Abstract
Porphyromonas gingivalis is one of the main etiological organisms in periodontal disease. On oral surfaces P. gingivalis is a component of multispecies biofilm communities and can modify the pathogenic potential of the community as a whole. Accumulation of P. gingivalis in communities is facilitated by interspecies binding and communication with the antecedent colonizer Streptococcus gordonii. In this study we screened a library of small molecules to identify structures that could serve as lead compounds for the development of inhibitors of P. gingivalis community development. Three small molecules were identified that effectively inhibited accumulation of P. gingivalis on a substratum of S. gordonii. The structures of the small molecules are derived from the marine alkaloids oroidin and bromoageliferin and contain a 2-aminoimidazole or 2-aminobenzimidazole moiety. The most active compounds reduced expression of mfa1 and fimA in P. gingivalis, genes encoding the minor and major fimbrial subunits respectively. These fimbrial adhesins are necessary for P. gingivalis coadhesion with S. gordonii. These results demonstrate the potential for a small molecular inhibitor based approach to the prevention of diseases associated with P. gingivalis.
Introduction
Bacterial community formation within the oral cavity represents one of the fundamental survival strategies for the more than 700 species (Aas et al., 2005) that can reside in this dynamic environment. The development of complex adherent multispecies microbial communities, otherwise known as biofilmss, occurs in an ordered manner on available surfaces. The attachment of streptococcal species to the salivary pellicle represents a cornerstone in the initial community development on tooth surfaces, and it is to this streptococcal substratum that many subsequent colonizers can attach (Kuboniwa & Lamont, 2010, Wright et al., 2013). In a healthy mouth, the plaque biofilm is controlled by host innate immunity and oral hygiene procedures. Problems arise for the host when this delicate balance is disturbed by such factors as smoking, poor oral hygiene and/or the colonization of pathogenic organisms, and the relationship between host and microbial community changes from symbiotic to dysbiotic (Hajishengallis & Lamont, 2012, Hajishengallis & Lamont, 2014).
Periodontitis is one of the more prevalent chronic conditions suffered by adults, with an incidence rate of around 50 % in the adult population of the United States (Albandar, 2005, Eke et al., 2012). Porphyromonas gingivalis, a gram-negative anaerobe, is strongly associated with chronic cases of periodontitis (Byrne et al., 2009). However, it worth noting that although P. gingivalis can be isolated from a large number of chronic periodontitis cases, it is sometimes present in relatively small numbers compared to other community members. It was proposed that virulence of P. gingivalis is expressed through modification of the pathogenicity of the previously commensal biofilm community (Hajishengallis et al., 2011). In addition, communication with oral streptococci can increase the pathogenicity of P. gingivalis (Whitmore & Lamont, 2011).
Colonization of P. gingivalis is enhanced through coadhesive interactions with a number of oral bacteria (Wright et al., 2013, Kuboniwa & Lamont, 2010, Rosan & Lamont, 2000). One well-studied example of inter-species coadhesion is that of P. gingivalis and S. gordonii. Binding is effectuated by two pairs of adhesins-receptors: the major fimbriae on the surface of P. gingivalis and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) on the surface of S. gordonii cells (Maeda et al., 2004, Lamont et al., 1993); and the minor fimbriae of P. gingivalis binding to the SspA/B protein of S. gordonii, a member of the antigen I/II family of proteins (Lamont et al., 2002, Park et al., 2005, Daep et al., 2006). The gene encoding the structural subunit of the major fimbriae, fimA, is part of a larger operon that responds to a number of environment cues including temperature and hemin (Xie et al., 1997, Park et al., 2007, Nishikawa et al., 2004). Mfa1 is the structural subunit of the minor fimbriae and the mfa1 gene is regulated by contact with S. gordonii through a pathway involving the Ltp1 tyrosine phosphatase and the transcription factor CdhR (Park et al., 2005, Maeda et al., 2008, Chawla et al., 2010, Simionato et al., 2006).
Control of P. gingivalis colonization and community development is a potential means to reduce the incidence and severity of periodontitis, and a number of strategies have been explored. One promising approach is based on small peptides representing the binding domain (BAR) of S. gordonii SspB, which can inhibit P. gingivalis-S. gordonii community development and reduce P. gingivalis colonization and bone loss in a mouse model (Daep et al., 2011, Daep et al., 2006). In addition, gallium and silver ions can inhibit both the planktonic and biofilm growth of P. gingivalis in a mixed species biofilm assay with S. gordonii (Valappil et al., 2012). In the current study we focused on a library of small molecule based primarily on the 2-aminoimidazole and 2-aminobenzimidazole scaffolds, and which have been shown to modulate biofilm development in a variety of model systems (Liu et al., 2011, Worthington et al., 2012). Three compounds were identified that specifically inhibited P. gingivalis community development with S. gordonii, and which regulated expression of P. gingivalis adhesins.
Material and Methods
Bacterial strains and growth conditions
P. gingivalis strains ATCC 33277 and A7436 were routinely cultured anaerobically at 37 °C in Trypticase soy broth (TSB) supplemented with 1 g yeast extract, 5 mg hemin and 1 mg menadione (per liter). Solid medium was supplemented with 5 % sheep blood and 1.5% agar. S. gordonii was cultured in brain heart infusion broth containing 0.5 % yeast extract.
Screen of small molecule library
An initial screen of the small molecule library of 506 compounds (Liu et al., 2011) for inhibition of P. gingivalis-S. gordonii community development utilized a dot blot format as previously described (Kuboniwa et al., 2006). Stock solution of inhibitors were at 10 mM in DMSO. S. gordonii cells were washed in PBS and 1x108 cells were applied to a nitrocellulose membrane. The membrane was blocked with 1.5% BSA, in Tris-buffered saline (TBS). P. gingivalis cells were labeled with N-hydroxysuccinimidobiotin and incubated 10 μM inhibitor (or vehicle alone) for 1 h. P. gingivalis cells (1x108) were reacted with the S. gordonii substratum for 12 h with rocking. P. gingivalis binding was visualized with alkaline phosphatase (AP)-conjugated streptavidin and AP-specific substrate (BCIP, Sigma).
Confocal laser scanning microscopy (CLSM) of P. gingivalis-S. gordonii communities
Mixed species communities of P. gingivalis and S. gordonii were generated and analyzed essentially as described previously (Kuboniwa et al., 2006). In brief, S. gordonii cells were stained with hexidium iodide (15 μg/ml−1, Invitrogen) and 2 × 108 cells were incubated on glass coverslips anaerobically for 16 h at 37 °C. Mid-log cultures of P. gingivalis were stained with 5-(and-6)-carboxyfluorescein, succinimidyl ester (4 μg/ml−1, Invitrogen) and 2 × 107 cells were incubated with inhibitors for 5 min before addition to the S. gordonii substrate. P. gingivalis-S. gordonii communities were incubated anaerobically for 24 h at 37 °C and viewed with an Olympus FV500 confocal microscope. XYZ stacks were digitally reconstructed using the Volocity analysis program (Improvision). Quantitation of the volume of P. gingivalis fluorescence was obtained using the Find Objects algorithm in the Volocity program. This process analyzed all P. gingivalis fluorescence in the 3D digitally re-created confocal images. To estimate microcolony formation, the Find Objects process was used with a threshold for 3D objects greater than 20 μm3.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from three independent cultures of P. gingivalis in heterotypic communities with S. gordonii as described previously (Hirano et al., 2012). RNA was converted to cDNA using the high capacity cDNA reverse transcription kit (Applied Biosystems) from 20 ng of RNA template. qRT-PCR was performed by StepOne plus by the ΔΔCt method using 16S rRNA as an internal control as described previously (Hirano et al., 2012, Wright et al., 2014). Primers are listed in Table S1.
Statistics
Experiments were carried out in triplicate and Prism 6 (Graphpad) software was used to analyze data displayed as mean ± standard deviation (SD). Multiple data sets were analyzed by ANOVA followed by Tukey post test.
Results
Identification of small molecule inhibitors that inhibit P. gingivalis heterotypic community development
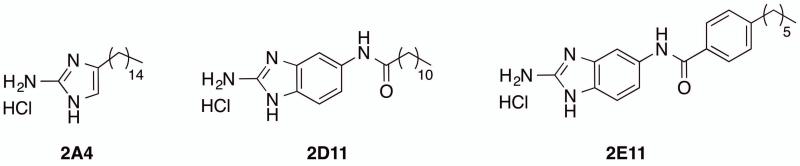
The small molecule library of 506 compounds was screened for inhibition of P. gingivalis accumulation into heterotypic communities with S. gordonii using a semi-quantitative dot blot. Three compounds, 2A4, 2D11 and 2E11 reduced the amount of P. gingivalis accumulation to background levels at 10 μM without affecting the integrity of the S. gordonii substratum (not shown). The structures of these three compounds are shown in Figure 1.
Figure 1. Structures of compounds that inhibited P. gingivalis-S. gordonii community formation.
Characteristics of active compounds
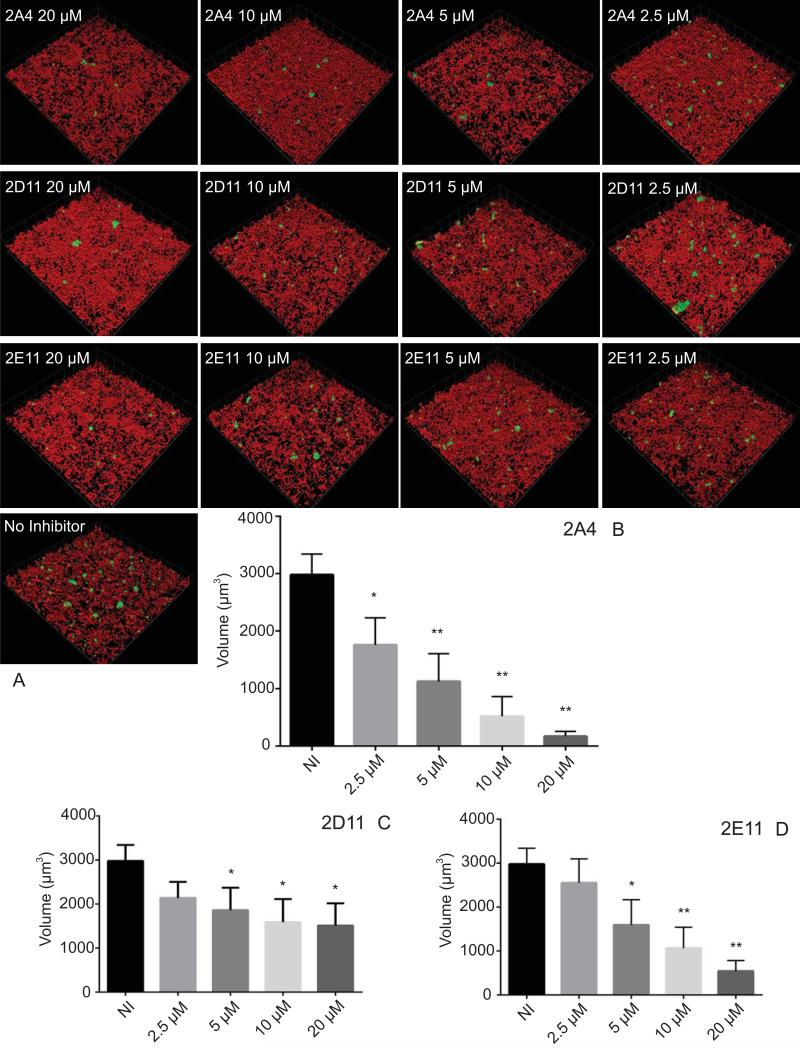
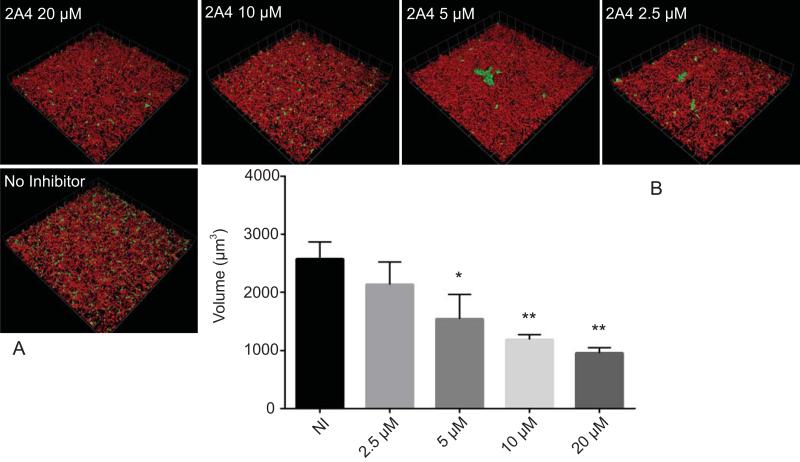
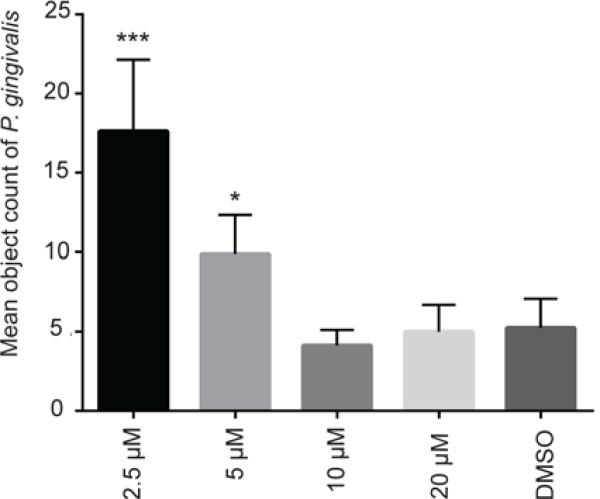
The effects of the three most potent inhibitors on P. gingivalis heterotypic community development were visualized and quantified by CLSM. As shown in Fig. 2, over a dose-response range, 2A4 reduced the total biovolume of P. gingivalis over 90% (P < 0.001) at 20 μM, and 40% at 2.5 μM (P < 0.05) with a 50 % inhibitory concentration (IC50) of 3.41 μM ±0.92. Inhibitors 2D11 and 2E11 also exhibited a dose dependent reduction in P. gingivalis biovolume with S. gordonii, although neither was as effective as 2A4 (Fig. 2). The IC50 value for 2E11 was 6.88 μM ± 1.45, while 2D11 gave an IC50 of 4.73 μM ± 1.17. As an additional negative control, compound 2B1 which was negative in the initial screen was also tested and found not to inhibit development of the dual species communities (not shown). To ensure that the effects of the inhibitors were not restricted to one strain of P. gingivalis, 2A4 was also tested with a disseminating strain of P. gingivalis, A7436, originally isolated from a refractory periodontitis patient (Genco et al., 1991). 2A4 inhibited heterotypic community development by A7436 (Fig. 3), although the level of reduction was less than that observed with strain 33277 and the IC50 was 4.07 μM ± 2.33. Changes in community architecture were also observed in the presence of inhibitors, with remaining microcolonies developing a greater abundance. The effect was particularly noticeable with A7436. At 5 μM and 2.5 μM concentrations of inhibitor, and an object count revealed a statistically significant increase in P. gingivalis A7436 objects over 20 μm3 (Fig. 4).
Figure 2. Effects of small molecule inhibitors on heterotypic community development.
P. gingivalis 33277 was incubated with the inhibitors at the concentrations indicated, or with vehicle (DMSO) alone, for 18 h. A) Visualization of dual species communities of P. gingivalis (green) with S. gordonii (red). A series of 20-30 μm-deep optical fluorescent x-y sections (213 × 213 μm) were collected to create digitally reconstructed 3D images with Volocity software. B-D) Total P. gingivalis biovolume in images represented in A) in the presence of inhibitors or control (NI) measured with Volocity software. Quantitative results are means with standard deviation of three independent experiments performed in triplicate * – P value of <0.05, ** – P value of <0.01, *** – P value <0.001.
Figure 3. Effect of 2A4 on P. gingivalis A7436 community development.
P. gingivalis A7436 was incubated with inhibitor 2A4 at the concentrations indicated, or DMSO alone (NI), for 18 h. A) Visualization of dual species communities of P. gingivalis (green) with S. gordonii (red). A series of 20-30 μm-deep optical fluorescent x-y sections (213 × 213 μm) were collected to create digitally reconstructed 3D images with Volocity software. B) Total P. gingivalis biovolume in images represented in A) in the presence of inhibitors or DMSO control measured with Volocity software. Results are means with standard deviation of three independent experiments performed in triplicate * – P value of <0.05, ** – P value of <0.01.
Figure 4. Mean object count of P. gingivalis A7436 microcolonies.
Volocity software was used to determine number of microcolonies larger than 20 μm3 from experiments shown in Figure 3. Quantitative results are means with standard deviation of three independent experiments performed in triplicate * – P value of <0.05, *** – P value <0.001.
Effect of inhibitors on growth of P. gingivalis
To verify that the effects of the inhibitors on community development were not due to a decrease in growth rate, 2A4, 2D11 and 2E11 were included in broth cultures of P. gingivalis, and growth monitored until stationary phase. When added at the IC50 concentration, 2A4, 2D11, and 2E11 did not impact growth of P. gingivalis (Fig. S1).
Changes in expression of community associated genes in P. gingivalis
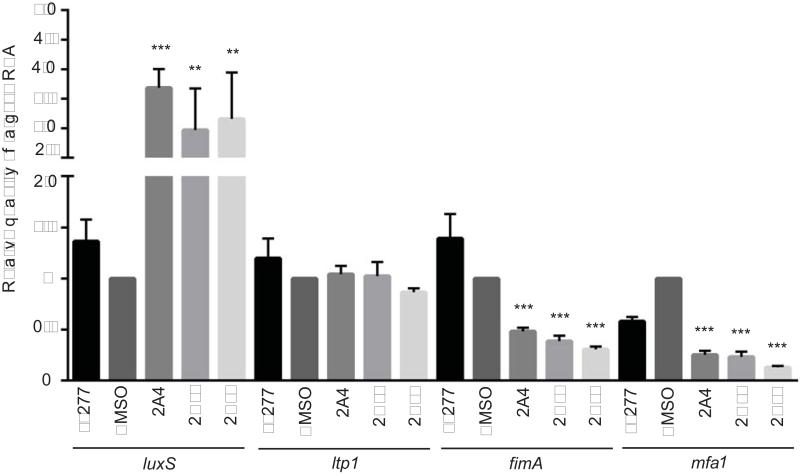
Heterotypic community formation by P. gingivalis involves the fimbrial adhesins Mfa1 and FimA, intracellular signal transduction through the Ltp1 tyrosine phosphatase, and cell-cell signaling through AI-2. To ascertain if the inhibitors were exerting an effect on these community mediators, quantitative RT-PCR was applied to P. gingivalis cells reacted with the inhibitors prior to community formation with S. gordonii (Fig. 5). Compared to DMSO vehicle alone, mRNA levels of fimA and mfa1 were reduced by 2A4, 2D11 and 2E11. In contrast expression of luxS was increased by all of the inhibitors while ltp1 was unaffected. Thus, the primary effect of the inhibitors may be on the initial adherence of P. gingivalis to S. gordonii. An increase in luxS expression could be a direct effect on gene transcription of the result of a feedback mechanism as the organism senses and responds to decreased adherence.
Figure 5. Analysis of differential gene expression by qRT-PCR.
mRNA extracted from P. gingivalis cells under biofilm conditions incubated with and without inhibitors was analyzed by qRT-PCR. 16s rRNA was used for normalization. ** – P value of <0.01, *** – P value <0.001 compared to DMSO control. Representative data are shown as means with standard deviation of 3 biological replicates.
Discussion
In this study, a small molecule library was screened for effectiveness against heterotypic community formation by the periodontal pathogen P. gingivalis. The library is comprised of a series of molecules derived from marine natural products and structurally based on the 2-aminoimidazole scaffold (Rogers & Melander, 2008), and components have been found to exert anti-biofilm activity against a variety of gram-negative and gram-positive pathogens (Richards et al., 2008, Rogers et al., 2009). In addition, a number of library constituents inhibit the formation of biofilms by the cariogenic organism Streptococcus mutans (Liu et al., 2011). While the etiology of periodontitis involves a complex multispecies community (Hajishengallis & Lamont, 2012, Hajishengallis & Lamont, 2014); within this dysbiotic community certain organisms can play a pivotal role. P. gingivalis is considered a keystone pathogen as it can elevate the virulence of the community as a whole (Hajishengallis et al., 2011). Other organisms act as accessory pathogens in that they can increase the virulence potential of P. gingivalis, but in the absence of overt pathogens usually exist in commensal balance with the host (Whitmore & Lamont, 2011). Hence, interference with the accumulation of P. gingivalis into a heterotypic community is an attractive target to disrupt the transition of a balanced periodontal community into a destructive one.
The model system that we utilized involved accumulation of microcolonies of P. gingivalis on a substratum of S. gordonii. It is well documented that P. gingivalis can bind to S. gordonii both in vitro and in vivo within supragingival plaque (Slots & Gibbons, 1978, Kuboniwa & Lamont, 2010, Maeda et al., 2008, Chawla et al., 2010, Daep et al., 2011). As oral streptococci such as S. gordonii can constitute up to 70% of the early bacterial community on supragingival tooth surfaces (Socransky et al., 1977), their interaction with P. gingivalis is considered an important initial colonization mechanism that allows the organism to become established on the tooth surface before spreading to the subgingival area (Kuboniwa & Lamont, 2010). Three small molecules, 2A4, 2D11 and 2E11 were capable of diminishing the biovolume of P. gingivalis in dual species communities, without affecting the underlying substratum of S. gordonii. Interestingly, two of these, 2A4 and 2D11, were also effective in reducing S. mutans accumulations. In the presence of 2A4, production of Antigen I/II and Gtf, two main surface adhesion molecules of S. mutans is significantly reduced, suggesting an effect on the bacterial surface (Liu et al., 2011). Hence, these compounds have potential for use in controlling two of the major diseases of microbial origin: caries and periodontal disease.
In the presence of the inhibitors, the expression of both fimbrial genes mfa1 and fimA was reduced. Lower levels of the major (FimA) and minor (Mfa1) fimbriae compromises the initial attachment of P. gingivalis to S. gordonii and impedes further development of heterotypic communities (Lamont et al., 1993, Lamont et al., 2002). Both fimA and mfa1 are regulated by the FimS/FimR two component system (TCS) (Hayashi et al., 2000, Wu et al., 2007) and the 2-aminoimidazole or 2-aminobenzimidazole derived compounds can inhibit TCS in other organisms (Worthington et al., 2013). Disruption of TCS control of fimA and mfa1 transcription, therefore, represents a potential mechanism of action of the inhibitors.
As well as observing a decrease in primary binding mechanisms to S. gordonii, an alteration in colony architecture was observed in P. gingivalis. P. gingivalis microcolonies that successfully developed in the presence of the inhibitors were significantly altered in colony architecture. Explanations for this phenomenon include a decrease in competition with other microcolonies or an increase in autoaggregation of P. gingivalis resulting from reduced amounts of FimA or Mfa1 (Kuboniwa et al., 2009). Alternatively, expression of the luxS gene was significantly increased by the inhibitors, and the higher degree of AI-2 signaling will increase the size of the microcolonies (Chawla et al., 2010). Moreover, previous reports have established an optimal level of AI-2 for oral microbial community development (Rickard et al., 2006) and disruption of AI-2 dependent communication by the inhibitors could therefore have adverse effects that extend beyond P. gingivalis-S. gordonii interactions.
The experimental system adopted for this study represents the first stage in a continuum from in vitro models, to in vivo testing in animals, to human intervention studies. As with all models, in order to be tractable, complexity is reduced. In vivo, the presence of saliva and crevicular fluid, or of other bacteria may modulate the effect of the inhibitors. In addition, bacterial cells in mature biofilms may behave differently to those simple communities. These factors will be investigated in future studies.
Supplementary Material
Figure S1. Growth curves of P. gingivalis 33277 in the presence of inhibitors. P. gingivalis was incubated with the inhibitors indicated at the IC50, or DMSO control, and OD600 recorded over time.
Table S1. Primers used in this study.
Acknowledgements
Supported by NIH NIDCR DE012505, DE023193 (RJL) and DE022350 (HW and CM).
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albandar JM. Dent Clin North Am. 2005;49:517–532. doi: 10.1016/j.cden.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, Reynolds EC. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol Immunol. 2009;24:469–477. doi: 10.1111/j.1399-302X.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- Chawla A, Hirano T, Bainbridge BW, Demuth DR, Xie H, Lamont RJ. Community signalling between Streptococcus gordonii and Porphyromonas gingivalis is controlled by the transcriptional regulator CdhR. Mol Microbiol. 2010;78:1510–1522. doi: 10.1111/j.1365-2958.2010.07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daep CA, James DM, Lamont RJ, Demuth DR. Structural characterization of peptide-mediated inhibition of Porphyromonas gingivalis biofilm formation. Infect Immun. 2006;74:5756–5762. doi: 10.1128/IAI.00813-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daep CA, Novak EA, Lamont RJ, Demuth DR. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect Immun. 2011;79:67–74. doi: 10.1128/IAI.00361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Genco CA, Cutler CW, Kapczynski D, Maloney K, Arnold RR. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991;59:1255–1263. doi: 10.1128/iai.59.4.1255-1263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Breaking bad: Manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44:328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J, Nishikawa K, Hirano R, Noguchi T, Yoshimura F. Identification of a two-component signal transduction system involved in fimbriation of Porphyromonas gingivalis. Microbiol Immunol. 2000;44:279–282. doi: 10.1111/j.1348-0421.2000.tb02496.x. [DOI] [PubMed] [Google Scholar]
- Hirano T, Beck DA, Demuth DR, Hackett M, Lamont RJ. Deep sequencing of Porphyromonas gingivalis and comparative transcriptome analysis of a LuxS mutant. Front Cell Infect Microbiol. 2012;2:79. doi: 10.3389/fcimb.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa M, Amano A, Hashino E, Yamamoto Y, Inaba H, Hamada N, et al. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by Porphyromonas gingivalis. BMC Microbiol. 2009;9:105. doi: 10.1186/1471-2180-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa M, Lamont RJ. Subgingival biofilm formation. Periodontol 2000. 2010;52:38–52. doi: 10.1111/j.1600-0757.2009.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa M, Tribble GD, James CE, Kilic AO, Tao L, Herzberg MC, et al. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol Microbiol. 2006;60:121–139. doi: 10.1111/j.1365-2958.2006.05099.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Bevan CA, Gil S, Persson RE, Rosan B. Involvement of Porphyromonas gingivalis fimbriae in adherence to Streptococcus gordonii. Oral Microbiol Immunol. 1993;8:272–276. doi: 10.1111/j.1399-302x.1993.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, El-Sabaeny A, Park Y, Cook GS, Costerton JW, Demuth DR. Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates. Microbiology. 2002;148:1627–1636. doi: 10.1099/00221287-148-6-1627. [DOI] [PubMed] [Google Scholar]
- Liu C, Worthington RJ, Melander C, Wu H. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrob Agents Chemother. 2011;55:2679–2687. doi: 10.1128/AAC.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Nagata H, Yamamoto Y, Tanaka M, Tanaka J, Minamino N, Shizukuishi S. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect Immun. 2004;72:1341–1348. doi: 10.1128/IAI.72.3.1341-1348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Tribble GD, Tucker CM, Anaya C, Shizukuishi S, Lewis JP, et al. A Porphyromonas gingivalis tyrosine phosphatase is a multifunctional regulator of virulence attributes. Mol Microbiol. 2008;69:1153–1164. doi: 10.1111/j.1365-2958.2008.06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Yoshimura F, Duncan MJ. A regulation cascade controls expression of Porphyromonas gingivalis fimbriae via the FimR response regulator. Mol Microbiol. 2004;54:546–560. doi: 10.1111/j.1365-2958.2004.04291.x. [DOI] [PubMed] [Google Scholar]
- Park Y, Simionato MR, Sekiya K, Murakami Y, James D, Chen W, et al. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun. 2005;73:3983–3989. doi: 10.1128/IAI.73.7.3983-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Xie H, Lamont RJ. Transcriptional organization of the Porphyromonas gingivalis fimA locus. FEMS Microbiol Lett. 2007;273:103–108. doi: 10.1111/j.1574-6968.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- Richards JJ, Ballard TE, Melander C. Inhibition and dispersion of Pseudomonas aeruginosa biofilms with reverse amide 2-aminoimidazole oroidin analogues. Org Biomol Chem. 2008;6:1356–1363. doi: 10.1039/b719082d. [DOI] [PubMed] [Google Scholar]
- Rickard AH, Palmer RJ, Jr., Blehert DS, Campagna SR, Semmelhack MF, Egland PG, et al. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol. 2006;60:1446–1456. doi: 10.1111/j.1365-2958.2006.05202.x. [DOI] [PubMed] [Google Scholar]
- Rogers SA, Huigens RW, 3rd, Melander C. A 2-aminobenzimidazole that inhibits and disperses gram-positive biofilms through a zinc-dependent mechanism. J Am Chem Soc. 2009;131:9868–9869. doi: 10.1021/ja9024676. [DOI] [PubMed] [Google Scholar]
- Rogers SA, Melander C. Construction and screening of a 2-aminoimidazole library identifies a small molecule capable of inhibiting and dispersing bacterial biofilms across order, class, and phylum. Angew Chem Int Ed Engl. 2008;47:5229–5231. doi: 10.1002/anie.200800862. [DOI] [PubMed] [Google Scholar]
- Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–1607. doi: 10.1016/s1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- Simionato MR, Tucker CM, Kuboniwa M, Lamont G, Demuth DR, Tribble GD, Lamont RJ. Porphyromonas gingivalis genes involved in community development with Streptococcus gordonii. Infect Immun. 2006;74:6419–6428. doi: 10.1128/IAI.00639-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J, Gibbons RJ. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978;19:254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Manganiello AD, Propas D, Oram V, van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977;12:90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Valappil SP, Coombes M, Wright L, Owens GJ, Lynch RJ, Hope CK, Higham SM. Role of gallium and silver from phosphate-based glasses on in vitro dual species oral biofilm models of Porphyromonas gingivalis and Streptococcus gordonii. Acta Biomater. 2012;8:1957–1965. doi: 10.1016/j.actbio.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Whitmore SE, Lamont RJ. The pathogenic persona of community-associated oral streptococci. Mol Microbiol. 2011;81:305–314. doi: 10.1111/j.1365-2958.2011.07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington RJ, Blackledge MS, Melander C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Future Med Chem. 2013;5:1265–1284. doi: 10.4155/fmc.13.58. [DOI] [PubMed] [Google Scholar]
- Worthington RJ, Richards JJ, Melander C. Small molecule control of bacterial biofilms. Org Biomol Chem. 2012;10:7457–7474. doi: 10.1039/c2ob25835h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, et al. Microbial interactions in building of communities. Mol Oral Microbiol. 2013;28:83–101. doi: 10.1111/omi.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Xue P, Hirano T, Liu C, Whitmore SE, Hackett M, Lamont RJ. Characterization of a bacterial tyrosine kinase in Porphyromonas gingivalis involved in polymicrobial synergy. Microbiologyopen. 2014 doi: 10.1002/mbo3.177. 10.1002/mbo3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lin X, Xie H. Porphyromonas gingivalis short fimbriae are regulated by a FimS/FimR two-component system. FEMS Microbiol Lett. 2007;271:214–221. doi: 10.1111/j.1574-6968.2007.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Cai S, Lamont R. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect Immun. 1997;65:2265–2271. doi: 10.1128/iai.65.6.2265-2271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Growth curves of P. gingivalis 33277 in the presence of inhibitors. P. gingivalis was incubated with the inhibitors indicated at the IC50, or DMSO control, and OD600 recorded over time.
Table S1. Primers used in this study.