the disulfide bond is lost by the C605Y substitution. Alternatively, the negative effect of the L593Q substitution in the absence of the disulfide bond can be partially overcome by acquisition of the compensatory Q591L substitution, as exemplified by two virus cultures that follow this pathway (fig. 3: X3 and Q, and fig. 4). However, given sufficient evolution time in the absence of the SOS disulfide bond, both 591 and 593 residues revert back to the wt sequence (fig. 3: culture Q).
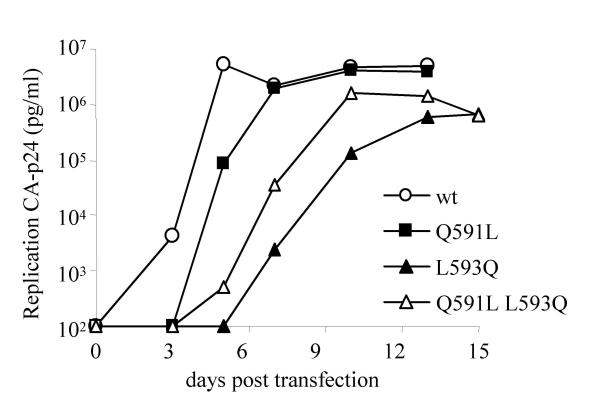
To analyze the effects of the L593Q and Q591L changes, we constructed molecular clones containing these substitutions, either individually or in combination, in the context of SOS (A501C T605C) and the revertant virus (A501C C605Y). However, the poor replication capacity of these viruses did not allow any significant further testing (results not shown). We therefore studied the effect of the L593Q and Q591L substitutions in the context of the wt virus. MT-2 T cells were transfected with the appropriate molecular clones and virus spread was measured (fig. 5). The L593Q mutant replicated with a delay of approximately 4 days compared to the wt virus. Replication of the Q591L mutant was significantly better, with a delay of only one day compared to the wt virus. Of note is that the double mutant L593Q Q591L had an intermediate phenotype, the delay being 3 days. Similar results were obtained in independent infection experiments (not shown). Thus, whereas the Q591L substitution is slightly disadvantageous for the wt Env protein, it can partially compensate for the defect caused by the L593Q substitution. The T719I substitution that was also found in the revertant SOS-X virus did not have any effect on replication of the wt virus (results not shown).
Figure 5.
Replication of the L593Q and Q591L mutant viruses. 5 × 106 MT-2 cells were transfected with 5 μg of the indicated molecular clones and virus spread was monitored for 15 days by CA-p24 ELISA.
Modeling of reversions in the gp41 structure model
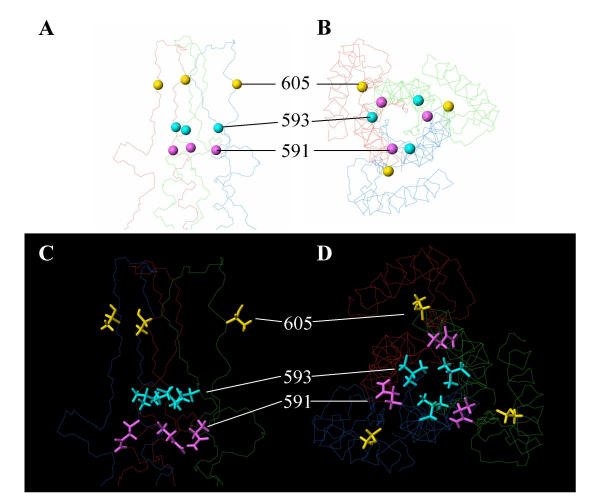
To better understand the molecular mechanisms of the oscillating 591 and 593 substitutions, we analyzed the substitutions at positions 591, 593 and 605 in a structure model of the HIV-1 gp41 loop region (fig. 6). The model is based on the SIV gp41 NMR structure and represents the post-fusion, six-helix bundle state of gp41 [18,19]. It was used because the available crystal structures of the six-helix bundle do not include the loop region [20-22]. Ideally, we would also like to model the substitutions in the pre-fusion structure of gp41 since they are likely to exert their effect on the Env complex at this stage. However, the structure of gp41 in the pre-fusion state is currently unknown. As reported previously, residue 605 (yellow in fig. 6) is on the outside of the gp41 molecule and thus available for an interaction with gp120 [18]. The side chain of residue 605 points outwards such that substitutions here would not be expected to disrupt the loop structure. Indeed, the cysteine-to-tyrosine reversion that we observed can easily be accommodated at position 605.
Figure 6.
Modeling of the SOS reversions in structure model of the HIV-1 gp41 ectodomain [18]. The Cα atoms of the relevant residues are indicated as spheres in fig. A and B, using the following color scheme: C605 is yellow, L593 is cyan, and Q591 is purple. The side chains in fig. C and D, use the same color scheme. Panels A and C depict a side view of the gp41 loop region, panels B and D a top view from the perspective of the target membrane (and of gp120).
Residues 591 and 593 are located at an equivalent position in the interior of the gp41 core, but the orientation of their side chains differs (fig. 6). The side chain of residue 593 (cyan in fig. 6) points towards the interior of the loop, thereby establishing an interaction with its counterparts in the other subunits at the trimer axis. This 593 Leu-Leu-Leu triplet stabilizes the loop structure by hydrophobic interactions. Similar hydrophobic Leu-Leu-Leu and Ile-Ile-Ile interactions stabilize the upstream coiled coil region (e.g. residues L545, I548, L555, I559, L566, I573, L576, L587). It is evident that L593 does not directly interact with residues 591 or 605. Leucine 593 can be replaced by glutamine without disrupting the backbone. This change might weaken the loop structure due to the introduction of hydrophilic side chains into the protein interior, but the glutamine side chains may rearrange to form hydrogen bonds to regain some of the lost energy. Similar to Gln-Gln-Gln interactions that are present in the coiled coil domain (e.g. residues Q552, Q562).
The side chain of residue 591 (purple in fig. 6) is located at the end of the N-terminal helix. It is partially occluded in the interior of gp41 and partially exposed on the surface. It does not directly interact with either residue 593 or 605. Replacing glutamine 591 with leucine is possible without perturbing the backbone (fig. 6C and 6D). In conclusion, the Q591L and L593Q substitutions do not appear to have dramatic effects on the gp41 post-fusion conformation, which is consistent with the notion that these reversions may exert their effects on the gp41 – gp120 interaction in the pre-fusion form of the Env complex.
Conclusions
The initial goal of our forced evolution studies was to generate SOS Env variants that could replicate despite having an intermolecular disulfide bond between gp120 and gp41. The presence of a disulfide bond between the SU and TM subunits of other viruses, including retroviruses, provides a rationale for this study [23-40]. The evolutionary selection of a disulfide bond-stabilized, but functional HIV-1 Env complex would have been useful for mechanistic studies and the design of variant SOS Env immunogens. A functional, covalently-linked Env complex would imply that gp120 shedding is not necessary for Env-mediated fusion to occur. This is still a matter of debate, but our results strongly suggest that gp120 dissociation from gp41 is required for fusion activity. A functional, covalently-linked Env complex would also be an interesting immunogen, since a functional Env complex should be a faithful mimic of the functional virion-associated Env complex. Note, however, that during the course of our evolution experiments, it became clear that unmodified SOS Env is in fact functional upon reduction of the disulfide bond, implying that it does truly mimic the functional Env complex on virions [11,12].
We did identify one SOS variant that replicated extremely poorly, but still retained the engineered cysteines (SOS-X, containing the L593Q reversion). This poorly replicating variant seemed a good candidate for subsequent evolution experiments. However, the cysteine at position 605 was always lost over time in multiple independent cultures. The L593Q reversion substitution may in fact destabilize the SOS disulfide bond (see below), thus biasing the subsequent evolution towards elimination of the disulfide bond. In conclusion, we were not able to obtain efficiently replicating viruses that retained the SOS disulfide bond. A rigid, covalent interaction between gp120 and gp41 is probably deleterious for HIV-1 replication. The dissociation of gp120 from gp41, or a significant shift in the geometry of the two subunits, may be essential for fusion to occur. This conclusion is supported by the observation that SOS Env will undergo fusion efficiently once a reducing agent is added to break the engineered disulfide bond subsequent to receptor engagement [11,12].
An intriguing question is why the loss of the SOS disulfide bond occurred in multiple independent cultures via a substitution of C605, but never of C501. This is a surprising finding given the fact that a virus with a single cysteine at position 605 is replication competent, whereas a virus with a single cysteine at position 501 is not (fig. 1). It is possible that the evolutionary possibilities at position 501 are more restricted. For example, it may take more than one nucleotide change in codon 501 to acquire a functional amino-acid. The wt A501 is strongly conserved in natural isolates and it would require at least 2 nucleotide changes to remake the C501 codon into a triplet that is present in natural virus isolates. The underlying Rev responsive element may impose additional constraints on the evolution of this codon. In contrast, the C605Y reversion is generated by a relatively easy G-to-A transition [41], and tyrosine is tolerated at this position, as exemplified by the presence of a tyrosine in subtype O isolates http://www.hiv.lanl.gov/content/index.
The evolutionary oscillation of the 591 and 593 residues (Q591 L593 or L591 Q593) has implications for understanding the molecular basis of the gp120-gp41 interaction. Molecular modeling indicated that these reversions do not have a drastic effect on the loop structure in the post-fusion, six-helix bundle configuration of gp41, although the initial L593Q substitution probably has a destabilizing effect. In the context of the SOS disulfide bond, destabilization of the loop region of gp41 could allow the disulfide bond-linked gp120 subunit to be more easily accommodated. However, inspection of the post-fusion gp41 structure does not readily explain why the Q591L secondary reversion compensates for the L593Q change in the absence of the SOS disulfide bond. We therefore favor an alternative explanation in which the initial L593Q change destabilizes the gp120-gp41 interaction. Of note is that the crystal structure of the SIV ectodomain places the side chain of residue 593 on the outside of the molecule in contrast to the NMR structure [42]. A destabilizing effect of L593Q would be consistent with previous mutagenesis studies [13,16]. For example, the L593A substitution virtually abolishes gp120-gp41 association [16]. The conservative L593V substitution also affects the gp120-gp41 interaction although the effect is more subtle [13]. Interestingly, the importance of residues involved in the gp120-gp41 interaction, including residue 593, can be dependent on the context of the particular Env, e.g. its coreceptor usage, and differs among viral isolates [13].
The L593Q reversion could either destabilize the SOS disulfide bond or prevent its formation. We were unable to detect such an effect in biochemical assays using soluble SOS gp140 (results not shown), but the effect may be marginal, since the positive effect on SOS virus replication is also minor. Substitutions at position 591 (Q591A and Q591K) are much better tolerated with regard to Env function [16], which may explain why the Q591L reversion could act as an intermediate in two independent evolution cultures. In another study on the idiotypic mimicry of two monoclonal antibodies, the stretch of residues 591–594 was shown to be an interaction site for gp120 [43]. Thus, previous mutagenesis studies, idiotypic mimicry and the forced evolution studies presented here all point to an important role for this gp41 domain in the interaction with gp120.
The stability of the gp120-gp41 interaction is delicately balanced. Too weak an interaction is deleterious to virus replication because it results in premature gp120 shedding, loss of Env function and loss of virus replication. However, a too rigid, and certainly a covalent interaction is also incompatible with HIV-1 Env function, probably because this impedes conformational changes that are necessary for fusion to occur, which may even include the complete dissociation of gp120 from gp41 [44,45].
Methods
Plasmid Constructs
The plasmid pRS1, generated to subclone mutant env genes, was generated as follows. First, the SalI-BamHI fragment from a molecular clone of HIV-1LAI (pLAI) [46] was cloned into pUC18 (Roche, Indianapolis, IN). A PstI-StuI fragment from the resulting plasmid was then cloned into a pBS-SK(+)-gp160 plasmid with the SalI-XhoI sequences of pLAI. Mutations were introduced in pRS1 using the Quickchange mutagenesis kit (Stratagene, La Jolla, CA) and verified by DNA sequencing. Mutant env genes in pRS1 were cloned into pLAI as SalI-BamHI fragments. The numbering of individual amino-acids is based on the HIV-1HXB2 gp160 sequence.
Cells and transfection
SupT1 T cells and C33A cervix carcinoma cells were maintained in RPMI 1640 medium and Dulbecco's modified eagle'S medium (DMEM), respectively (Life Technologies Ltd., Paisley, UK), supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml) and streptomycin (100 μg/ml) as previously described [47]. SupT1 and C33A cells were transfected with pLAI constructs by electroporation and Ca2(PO4)3 precipitation, respectively, as described elsewhere [48].
Virus entry and infection
Virus stocks were produced by transfecting C33A cells with the appropriate pLAI constructs. The virus containing supernatant was harvested 3 days post-transfection, filtered and stored at -80°C. The virus concentration was quantified by capsid CA-p24 ELISA as described previously [49]. The resulting values were used to normalize the amount of virus in subsequent infection experiments, which were performed as follows. T cells (3.75 × 105) were infected with 1.5 ng CA-p24 of HIV-1LAI (produced in C33A cells) per well of a 24-well plate. Subsequent virus spread was monitored by CA-p24 ELISA for 14 days. LuSIV cells, stably transfected with an LTR-luciferase construct [50], were infected with 200 ng CA-p24/300 × 103 cells/ml in a 48 well plate. Cells were maintained in the presence of 200 nM saquinavir to prevent additional rounds of virus replication. Luciferase activity was measured after 48 hrs.
Virus evolution
For evolution experiments, 5 × 106 SupT1 cells were transfected with 40 μg pLAI by electroporation. The cultures were inspected regularly for the emergence of revertant viruses, using CA-p24 ELISA and/or the appearance of syncytia as indicators of virus replication. At regular intervals, cells and filtered supernatant were stored at -80°C and virus was quantitated by CA-p24 ELISA. When a revertant virus was identified, DNA was extracted from infected cells [51], then proviral env sequences were PCR-amplified and sequenced. The complete env genes of the proviral DNA of cultures X, X3 and X4 were sequenced. Only the C5 region and gp41 were sequenced in subsequent evolution experiments.
Authors contributions
RWS carried out the initial replication and evolution experiments and drafted the manuscript. MMD carried out part of the evolution experiments and constructed the molecular clones containing the revertant amino-acids. EB performed the virus replication and virus entry studies. MC performed the modelling studies and participated in the general discussion involved in the study. JPM participated in the study design and coordination. BB supervised the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgments
We thank Stephan Heynen for technical assistance. This work was sponsored by the Dutch AIDS Fund (Amsterdam) and by NIH grants AI 39420, AI 45463 and AI 54159.
Contributor Information
Rogier W Sanders, Email: r.w.sanders@amc.uva.nl.
Martijn M Dankers, Email: mdankers@pamgene.com.
Els Busser, Email: eisje@hotmail.com.
Michael Caffrey, Email: caffrey@uic.edu.
John P Moore, Email: jpm2003@mail.med.cornell.edu.
Ben Berkhout, Email: b.berkhout@amc.uva.nl.
References
- Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003;4:309–319. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003;1614:36–50. doi: 10.1016/S0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- Parren PW, Burton DR, Sattentau QJ. HIV-1 antibody--debris or virion? Nat Med. 1997;3:366–367. doi: 10.1038/nm0497-366d. [DOI] [PubMed] [Google Scholar]
- Parren PW, Gauduin MC, Koup RA, Poignard P, Fisicaro P, Burton DR, Sattentau QJ. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol Lett. 1997;57:105–112. doi: 10.1016/S0165-2478(97)00043-6. [DOI] [PubMed] [Google Scholar]
- Parren PWHI, Moore JP, Burton DR, Sattentau QJ. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13 (Suppl. A):S137–S162. [PubMed] [Google Scholar]
- Sakurai H, Williamson RA, Crowe JE, Beeler JA, Poignard P, Bastidas RB, Chanock RM, Burton DR. Human antibody responses to mature and immature forms of viral envelope in respiratory syncytial virus infection: significance for subunit vaccines. J Virol. 1999;73:2956–2962. doi: 10.1128/jvi.73.4.2956-2962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. A recombinant HIV-1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–643. doi: 10.1128/JVI.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Schiffner L, Master A, Kajumo F, Guo Y, Dragic T, Moore JP, Binley JM. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J Virol. 2000;74:5091–5100. doi: 10.1128/JVI.74.11.5091-5100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Cayanan CS, Wiley C, Schulke N, Olson WC, Burton DR. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J Virol. 2003;77:5678–5684. doi: 10.1128/JVI.77.10.5678-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamyan LG, Markosyan RM, Moore JP, Cohen FS, Melikyan GB. Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion. J Virol. 2003;77:5829–5836. doi: 10.1128/JVI.77.10.5829-5836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poumbourios P, Maerz AL, Drummer HE. Functional evolution of the HIV-1 envelope glycoprotein gp120-association site of gp41. J Biol Chem. 2003;278:42149–42160. doi: 10.1074/jbc.M305223200. [DOI] [PubMed] [Google Scholar]
- Cao J, Bergeron L, Helseth E, Thali M, Repke H, Sodroski J. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J Virol. 1993;67:2747–2755. doi: 10.1128/jvi.67.5.2747-2755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SS. Functional role of the zipper motif region of human immunodeficiency virus type 1 transmembrane protein gp41. J Virol. 1994;68:2002–2010. doi: 10.1128/jvi.68.3.2002-2010.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerz AL, Drummer HE, Wilson KA, Poumbourios P. Functional analysis of the disulfide-bonded loop/chain reversal region of human immunodeficiency virus type 1 gp41 reveals a critical role in gp120-gp41 association. J Virol. 2001;75:6635–6644. doi: 10.1128/JVI.75.14.6635-6644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Korber B, Lu M, Berkhout B, Moore JP. Mutational analyses and natural variablility of the gp41 ectodomain. In: KuikenC, FoleyB, FreedE, HahnB, MarxP, McCutchanF, MellorsJ, WolinskyS and KorberB, editor. HIV Sequence compendium 2002. Los Alamos, New Mexico, Los Alamos National Laboratory, Theoretical Biology and Biophysics Group; 2002. pp. 43–68. [Google Scholar]
- Caffrey M. Model for the structure of the HIV gp41 ectodomain: insight into the intermolecular interactions of the gp41 loop. Biochim Biophys Acta. 2001;1536:116–122. doi: 10.1016/S0925-4439(01)00042-4. [DOI] [PubMed] [Google Scholar]
- Caffrey M, Cai M, Kaufman J, Stahl SJ, Wingfield PT, Covell DG, Gronenborn AM, Clore GM. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 1998;17:4572–4584. doi: 10.1093/emboj/17.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/S0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- Gretch DR, Gehrz RC, Stinski MF. Characterization of a human cytomegalovirus glycoprotein complex (gcI) J Gen Virol. 1988;69 ( Pt 6):1205–1215. doi: 10.1099/0022-1317-69-6-1205. [DOI] [PubMed] [Google Scholar]
- Gruber C, Levine S. Respiratory syncytial virus polypeptides. III. The envelope-associated proteins. J Gen Virol. 1983;64 (Pt 4):825–832. doi: 10.1099/0022-1317-64-4-825. [DOI] [PubMed] [Google Scholar]
- Hardwick JM, Bussell RH. Glycoproteins of measles virus under reducing and nonreducing conditions. J Virol. 1978;25:687–692. doi: 10.1128/jvi.25.2.687-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamnson RN, Halpern MS. Subunit structure of the glycoprotein complex of avian tumor virus. J Virol. 1976;18:956–968. doi: 10.1128/jvi.18.3.956-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamnson RN, Shander MH, Halpern MS. A structural protein complex in Moloney leukemia virus. Virol. 1977;76:437–439. doi: 10.1016/0042-6822(77)90318-X. [DOI] [PubMed] [Google Scholar]
- Meredith DM, Stocks JM, Whittaker GR, Halliburton IW, Snowden BW, Killington RA. Identification of the gB homologues of equine herpesvirus types 1 and 4 as disulphide-linked heterodimers and their characterization using monoclonal antibodies. J Gen Virol. 1989;70 ( Pt 5):1161–1172. doi: 10.1099/0022-1317-70-5-1161. [DOI] [PubMed] [Google Scholar]
- Montelaro RC, Sullivan SJ, Bolognesi DP. An analysis of type-C retrovirus polypeptides and their associations in the virion. Virol. 1978;84:19–31. doi: 10.1016/0042-6822(78)90215-5. [DOI] [PubMed] [Google Scholar]
- Opstelten DJ, Wallin M, Garoff H. Moloney murine leukemia virus envelope protein subunits, gp70 and Pr15E, form a stable disulfide-linked complex. J Virol. 1998;72:6537–6545. doi: 10.1128/jvi.72.8.6537-6545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A, Fleissner E. The presence of disulfide-linked gp70-p15(E) complexes in AKR murine leukemia virus. Virol. 1977;83:417–422. doi: 10.1016/0042-6822(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Pinter A, Fleissner E. Structural studies of retroviruses: characterization of oligomeric complexes of murine and feline leukemia virus envelope and core components formed upon cross-linking. J Virol. 1979;30:157–165. doi: 10.1128/jvi.30.1.157-165.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ. Comparison of structural domains of gp70s of ecotropic Akv and dualtropic MCF-247 MuLVs. Virol. 1983;129:40–50. doi: 10.1016/0042-6822(83)90394-X. [DOI] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ. Characterization of structural and immunological properties of specific domains of Friend ecotropic and dual-tropic murine leukemia virus gp70s. J Virol. 1984;49:452–458. doi: 10.1128/jvi.49.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A, Choppin PW. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virol. 1977;80:54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Storey DG, Dimock K, Kang CY. Structural characterization of virion proteins and genomic RNA of human parainfluenza virus 3. J Virol. 1984;52:761–766. doi: 10.1128/jvi.52.3.761-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurk van Drunen Littel-van den, Babiuk LA. Synthesis and processing of bovine herpesvirus 1 glycoproteins. J Virol. 1986;59:401–410. doi: 10.1128/jvi.59.2.401-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M, Scrace G, Skehel J. Disulphide bonds of haemagglutinin of Asian influenza virus. Nature. 1981;289:422–424. doi: 10.1038/289422a0. [DOI] [PubMed] [Google Scholar]
- Waxham MN, Wolinsky JS. Immunochemical identification of rubella virus hemagglutinin. Virol. 1983;126:194–203. doi: 10.1016/0042-6822(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Witte ON, Tsukamoto-Adey A, Weissman IL. Cellular maturation of oncornavirus glycoproteins: topological arrangement of precursor and product forms in cellular membranes. Virol. 1977;76:539–553. doi: 10.1016/0042-6822(77)90236-7. [DOI] [PubMed] [Google Scholar]
- Berkhout B, Das AT, Beerens N. HIV-1 RNA editing, hypermutation and error-prone reverse transcription. Science. 2001;292:7. doi: 10.1126/science.292.5514.7a. [DOI] [PubMed] [Google Scholar]
- Yang ZN, Mueser TC, Kaufman J, Stahl SJ, Wingfield PT, Hyde CC. The crystal structure of the SIV gp41 ectodomain at 1.47 A resolution. J Struct Biol. 1999;126:131–144. doi: 10.1006/jsbi.1999.4116. [DOI] [PubMed] [Google Scholar]
- Lopalco L, Longhi R, Ciccomascolo F, De Rossi A, Pelagi M, Andronico F, Moore JP, Schulz T, Beretta A, Siccardi AG. Identification of human immunodeficiency virus type 1 glycoprotein gp120/gp41 interacting sites by the idiotypic mimicry of two monoclonal antibodies. AIDS Res Hum Retroviruses. 1993;9:33–39. doi: 10.1089/aid.1993.9.33. [DOI] [PubMed] [Google Scholar]
- McKeating JA, McKnight A, Moore JP. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65:852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, McKeating JA, Weiss RA, Sattentau QJ. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virol. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-L. [DOI] [PubMed] [Google Scholar]
- Sanders RW, de Jong EC, Baldwin CE, Schuitemaker JH, Kapsenberg ML, Berkhout B. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J Virol. 2002;76:7812–7821. doi: 10.1128/JVI.76.15.7812-7821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AT, Klaver B, Berkhout B. A hairpin structure in the R region of the Human Immunodeficiency Virus type 1 RNA genome is instrumental in polyadenylation site selection. J Virol. 1999;73:81–91. doi: 10.1128/jvi.73.1.81-91.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeeninga RE, Hoogenkamp M, Armand-Ugon M, de Baar M, Verhoef K, Berkhout B. Functional differences between the LTR transcriptional promoters of HIV-1 subtypes A through G. J Virol. 2000;74:3740–3751. doi: 10.1128/JVI.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos JW, Maughan MF, Liao Z, Hildreth JE, Clements JE. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virol. 2000;273:307–315. doi: 10.1006/viro.2000.0431. [DOI] [PubMed] [Google Scholar]
- Das AT, Klaver B, Klasens BIF, van Wamel JLB, Berkhout B. A conserved hairpin motif in the R-U5 region of the human immunodeficiency virus type 1 RNA genome is essential for replication. J Virol. 1997;71:2346–2356. doi: 10.1128/jvi.71.3.2346-2356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]