Abstract
Background
Human herpesvirus 6 (HHV-6) is an opportunistic pathogen after hematopoietic cell transplantation (HCT) that is associated with central nervous system (CNS) dysfunction.
Objectives
The aim of this study was to determine the frequency and significance of HHV-6 DNA detection in cerebrospinal fluid (CSF) after HCT.
Study design
We identified patients with HHV-6 DNA in CSF using quantitative PCR. Patients with neurologic symptoms and HHV-6 DNA in CSF without identification of an alternative etiology were categorized as having HHV-6 CNS dysfunction.
Results
Among 3,902 allogeneic HCT recipients from 1998-2012, 51 of 124 tested patients had HHV-6 DNA in CSF; 37 met criteria for HHV-6 CNS dysfunction and 14 (27%) did not. Patients with an alternative diagnosis had longer time to HHV-6 detection and lower viral load in CSF. Six patients without HHV-6 CNS dysfunction were not treated and had no morbidity attributable to HHV-6. Kaplan-Meier analysis demonstrated poor overall survival among all patients. Variables associated with higher all-cause mortality in a multivariable Cox model included alternative diagnosis (adjusted hazard ratio [aHR], 8.4; 95% CI, 1.7-40.9; P = 0.009) and higher peak plasma viral load (log10 scale) (aHR, 1.4; 95% CI, 1.1-1.9; P = 0.01).
Conclusion
We identified a number of allogeneic HCT recipients with HHV-6 DNA in CSF who did not meet criteria for HHV-6 CNS dysfunction. All patients had poor survival. Whether CSF HHV-6 DNA detection in patients without associated CNS dysfunction independently contributes to mortality and warrants treatment is unclear; management of these patients warrants further investigation.
Keywords: herpesvirus, hhv-6, transplant, encephalitis, CNS, neurologic
1. Background
Human herpesvirus 6 (HHV-6) latently infects over 90% of the population and is an opportunistic pathogen in patients undergoing allogeneic hematopoietic cell transplantation (HCT) [1,2]. Approximately 30-80% of patients develop HHV-6B viremia within six weeks of HCT, and this has been associated with multiple complications, including a spectrum of central nervous system (CNS) effects.
HHV-6-associated CNS dysfunction after HCT is well described and has significant morbidity [1–5]. Diagnosis of HHV-6 CNS dysfunction is predicated on detection of HHV-6 DNA in cerebrospinal fluid (CSF), which usually prompts treatment. There is a dearth of data regarding the frequency of HHV-6 DNA detection in CSF in the absence of associated CNS dysfunction; two studies found this to occur in 0-0.9% of immunocompromised patients [6,7]. We assessed the clinical significance of HHV-6 DNA detection in CSF from allogeneic HCT recipients.
2. Objectives
The aim of this study was to determine the frequency and significance of HHV-6 DNA detection in CSF after HCT.
3. Study design
3.1. Materials and methods
We identified all allogeneic HCT recipients who had HHV-6 DNA detected in CSF between 1998 and 2012 at Fred Hutchinson Cancer Research Center. Eleven patients were previously described [8]. Clinical testing of CSF for HHV-6 DNA was routinely performed during this period in post-HCT patients with neurologic symptoms using a previously described quantitative polymerase chain reaction (qPCR) assay that detected, but did not discriminate between, HHV-6B and A [8]. Semi-quantitative methods were replaced by real-time qPCR assays after 2000, and the testing procedure has otherwise been largely unchanged. Patients and their donors were evaluated for chromosomally integrated HHV-6 by determining the ratio of HHV-6 DNA to cell genome equivalents and excluded if positive [9].
We extracted patient demographics and clinical characteristics from a prospectively maintained database and chart review. CNS dysfunction was defined as a spectrum of symptoms ranging from headache to encephalopathy. Patients with persistent (>48 hours) symptoms of CNS dysfunction and HHV-6 DNA detection in CSF by qPCR without identification of an alternative etiology after extensive evaluation were categorized as having HHV-6 CNS dysfunction.
We compared risk factors and clinical outcomes in patients with and without HHV-6 CNS dysfunction. Demographics and clinical characteristics were compared using Fisher's exact test, chi-square test, or Wilcoxon rank-sum test as appropriate for the variable. We used Kaplan-Meier analyses to estimate survival probabilities and Cox proportional hazards models to evaluate univariate and multivariable hazard ratios (HRs) for all-cause mortality at 200 days after the first CSF sample with HHV-6 DNA detected. We included variables in the multivariable analysis if they had a P-value <.2 in univariate analysis. Statistical significance was defined as P <.05. SAS version 9.3 (SAS Institute, Cary, NC) was used for all statistical analyses.
4. Results
Among 3,902 allogeneic HCT recipients from 1998-2012, 388 had CSF tested for HHV-6, and 56 had HHV-6 DNA detected; five patients with pre-HCT or donor-derived ciHHV-6 were excluded. In this cohort of 51 patients, 37 (73%) met criteria for HHV-6 CNS dysfunction and 14 (27%) did not. Patient characteristics are presented in Supplementary Table 1. Among the 14 patients with HHV-6 DNA in CSF without HHV-6 CNS dysfunction, eight patients had an alternative diagnosis for their neurologic symptoms (infection [n=5] or drug toxicity [n=3]), three had spontaneous resolution of their symptoms prior to antiviral therapy, and three had no neurologic symptoms (CSF testing prompted by HHV-6 viremia). CSF testing among patients with no or spontaneous resolution of neurologic symptoms was negative for other infectious causes. Magnetic resonance imaging revealed evidence of limbic encephalitis in 14 of 29 tested patients with HHV-6 CNS dysfunction versus zero of nine tested patients without HHV-6 CNS dysfunction. Among identified cord blood recipients, 16/17 had HHV-6 CNS dysfunction.
Patients with HHV-6 CNS dysfunction had significantly higher HHV-6 VL in CSF (median peak copies/ml, 9,050; range, 54-450,000) compared to all patients without HHV-6 CNS dysfunction (median peak copies/ml, 655; range, 25-260,000; P = 0.05; Table 1, Fig. 1). This difference was driven primarily by patients with an alternative diagnosis (median peak copies/ml, 314; range, 45-9,200;= 0.02), who also had longer time after HCT to HHV-6 DNA detection in plasma (median day, 161 vs. 22; P = 0.03) and CSF (median day, 114 vs. 24; P = 0.002). Plasma HHV-6 VL did not distinguish between patients with and without CNS dysfunction, and there was no correlation between plasma and CSF HHV-6 VL in this cohort. Patients with HHV-6 CNS dysfunction compared to those with no or spontaneous resolution of neurologic symptoms had no significant differences.
Table 1. Characteristics of Patients With HHV-6 DNA in CSF After Allogeneic HCT and Comparison of Those With HHV-6 CNS Dysfunction to Other Patient Groups.
| No HHV-6 CNS dysfunction (n = 14) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | HHV-6 CNS dysfunction (n = 37) | No HHV-6 CNS dysfunction (n = 14) | P | Spontaneous resolution or no neurologic symptoms (n = 6 of 14) | P | Alternative diagnosis (n = 8 of 14) | P |
| Plasma HHV-6 | |||||||
| Day detected, median (range) | 22 (12-41) | 25 (13-206) | 0.49 | 18 (13-25) | 0.40 | 116, 206a | 0.03 |
| VL ≥ 1,000 copies/ml (%)b | 20 (67) | 3 (43) | 0.39 | 2 (50) | 0.60 | 1 (33) | 0.54 |
| VL, peak; median (range, copies/ml)b | 6,000 (35-520,000) | 24,700 (490-120,000) | 0.62 | 40,000 (490-120,000) | 0.52 | 9,400c | 0.84 |
| CSF HHV-6 | |||||||
| Day detected, median (range) | 24 (12-111) | 58 (14-209) | 0.03 | 24 (14-63) | 0.86 | 114 (14-209) | 0.002 |
| VL ≥ 1,000 copies/ml (%)d | 25 (69) | 5 (42) | 0.10 | 4 (67) | 1.00 | 1 (17) | 0.02 |
| VL, peak; median (range, copies/ml)d | 9,050 (54-450,000) | 655 (25-260,000) | 0.05 | 11,100 (25-260,000) | 0.58 | 314 (45-9,200) | 0.02 |
| Treated, no. (%) | 37 (100) | 8 (56) | … | 3 (50) | … | 5 (63) | … |
P values indicate comparisons between the HHV-6 CNS dysfunction group and each other group, respectively.
Abbreviations: HHV-6 CNS dysfunction, human herpesvirus 6-associated central nervous system dysfunction; CSF, cerebrospinal fluid; HCT, hematopoietic cell transplant; PBSC, peripheral blood stem cell; BM, bone marrow; VL, viral load.
Only two values available.
Quantitative results available for 30 patients with and seven without HHV-6 CNS dysfunction (four of six patients with spontaneous resolution or no neurologic symptoms and three of eight with an alternative diagnosis). Only positive results displayed.
Only one value available.
Quantitative results available for 36 patients with and 12 without HHV-6 CNS dysfunction (six of six patients with spontaneous resolution or no neurologic symptoms and six of eight with an alternative diagnosis).
Figure 1.
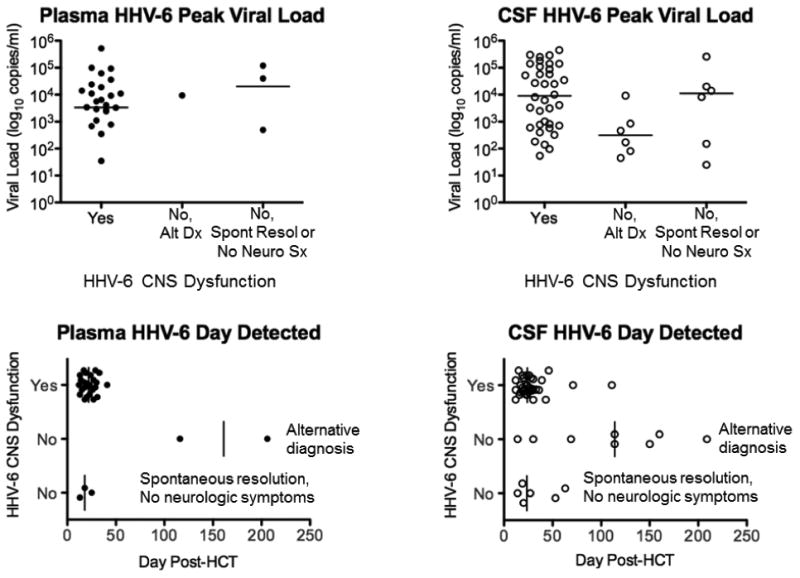
Comparisons of HHV-6 median peak viral load and median day of detection in plasma and CSF samples among patients with HHV-6 CNS dysfunction and those without HHV-6 CNS dysfunction (stratified into alternative diagnosis group and no neurologic symptoms or sponatenous resolution group). Patients with HHV-6 CNS dysfunction had significantly higher CSF HHV-6 peak viral load (P = 0.02) and earlier CSF day of detection (P = 0.002) than patients with an alternative diagnosis.
Ganciclovir and/or foscarnet were administered to all patients with and eight (57%) without HHV-6 CNS dysfunction for at least one week. Six patients without HHV-6 CNS dysfunction were not treated and had no morbidity attributable to HHV-6. Kaplan-Meier survival curves for patients with HHV-6 DNA detected in CSF and a contemporaneous group who were tested and negative demonstrated poor overall survival among all patient groups (Fig. 2). In a multivariable Cox proportional hazards model considering demographic and clinical characteristics (including antiviral treatment, plasma VL, and CSF VL), variables associated with higher all-cause mortality within 200 days of HHV-6 CSF detection were alternative diagnosis (adjusted hazard ratio [aHR], 8.4; 95% confidence interval [CI], 1.7-40.9; P = 0.009), peak plasma viral load (log10 scale) (aHR, 1.4; 95% CI, 1.1-1.9; P = 0.01), and male sex (aHR, 3.6; 95% CI, 1.5-8.2; P = 0.003).
Figure 2.
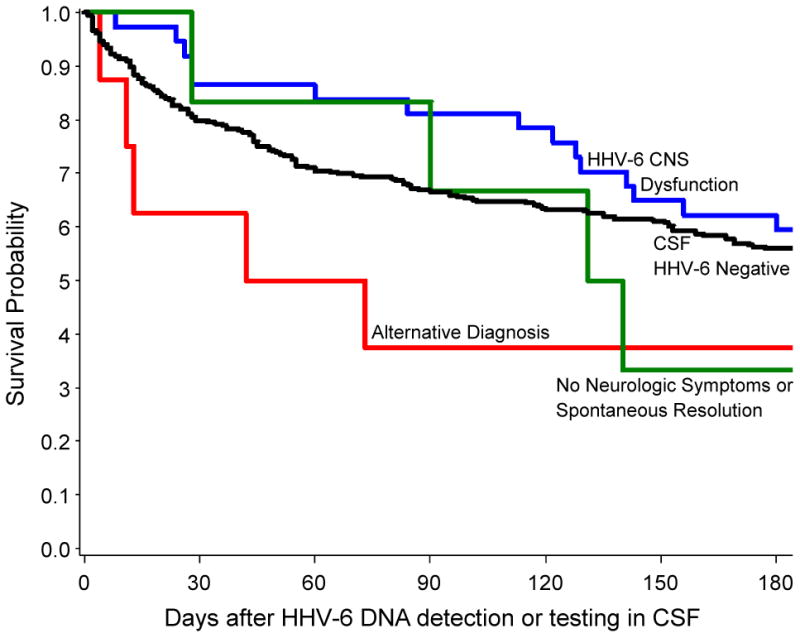
Kaplan-Meier survival curves for all-cause mortality through 180 days after HHV-6 DNA detection or testing in CSF, stratified by patient group. There were no significant differences between the curves by log-rank testing.
5. Discussion
Our findings suggest that HHV-6 DNA detection in CSF without associated CNS dysfunction may be relatively frequent, and clinical characteristics can be useful in identifying patients at higher risk for HHV-6 CNS dysfunction. We also found that both patients with and without HHV-6 CNS dysfunction had similarly poor survival.
Differences between patients with and without HHV-6 CNS dysfunction were driven by patients with an alternative diagnosis who had later HHV-6 DNA detection in plasma and CSF with lower CSF VL, although ranges were large and overlapping. Mortality rates did not significantly differ between those with and without HHV-6 CNS dysfunction and were similar to those who were tested and found negative for HHV-6 in CSF. This suggests that patients undergoing evaluation for neurological dysfunction after allogeneic HCT represent a high-risk cohort. We also showed that most cord blood recipients had HHV-6 CNS dysfunction if HHV-6 was detected in their CSF, consistent with studies underscoring the increased risk for HHV-6 reactivation and disease in these patients [1,3,4].
There was no association between antiviral treatment and decreased mortality among patients with HHV-6 DNA in CSF without CNS dysfunction, but this comparison was limited by small sample size and possibility of treatment bias. Notably, six patients without CNS dysfunction who were not treated had no morbidity attributable to HHV-6. The lack of correlation between plasma and CSF HHV-6 VL and low rate of traumatic lumbar punctures argue against HHV-6 spill over or contamination from blood. Whether CSF HHV-6 DNA detection in patients without associated CNS dysfunction independently contributes to mortality and warrants treatment is unclear. However, HHV-6 DNA detection in body fluids may underestimate organ-level replication [10], and HHV-6 viremia was independently associated with increased mortality in this and larger cohort studies [1].
Limitations of this study include small sample size and the administration of antiviral therapy to some patients who did not meet our post-hoc criteria for HHV-6 CNS dysfunction, possibly preventing development of qualifying symptoms. However, this is the largest cohort of patients with HHV-6-associated CNS dysfunction from a single center using sensitive qPCR assays to detect HHV-6 DNA and exclude patients with ciHHV-6.
In conclusion, patients may have HHV-6 DNA in CSF without clinically apparent HHV-6 CNS dysfunction, but these patients have similarly poor survival as those with HHV-6 CNS dysfunction. The epidemiology and significance of HHV-6 DNA detection in CSF remains incompletely understood, and management of these patients warrants further investigation.
Supplementary Material
Highlights.
We report the frequency of HHV-6 detection in CSF without HHV-6 CNS dysfunction.
We examine the clinical significance of HHV-6 detection in CSF.
27% of patients with HHV-6 in CSF did not have HHV-6 CNS dysfunction.
Patients with an alternative diagnosis had unique clinical findings.
Kaplan-Meier curves demonstrated poor overall survival among all patients.
Acknowledgments
We would like to thank Zack Stednick for his help with data retrieval for this study.
Funding: This work was supported by a Pilot Grant from the HHV-6 Foundation (J.A.H.) and by the National Institutes of Health (grant numbers CA18029 and HL093294, M.B.).
Footnotes
Presented in abstract form at the 40th Annual Meeting of the European Society for Blood and Marrow Transplantation, Milan, Italy. 2014: Abstract PH-O024.
Competing Interests: M. B. has served as a consultant and has received research support from Chimerix Inc., Genentech/Roche, and Gilead in addition to consulting for Clinigen. All other authors report no potential conflicts.
Ethical approval: All patients provided written consent, and the Fred Hutchinson Cancer Research Institute institutional review board approved this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zerr DM. Human herpesvirus 6 (HHV-6) disease in the setting of transplantation. Current Opinion in Infectious Diseases. 2012;25:438–44. doi: 10.1097/QCO.0b013e3283553362. [DOI] [PubMed] [Google Scholar]
- 2.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clinical Microbiology Reviews. 2005;18:217–45. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill Ja, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biology of Blood and Marrow Transplantation. Journal of the American Society for Blood and Marrow Transplantation. 2012;18:1638–8. doi: 10.1016/j.bbmt.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogata M, Satou T, Kadota JI, Saito N, Yoshida T, Okumura H, et al. Human Herpesvirus 6 (HHV-6) Reactivation and HHV-6 Encephalitis After Allogeneic Hematopoietic Cell Transplantation: A Multicenter, Prospective Study. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2013;57:671–81. doi: 10.1093/cid/cit358. [DOI] [PubMed] [Google Scholar]
- 5.Zerr DM, Fann JR, Breiger D, Boeckh M, Adler AL, Xie H, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117:5243–9. doi: 10.1182/blood-2010-10-316083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69:156–65. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- 7.Wang FZ, Linde a, Hägglund H, Testa M, Locasciulli a, Ljungman P. Human herpesvirus 6 DNA in cerebrospinal fluid specimens from allogeneic bone marrow transplant patients: does it have clinical significance? Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 1999;28:562–8. doi: 10.1086/515142. [DOI] [PubMed] [Google Scholar]
- 8.Zerr DM, Gupta D, Huang ML, Carter R, Corey L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2002;34:309–17. doi: 10.1086/338044. [DOI] [PubMed] [Google Scholar]
- 9.Sedlak RH, Cook L, Huang ML, Magaret A, Zerr DM, Boeckh M, et al. Identification of Chromosomally Integrated Human Herpesvirus 6 by Droplet Digital PCR. Clinical Chemistry. 2014 doi: 10.1373/clinchem.2013.217240. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fotheringham J, Akhyani N, Vortmeyer A, Donati D, Williams E, Oh U, et al. Detection of active human herpesvirus-6 infection in the brain: correlation with polymerase chain reaction detection in cerebrospinal fluid. The Journal of Infectious Diseases. 2007;195:450–4. doi: 10.1086/510757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


