Abstract
Purpose
This study investigated the trade-off in tumor coverage and organ-at-risk sparing when applying dose escalation for concurrent chemoradiation therapy (CRT) of mid-esophageal cancer, using radiobiological modeling to estimate local control and normal tissue toxicity.
Methods and Materials
Twenty-one patients with mid-esophageal cancer were selected from the SCOPE1 database (International Standard Randomised Controlled Trials number 47718479), with a mean planning target volume (PTV) of 327 cm3. A boost volume, PTV2 (GTV + 0.5 cm margin), was created. Radiobiological modeling of tumor control probability (TCP) estimated the dose required for a clinically significant (+20%) increase in local control as 62.5 Gy/25 fractions. A RapidArc (RA) plan with a simultaneously integrated boost (SIB) to PTV2 (RA62.5) was compared to a standard dose plan of 50 Gy/25 fractions (RA50). Dose-volume metrics and estimates of normal tissue complication probability (NTCP) for heart and lungs were compared.
Results
Clinically acceptable dose escalation was feasible for 16 of 21 patients, with significant gains (>18%) in tumor control from 38.2% (RA50) to 56.3% (RA62.5), and only a small increase in predicted toxicity: median heart NTCP 4.4% (RA50) versus 5.6% (RA62.5) P<.001 and median lung NTCP 6.5% (RA50) versus 7.5% (RA62.5) P<.001.
Conclusions
Dose escalation to the GTV to improve local control is possible when overlap between PTV and organ-at-risk (<8% heart volume and <2.5% lung volume overlap for this study) generates only negligible increase in lung or heart toxicity. These predictions from radiobiological modeling should be tested in future clinical trials.
Summary.
Radiobiological modeling suggests that dose escalation to the gross tumor volume in esophageal cancer has the potential to produce significant gains in tumor control with only a minor increase in lung or heart toxicity for the majority of patients. The relationship between tumor response and normal tissue toxicity during dose escalation should be carefully validated in clinical trials.
Introduction
Chemoradiation therapy (CRT) is becoming established as an effective treatment for locally advanced esophageal cancer, as a neoadjuvant strategy for operable adenocarcinomas or as a definitive treatment when there is a high risk of surgical morbidity and mortality (1). Long-term survival for operable squamous cell carcinomas treated with definitive CRT (dCRT) is comparable to surgery alone (2). Although CRT is more effective than either radiation therapy or chemotherapy alone 3, 4, locoregional control rates with the standard radiation therapy dose of ≈50 Gy are still low, and >75% of failures occur within the gross tumor volume (GTV) 5, 6.
A correlation between higher dose and improved tumor control and survival was described by Zhang et al (7) when patients were divided into low-dose (≤51 Gy) and high-dose (>51 Gy) groups. Further evidence of a radiation dose response has been found by a systematic review (8), which looked at rates of pathological complete response (pCR) in preoperative CRT. Fitting the data to a radiobiological model suggested that increasing the radiation dose prescription from the standard ≈50 Gy could result in significant improvement in tumor control probability (TCP).
Conversely, data from a phase 3 clinical trial, Radiation Therapy Oncology Group (RTOG) 9405, investigating the use of higher radiation dose (64.8 Gy) versus standard dose (50.4 Gy) found no improvement in survival or local control, and a relatively high comorbidity in the high-dose arm (9). However, the radiation therapy treatment technique at this time was based on 2D planning (using relatively large treatment fields) and a sequential dose boost regimen. Occurrence of several deaths in the high-dose arm before reaching 50.4 Gy has limited further investigation of dose escalation in spite of general understanding of 2D planning insufficiency.
The role of radiation therapy dose escalation in improving outcomes for definitive CRT has been recently identified as one of the priorities for research in esophageal cancer in the United Kingdom (10). Nevertheless, the optimal combination of radiation therapy and chemotherapy doses needs to be carefully established in order to improve locoregional control.
Long-term toxicities, particularly to the cardiorespiratory system have been described following dCRT for esophageal cancer. Acute toxicity related to substructures of the heart has been studied specifically, for example, pericardial effusion (onset within 6 months) 11, 12, 13. These studies suggest a higher mean pericardial dose is associated with increased risk of pericardial effusion. Left ventricular mean dose (14) has also been found to be related to acute cardiac impairment observed by MRI for patients undergoing CRT for esophageal cancer. Radiobiological models of long-term cardiac toxicity have also been presented using dose to whole heart (15).
Radiation-induced lung toxicity is important, not just as a result of the proximity of lung tissue to the esophageal target volume (16) but also as newer radiation therapy techniques such as IMRT or volumetric arc, generate a low dose bath to a large region of lung tissue. Recent studies of lung cancer also indicate that radiation-induced lung toxicity may be related to either pre-existing cardiac co-morbidity (17) or to concomitant irradiation of the heart (18). Although the biological mechanism is as yet unclear, there is a statistically significant association between risk of radiation pneumonitis and dose-volume variables for both heart and lung for these patients.
This study had 2 aims: (1) to estimate the level of dose escalation required for a clinically significant increase in tumor control using radiobiological modeling across a group of representative patients, and to examine whether this dose could be safely delivered by calculating normal tissue complication probability (NTCP) for heart and lungs; and (2) to evaluate the feasibility of a clinical trial using dose escalation for esophageal cancer patients treated with definitive CRT by comparing results for individual patients.
Methods and Materials
A subset of 21 mid-thoracic esophageal cancer patients (10% of the total radiation therapy group) was selected from the SCOPE1 database (ISRCTN 47718479). This subset had a range of planning target volumes of 140-591 cm3 (Table 1) and a mean PTV (327 cm3) consistent with the entire original cohort (mean 334 cm3). Trial-derived gross tumor volumes (GTV) were used, and visual assessment of normal tissue contours was undertaken. The SCOPE protocol standard margins were re-applied without modification to generate the clinical target volume (CTV) and planning target volume (PTV1) (19). Several additional volumes were contoured: PTV2 (boost volume) created by adding a 0.5 cm margin in all directions to the GTV, and a pericardium structure comprised of a 1 cm rind inside (12) the whole heart outline.
Table 1.
Patient target volume characteristics and overlap with heart and lung structures in order of increasing PTV1 size∗
| Patient | Volume (cm3) |
% Volume overlap |
|||
|---|---|---|---|---|---|
| GTV | PTV2 | PTV1 | Lung in PTV1 | Heart in PTV1 | |
| 1 | 4.7 | 18.4 | 140.1 | 0.5 | 1.0 |
| 2 | 4.1 | 16.7 | 146.8 | 1.0 | 4.9 |
| 3 | 7.2 | 28.8 | 195.2 | 0.2 | 6.7 |
| 4 | 10.5 | 32.7 | 195.6 | 0.5 | 5.5 |
| 5 | 8.8 | 29.0 | 205.2 | 0.4 | 7.6 |
| 6 | 15.3 | 43.4 | 218.6 | 0.4 | 3.8 |
| 7 | 12.1 | 43.1 | 233.4 | 0.2 | 9.6 |
| 8 | 16.5 | 48.9 | 239.0 | 0.8 | 5.2 |
| 9 | 27.7 | 66.1 | 297.7 | 2.0 | 8.6 |
| 10 | 30.2 | 71.6 | 301.1 | 1.3 | 6.3 |
| 11 | 23.3 | 66.1 | 311.7 | 0.8 | 16.5 |
| 12 | 29.3 | 76.8 | 329.8 | 2.0 | 4.6 |
| 13 | 37.2 | 92.7 | 356.1 | 0.8 | 4.6 |
| 14 | 45.1 | 101.2 | 374.9 | 0.5 | 5.5 |
| 15 | 52.3 | 111.0 | 405.2 | 1.9 | 4.9 |
| 16 | 43.9 | 105.3 | 408.8 | 2.5 | 8.2 |
| 17 | 46.0 | 109.8 | 434.2 | 1.4 | 11.3 |
| 18 | 35.4 | 94.1 | 443.1 | 1.5 | 3.6 |
| 19 | 65.5 | 140.3 | 453.6 | 1.7 | 2.6 |
| 20 | 95.4 | 175.2 | 544.9 | 1.8 | 5.0 |
| 21 | 104.4 | 199.5 | 590.8 | 3.2 | 8.5 |
Abbreviations: GTV = gross tumor volume; PTV = planning target volume.
Patients for whom dose escalation was unsuccessful are marked in boldface type.
Radiobiological modeling of TCP to determine the level of dose escalation to the GTV was carried out using the parameters from Geh et al (8). This is a multivariate logisitic regression model fitted to data from 26 CRT trials for preoperative esophageal cancer. Analysis was based on the protocol-prescribed doses of radiation therapy and chemotherapy (5-fluorouracil and cisplatin) to predict the TCP (TCP Geh) of pCR, and included total dose, dose per fraction and duration among the fitting parameters. The values of the covariates and coefficients used were those listed in the original paper (8).
where
A clinically significant 20% increase in local control requires 67.0 Gy, but this would considerably lengthen the total treatment time if delivered in 2-Gy fractions. We therefore calculated the equivalent dose to be delivered in 25 fractions (2.5 Gy per fraction) as 62.5 Gy, maintaining the same duration (33 days) as the standard plans of 50 Gy.
Treatment planning was performed using Eclipse, version 10 (Varian, Palo Alto, CA). RapidArc (RA) plans were created using 2 arcs of 360°, clockwise and counter-clockwise, with a collimator rotation of ±10°. Dose calculation was performed using the AAA algorithm using a 2.5-mm grid. A standard plan (RA50) was created (dose prescription 50 Gy/25 fractions to PTV1) and compared to a plan with an additional simultaneously integrated boost (SIB) of 62.5 Gy to PTV2 (RA62.5). Dose constraints are listed in Table 2, and additional dose-volume metrics were recorded for pericardium (mean dose, V30Gy, V45Gy) and lung V13Gy.
Table 2.
Dose constraints used in treatment planning for RA50 and RA62.5 radiation therapy plans
| Dose-volume | Constraint |
|---|---|
| PTV1 (50 Gy) | V95% (47.5 Gy) > 95% Dmax (0.1 cc) < 107% (53.5 Gy) |
| PTV2 (GTV + 0.5 cm) (62.5 Gy) | V95% (59.375 Gy) > 95% Dmax (0.1 cc) < 107% (66.875 Gy) |
| Lung | Mean dose < 20 Gy V20Gy < 25% |
| Heart | Mean dose < 25 Gy V30Gy < 45% |
| CordPRV | Dmax (0.1 cc) < 40 Gy (45 Gy permitted) |
Abbreviations: CordPRV = Cord planning organ at risk volume; Dmax = maximum dose; RA50 = RapidArc plan to 50 Gy; RA62.5 = RapidArc plan with boost to 62.5 Gy; V30Gy = volume receiving 30 Gy.
The differential dose-volume histogram (DVH) for the GTV was exported for each dose plan. TCP calculations were performed bin-wise using the Webb-Nahum model (20) with parameters from reference (21) (TCP Bedford) and also the Geh et al (8) model (TCP Geh). NTCP modeling was carried out for heart, using the whole-heart volume model of Gagliardi et al (15), for the pericardium (12), and for lung using the model parameters from De Jaeger et al (22), which predict radiation pneumonitis (RP) of grade 2 or higher (symptoms requiring steroids). In addition, we applied a combined heart and lung irradiation model (18) to calculate predicted risk of RP in these patients, where the dose to the “hottest” 10% of the heart volume (D10H), and the mean lung dose (MLD) in Gy are used to calculate NTCP
where
As the values of dose-volume metrics, TCP and NTCP across all patients were not normally distributed for all sets of data (Shapiro-Wilk test), we used the Wilcoxon signed rank test to compare RA50 versus RA62.5 plans for N=21 patients. Data were analyzed using SPSS, version 20.0.0 (IBM), and results are reported as median (range) values, with Z and P listed in Table 3.
Table 3.
Comparison of dose-volume metrics, TCP, and NTCP values∗
| Dose-volume | Plan |
||
|---|---|---|---|
| Median RA50 (range) | Median RA62.5 (range) | RA62.5-RA50 (Wilcoxon signed-rank test) | |
| PTV1 | |||
| V95% | 98.3 (95.3-100) | 96.7 (90.5-98.7) | Z = 2.63, P<.001 |
| PTV2 | |||
| V95% | 97.2 (93.9-99.3) | ||
| TCP Geh et al (8) (%) | 38.2 (37.6-39.6) | 56.3 (55.1-57.2) | Z = 4.02, P<.001 |
| TCP Bedford et al (21) (%) | 40.3 (39.2-43.2) | 71.7 (70.1-72.9) | |
| Lung | |||
| Mean dose (Gy) | 12.6 (8.4-18.2) | 13.3 (8.4-17.9) | Z = 3.77, P<.001 |
| V13Gy (%) | 42.2 (19.7-68.6) | 46.4 (19.7-68.8) | Z = 3.82, P<.001 |
| V20Gy (%) | 12.6 (5.9-29.9) | 15.9 (5.8-29.1) | Z = 3.74, P<.001 |
| NTCP (%) De Jaeger et al (22) | 6.5 (3.9-12.9) | 7.5 (3.9-12.6) | Z = 3.81, P<.001 |
| NTCP (%) Huang et al (18) | 14.2 (10.9-22.0) | 15.5 (10.8-21.5) | Z = 3.84, P<.001 |
| Heart | |||
| Mean dose (Gy) | 20.4 (13.2-27.9) | 20.3 (12.9-30.0) | Z = 1.41, P=.16 |
| V30Gy (%) | 16.6 (10.3-33.1) | 18.0 (10.5-38.1) | Z = 2.38, P=.02 |
| NTCP (%) Gagliardi et al (15) | 4.4 (2.3-10.4) | 5.6 (2.5-14.8) | Z = 3.98, P<.001 |
| Pericardium (1cm inner) | |||
| Mean dose (Gy) | 20.8 (13.8-27.9) | 22.0 (14.2-29.6) | Z = 2.86, P=.004 |
| V30Gy (%) | 19.6 (12.1-33.4) | 20.7 (12.3-35.0) | Z = 3.29, P=.001 |
| V45Gy (%) | 10.9 (6.5-23.2) | 10.7 (6.0-23.7) | Z = 1.14, P=.25 |
| CordPRV | |||
| Dmax 0.1 cc (Gy) | 34.9 | 36.1 | Z = 2.54, P=.01 |
Abbreviations: CordPRV = Cord planning organ at risk volume; Dmax = maximum dose; NTCP = normal tissue complication probability; PTV = planning target volume; RA50 = RapidArc boost to 50 Gy; RA62.5 = RapidArc plan with boost to 62.5 Gy; TCP = tumor control probability; V45Gy = volume receiving 45 Gy.
Although the median values for dose to organs at risk for RA50 and RA62.5 plans are statistically different (P<.05), except for heart mean dose, the differences in median dose across all patients are not clinically significant.
Results
Dose boosting to 62.5 Gy with adequate target dose coverage was possible for 20 out of 21 patients. For patient 21 (largest PTV, with 18.6% of PTV1 in lung) only 90.5% coverage by the 95% isodose contour for PTV1 was obtained. For all other patients the minimum V95% was 94.7% (RA62.5) versus 95.3% (RA50). For 4 additional patients (7, 11, 16, 17) it was not possible to increase dose to the GTV without exceeding other dose constraints. Failure was due to lung V20Gy > 25% (patient 16) and mean heart dose > 25 Gy (patients 7, 11, and 17). The dose escalation strategy was therefore successful for 16/21 (76%) of patients.
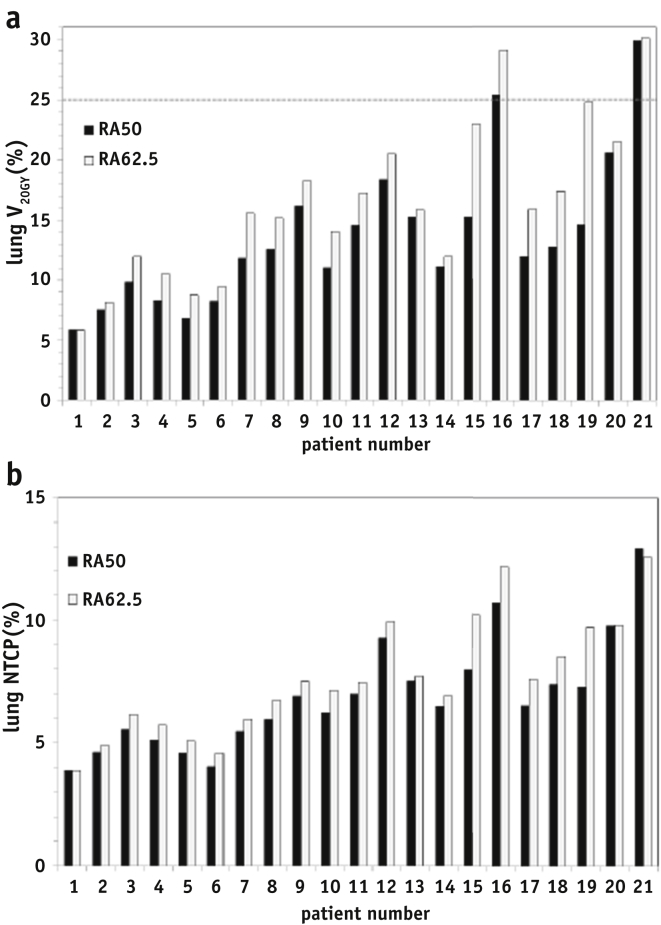
The calculated TCP (Table 3) increased by 18% (from 38.2% to 56.3%) on average for the Geh model, and 31% (from 40.3% to 71.7%) on average for the Bedford model. Figure 1 shows patients listed in order of increasing PTV1 size and illustrates the general trend of increased irradiation of the lung with increasing target volume size, although mean lung dose is <20 Gy for all patients, and the average increase with dose escalation is less than 1 Gy. The lung V20Gy constraint was exceeded only for patients 16 and 21 (RA50 and RA62.5 plans), who have the highest percentage of lung overlap in PTV1 of 2.5% and 3.2% respectively (Table 1). Lung V13Gy shows an average increase of 4.2% (Table 3) for the higher dose plans.
Fig. 1.
(a) Lung V20Gy values for each patient in order of increasing PTV size for plans RA50 (black bars) and RA62.5 (gray bars). The dose-volume constraint of 25% is shown as a dashed line. (b) Lung NTCP was calculated using the model parameters of De Jaeger for plans RA50 (black bars) and RA62.5 (gray bars) for each patient. NTCP = normal tissue complication probability; PTV = planning target volume; RA50 = RapidArc plan to 50 Gy; RA62.5 = RapidArc plan with boost to 62.5 Gy; V20Gy = volume receiving 20 Gy.
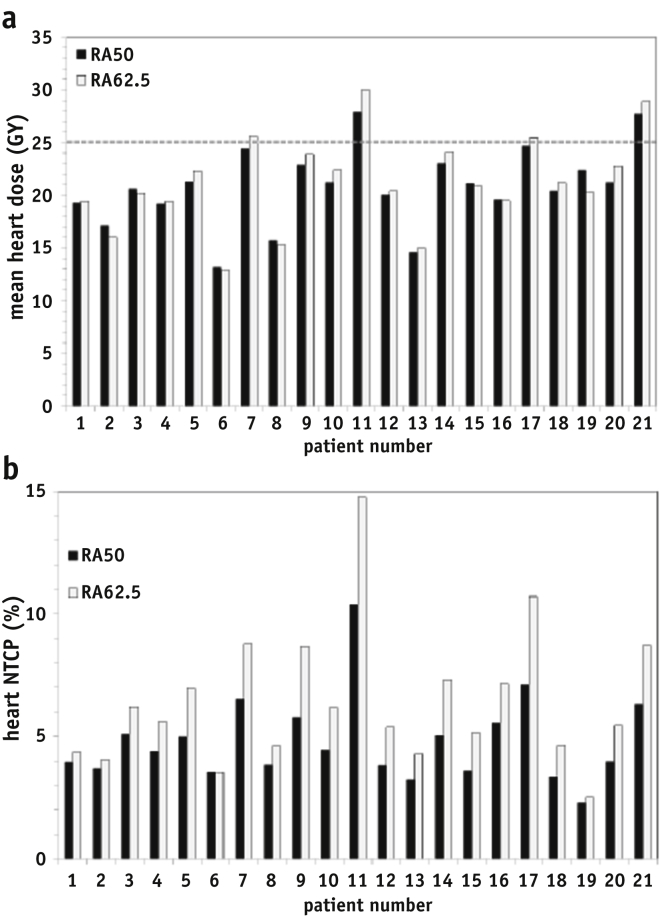
The SIB plans (RA62.5) result in an increase on average of less than 1 Gy in mean dose to heart and pericardium. Irradiation of the heart was not directly dependent on tumor size (Table 1), but on the overlap with the PTV1 volume (Fig. 2a). Patients 7, 11, and 17 have the highest % heart overlap in PTV1 (9.6%, 16.5%, and 11.3% respectively) and mean heart dose was exceeded when the boost dose was applied (plan RA62.5). For patient 11, the mean heart dose constraint could not be met for either plan, due to the GTV abutting the heart contour for this patient. Heart V30Gy was well below the 45% limit for all patients. Mean dose to the pericardium inner rind (12) was less than the recommended 27.1 Gy dose constraint for all patients except patient 11 (27.7 Gy) and patient 21 (27.9 Gy).
Fig. 2.
(a) Mean heart dose (Gy) for each patient for plans using RA50 (black bars) and RA62.5 (gray bars). The dose-volume constraint of 25 Gy is shown as a dashed line; (b) predicted heart NTCP for each patient using the whole heart contour and the NTCP model from Gagliardi et al (15). NTCP = normal tissue complication probability; RA50 = RapidArc plan to 50 Gy; RA62.5 = RapidArc plan with boost to 62.5 Gy.
Heart mortality predicted using the criteria by Gagliardi et al (15) showed a statistically significant increase of (on average) 1.2% with increased dose to the GTV (Table 3), though the predicted increase in heart mortality for some individual patients can be larger (Fig. 2b) due to overlap of heart with PTV1 (listed in Table 1). NTCP modeling for pericardial effusion (12) applied to the pericardial structure predicted a risk of pericarditis of zero for all patients.
For lung, the changes in NTCP (22) vary from patient to patient (Fig. 1b). On average, risk of grade 2 (or higher) radiation pneumonitis increased from 6.5% (RA50) to 7.5% (RA62.5) P<.001 (Table 2). Patients 20 and 21 with large PTV show no increase in predicted lung NTCP with higher GTV dose, due to the trade-off with lower target coverage. Using the combined heart and lung irradiation model for radiation pneumonitis (23), the predicted risks of RP are greater than for the lung only model, though the increase in predicted RP with dose escalation is on average < 1% (median NTCP: 14.2% [RA50] versus 15.5% [RA62.5] P<.001).
Discussion
Our analysis of the dose-volume metrics and radiobiological modeling of dose escalation for esophageal cancer suggests that a significant increase (+18%) in tumor control can potentially be achieved with only a modest increase in the risk of cardiac and lung toxicities for 76% of patients in our study of a subset of the SCOPE clinical trial database. Other authors have also shown that IMRT may reduce dose to critical structures such as heart and coronary arteries, when compared to conformal radiation therapy techniques 24, 25, but our study is the first to use radiobiological modeling to estimate both the level of dose escalation, and to predict heart and lung toxicities arising from this increase in dose. Patient outcomes and dose response will be monitored in the proposed randomized clinical trial investigating dose escalation for esophageal cancer (SCOPE-2).
The results of our analysis are, of course, dependent on the choice of the dose-volume and radiobiological parameters and models used, and we have therefore used a range of models and values from the literature. We have also shown that the relative comparison of 2 fractionation schemes is valid using 2 independent TCP models, although the absolute values of predicted TCP may be different, depending on the model parameters. By comparing fraction schedules with the same duration, we have also limited any error due to clonogenic repopulation rates and uncertainty in kick-off time. Both TCP models assume an identical dose response for adenocarcinomas and squamous cell esophageal cancers, and our analysis assumed a homogeneous distribution of clonogens within the GTV. If the 0.5 cm margins applied to create the boost volume around the GTV are insufficient to ensure adequate dose coverage in the presence of tumor motion, this may reduce the predicted increase in TCP, such that these values represent the upper limit of the benefits conferred by dose escalation.
Recent data from cine-MRI suggest a margin of 10.7 ± 0.4 mm in the cranial-caudal direction might be required to cover 95% of tumor movement (26), but this would imply an increase in dose to a significant portion of esophagus located immediately superior and inferior to the primary tumor, which may be a dose limiting factor in these patients. A margin of 0.5 cm was chosen in order to limit the volume of “healthy” esophagus irradiated and to generate a gradual drop-off in dose around the GTV. Data available in the literature concerning the dose-volume relationship for esophageal toxicity are from lung cancer patients 27, 28, and it is not clear if the dose limits would be applicable to patients with esophageal tumors. The results of a clinical trial using hypofractionation in non-small cell lung cancer (NSCLC) observed several grade 4 and 5 toxicities to central structures when doses were increased to 75 Gy and above but suggested 63.25 Gy/25 fractions should be well-tolerated in terms of both acute and late toxicity (29), which would suggest our proposed dose prescription of 62.5 Gy/25 fractions may be acceptable. We have in addition used stricter dose-volume constraints for plan optimization than specified in the original SCOPE protocol, but careful monitoring of esophageal toxicity during and after CRT will be necessary for the safe application of any dose escalation scheme.
For patients with larger PTVs with significant overlap in lung (>540 cm3 and 18% overlap in our patient subset), dose escalation using arc therapy may not be possible without a significant increase in dose delivered to surrounding lung. Hybrid static IMRT/RA irradiation techniques might provide better lung sparing for these patients (30). Also, the CTVs created in our study to encompass regions of microscopic tumor spread, were generated using isotropic geometrical margins. A further improvement would be to delineate the CTV for each patient on each slice of the CT scan to respect known anatomical boundaries, and thereby reduce the amount of normal tissue irradiated.
For patients where the heart overlaps the PTV (>8.5% heart in PTV overlap for this subset) dose escalation would exceed recommended cardiac dose constraints, even using advanced treatment planning and delivery techniques. Dose to the pericardium might be considered more relevant for modeling of acute toxicity (pericardial effusion) than dose to the whole heart, although our data suggests that for all patients, dose to pericardium is well within the various dose-volume constraints recommended in the literature 11, 12, 13. This may be due to the use of IMRT/RA, which delivers significantly lower dose to heart than previous 2D or 3D conformal radiation therapy techniques. We have therefore used the whole heart to model cardiac mortality, although as a long-term effect (5-10 years), this may be less clinically relevant for esophageal cancer patients where median survival is around 25 months (31).
Acute radiation-induced lung toxicity may therefore be more important for these patients and our data suggest that even with higher dose to the tumor, the increase in risk of radiation pneumonitis is small, and might be considered acceptable for patients with limited organ-at-risk overlap inside the PTV (<8% heart volume and <2.5% lung volume overlap for this study), given the predicted gains in tumor control. Concomitant irradiation of the heart or a pre-existing cardiac pathology have recently been identified as risk factors in radiation pneumonitis (18), and this should be assessed for each patient during planning.
Conclusions
Radiobiological modeling suggests that dose escalation to the GTV in esophageal cancer has the potential to produce significant gains in tumor control with only a minor increase in lung or heart toxicity for the majority of patients. The relationship between tumor response and normal tissue toxicity during dose escalation should be carefully validated in clinical trials.
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
This work was supported by Cancer Research UK, Medical Research Council, Drs Warren and Partridge are supported by Cancer Research UK grant C5255/A15935.
Dr Carrington received a PhD studentship grant from Cancer Research Wales. Dr Hawkins received MRC fellowship MC_PC_12001/2.
Conflict of interest: none.
References
- 1.Wolf M.C., Stahl M., Krause B.J. Curative treatment of esophageal carcinoma: Current options and future developments. Radiat Oncol. 2011;6:55. doi: 10.1186/1748-717X-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teoh A.Y., Chiu P.W., Yeung W.K. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: Results from a randomized controlled trial. Ann Oncol. 2013;24:165–171. doi: 10.1093/annonc/mds206. [DOI] [PubMed] [Google Scholar]
- 3.Cooper J.S., Guo M.D., Herskovic A. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D., Starling N., Rao S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 5.Welsh J., Settle S.H., Amini A. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632–2640. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Button M.R., Morgan C.A., Croydon E.S. Study to determine adequate margins in radiotherapy planning for esophageal carcinoma by detailing patterns of recurrence after definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:818–823. doi: 10.1016/j.ijrobp.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Liao Z., Jin J. Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:656–664. doi: 10.1016/j.ijrobp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Geh J.I., Bond S.J., Bentzen S.M. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: Evidence of a radiation and chemotherapy dose response. Radiother Oncol. 2006;78:236–244. doi: 10.1016/j.radonc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Minsky B.D., Pajak T.F., Ginsberg R.J. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 10.Gwynne S., Falk S., Gollins S. Oesophageal chemoradiotherapy in the UK—current practice and future directions. Clin Oncol (R Coll Radiol) 2013;25:368–377. doi: 10.1016/j.clon.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Wei X., Liu H.H., Tucker S.L. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 12.Martel M.K., Sahijdak W.M., Ten Haken R.K. Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys. 1998;40:155–161. doi: 10.1016/s0360-3016(97)00584-1. [DOI] [PubMed] [Google Scholar]
- 13.Fukada J., Shigematsu N., Takeuchi H. Symptomatic pericardial effusion after chemoradiation therapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys. 2013;87:487–493. doi: 10.1016/j.ijrobp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Hatakenaka M., Yonezawa M., Nonoshita T. Acute cardiac impairment associated with concurrent chemoradiotherapy for esophageal cancer: Magnetic resonance evaluation. Int J Radiat Oncol Biol Phys. 2012;83:e67–e73. doi: 10.1016/j.ijrobp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Gagliardi G., Lax I., Ottolenghi A. Long-term cardiac mortality after radiotherapy of breast cancer–application of the relative seriality model. Br J Radiol. 1996;69:839–846. doi: 10.1259/0007-1285-69-825-839. [DOI] [PubMed] [Google Scholar]
- 16.Marks L.B., Bentzen S.M., Deasy J.O. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(suppl 3):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalbantov G., Kietselaer B., Vandecasteele K. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother Oncol. 2013;109:100–106. doi: 10.1016/j.radonc.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Huang E.X., Hope A.J., Lindsay P.E. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol. 2011;50:51–60. doi: 10.3109/0284186X.2010.521192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurt C.N., Nixon L.S., Griffiths G.O. SCOPE1: A randomised phase II/III multicentre clinical trial of definitive chemoradiation, with or without cetuximab, in carcinoma of the oesophagus. BMC Cancer. 2011;11:466. doi: 10.1186/1471-2407-11-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb S., Nahum A.E. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol. 1993;38:653–666. doi: 10.1088/0031-9155/38/6/001. [DOI] [PubMed] [Google Scholar]
- 21.Bedford J.L., Viviers L., Guzel Z. A quantitative treatment planning study evaluating the potential of dose escalation in conformal radiotherapy of the oesophagus. Radiother Oncol. 2000;57:183–193. doi: 10.1016/s0167-8140(00)00258-9. [DOI] [PubMed] [Google Scholar]
- 22.De Jaeger K., Hoogeman M.S., Engelsman M. Incorporating an improved dose-calculation algorithm in conformal radiotherapy of lung cancer: Re-evaluation of dose in normal lung tissue. Radiother Oncol. 2003;69:1–10. doi: 10.1016/s0167-8140(03)00195-6. [DOI] [PubMed] [Google Scholar]
- 23.Huang E.X., Bradley J.D., El Naqa I. Modeling the risk of radiation-induced acute esophagitis for combined Washington University and RTOG trial 93-11 lung cancer patients. Int J Radiat Oncol Biol Phys. 2012;82:1674–1679. doi: 10.1016/j.ijrobp.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welsh J., Palmer M.B., Ajani J.A. Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phys. 2012;82:468–474. doi: 10.1016/j.ijrobp.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kole T.P., Aghayere O., Kwah J. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1580–1586. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 26.Lever F.M., Lips I.M., Crijns S.P. Quantification of esophageal tumor motion on cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2014;88:419–424. doi: 10.1016/j.ijrobp.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Belderbos J., Heemsbergen W., Hoogeman M. Acute esophageal toxicity in non-small cell lung cancer patients after high dose conformal radiotherapy. Radiother Oncol. 2005;75:157–164. doi: 10.1016/j.radonc.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Nijkamp J., Rossi M., Lebesque J. Relating acute esophagitis to radiotherapy dose using FDG-PET in concurrent chemo-radiotherapy for locally advanced non-small cell lung cancer. Radiother Oncol. 2013;106:118–123. doi: 10.1016/j.radonc.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Cannon D.M., Mehta M.P., Adkison J.B. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343–4348. doi: 10.1200/JCO.2013.51.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin S., Chen J.Z., Rashid Dar A. Dosimetric comparison of helical tomotherapy, RapidArc, and a novel IMRT & Arc technique for esophageal carcinoma. Radiother Oncol. 2011;101:431–437. doi: 10.1016/j.radonc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Crosby T., Hurt C.N., Falk S. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): A multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]