Abstract
Background
Diabetics patients who undergo lower extremity surgical revascularization for critical limb ischemia (CLI) are at high-risk for amputation or death, even when their inpatient procedures are successful. We hypothesized that post-operative outcomes might be improved in regions where diabetics with CLI receive more frequent high-quality outpatient care.
Methods
A retrospective cohort study was performed among 172,134 patients with CLI (52% male, 15% black, mean age 76 years) who underwent open and endovascular lower extremity revascularization procedures using Medicare claims (2004–2007), which included 84,653 (49%) beneficiaries who were diabetic. Regional utilization of annual serum cholesterol and hemoglobin A1c testing were used to assess the quality of outpatient diabetic care. We examined relationships between frequency of diabetic testing with amputation-free survival (AFS), major adverse limb events (MALE), and rates of readmission across all U.S. hospital referral regions.
Results
There was significant regional variation in annual serum cholesterol and hemoglobin A1c testing across the U.S. (87% highest quartile vs. 59% lowest quartile, p<0.01). Compared with the lowest quartile of diabetic testing, diabetic patients undergoing lower extremity revascularization in regions with the highest quartile of diabetic testing had significantly improved AFS [HR:0.94 (95%CI:0.90–0.97);P<0.01] and MALE [HR:0.92 (95%CI:0.89–0.96);P<0.01] persisting up to two years after lower extremity revascularization, even after adjusting for procedure type, gender, age, race and comorbidities. Moreover, the risk of 30-day readmission was significant reduced in regions with the highest vs. lowest quartile of diabetic testing [OR:0.91 (95%CI:0.85–0.97);P<0.01]. Non-diabetic patients with CLI, in comparison, did not benefit to the same extent from undergoing revascularization in regions with high quality outpatient diabetic care.
Conclusions
Diabetic patients undergoing lower extremity revascularization in regions with higher utilization of diabetic care quality measures have significantly better long-term limb-salvage and readmission outcomes. Our study underscores the importance of providing optimal outpatient care to diabetics following vascular surgery and outlines a potential strategy for quality improvement in these high-risk patients.
INTRODUCTION
The management of peripheral arterial disease in diabetic patients presents a formidable challenge for medical providers and vascular surgeons alike. Diabetic patients experience accelerated atherosclerosis in the micro and macrovascular circulation of the lower extremity, resulting in the development of critical limb ischemia (CLI) in over 25% of patients during their lifetime.1,2 Control of infections and wound healing in these high-risk patients is further impaired by chronic elevations in serum glucose and lipid levels. These risk factors limit the success of lower extremity revascularization procedures in diabetics with CLI, resulting in a significantly higher risk of limb loss and/or mortality when compared to non-diabetic patients.3–5
It is well recognized that diabetic patients with CLI require a multidisciplinary and comprehensive approach to achieve limb salvage.2,6 Prevention of infections and wound complications involve aggressive glycemic control, and management of associated risk factors such as smoking, hypertension, and hyperlipidemia. Benchmarks and guidelines to measure the quality of outpatient diabetic care have been established and widely implemented throughout healthcare systems in the U.K. and the U.S. These include quality measures for comprehensive diabetic care established by the U.K. National Institute for Health and Care Excellence (NICE) as well as the U.S. National Committee for Quality Assurance (NCQA), via the Healthcare Effectiveness & Information Set (HEDIS).7–9 While most healthcare practitioners and policy makers agree on the usefulness of guidelines and preventive strategies, it is unknown if diabetic care quality measures are effective in helping to prevent amputation or death among patients with CLI requiring revascularization procedures.
We hypothesized that diabetic patients with CLI who were treated in regions with more frequent utilization of high-quality outpatient care would benefit from improved limb salvage and survival following lower extremity open and endovascular revascularization procedures. To test this hypothesis, we designed a study to evaluate the compliance of hospital referral regions throughout the U.S. with two diabetic care quality measures – annual hemoglobin A1c testing and annual serum cholesterol testing – and to determine whether these quality indicators could be used predict the risk of amputation or death following lower extremity revascularization procedures.
METHODS
Study Design
We designed a retrospective cohort study to evaluate the relationship between regional utilization of diabetic care quality measures and limb related outcomes following LE revascularization procedures for CLI. We assembled a cohort comprised of both diabetic patients with CLI as well as non-diabetic patients with CLI in order to examine the specificity of diabetic care quality measures on limb-related outcomes within the target population. Moreover, non-diabetic patients with CLI served as a control group to assess for selection bias within the cohort. Among this entire cohort, we studied limb related outcomes following both open bypass and endovascular treatments. We performed analyses after stratifying by whether the procedure was performed using open versus endovascular techniques, as well as when both types of interventions were combined for analyses given that both open bypass and endovascular treatments for CLI have been associated with similar outcomes after up to 2 years of follow-up.10 Our study protocol was approved by the Dartmouth Institutional Review Board.
Data Sources and Study Population
We used the Centers for Medicare and Medicaid Services (CMS) Medicare Provider Analysis and Review (MedPAR) database to identify 172,134 patients > 65 years old undergoing open and endovascular LE revascularization procedures between January 1, 2004 and December 31, 2007. This database contains patient and hospital identifiers, demographics, cardiovascular risk factors and comorbidities, hospitalization and procedure dates, complications, and discharge status for patients’ ≥ 65 years of age undergoing LE revascularization procedures for CLI in all regions throughout the U.S. International Classification of Diseases, 9th Revision (ICD-9) procedure codes were used to identify patients who underwent endovascular percutaneous interventions as well as open bypass procedures from the CMS dataset. This included both inflow and outflow procedures performed using open and endovascular techniques. In addition to these procedural codes for LE revascularization procedures, each patient was required to have a diagnosis code for critical lower limb ischemia. This included patients with diagnoses of rest pain, non-healing wounds and gangrene of the extremity. Patients with claudication and those undergoing revascularization for upper extremity CLI were excluded from analysis. In addition, we excluded from analysis records with missing values for primary outcome variables as well as variables for gender, age, and race strata.
In order to obtain patient demographic data, the MedPAR dataset was linked by patient zip codes to the 2007 U.S. Census Bureau. This dataset was used to obtain per-capita income for all zip codes where patients underwent lower extremity revascularization.
Exposure Variable: Regional Utilization of Diabetic Testing
We examined nationwide variation in the utilization of diabetic care quality measures at the level of hospital referral regions (HRR) using the quality of care dataset available from the Dartmouth Atlas for Healthcare.11 HRRs are 306 large regionalized health care markets across the entire U.S., which are defined by patient travel to receive tertiary care services. Each HRR has at least one tertiary referral center and several smaller hospitals. Utilization of diabetic care services for all 306 HRRs were obtained from part A and part B Medicare claims for all eligible beneficiaries within each region who underwent annual hemoglobin A1c testing and blood lipid testing between 2003 and 2007. In the U.S., performance on these two variables is measured as part of HEDIS and used along with other measures to compare comprehensive diabetic care between health plans.7
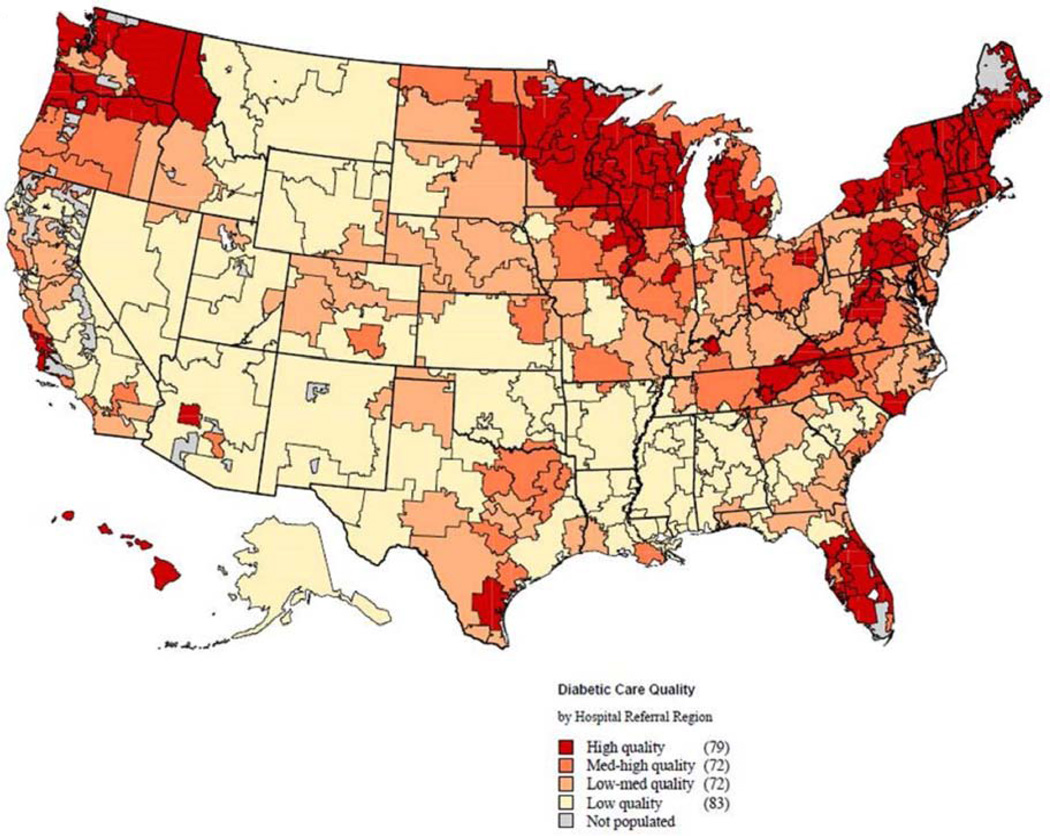
We generated a composite measure of regional diabetic care quality using the annual mean rate of serum lipid and hemoglobin A1c testing within each region, and then calculated this quality measure across the 306 HRRs in the United States. HRRs were then ranked according to this diabetic quality metric and stratified into four equal quartiles, which we deemed “low”, “low-medium”, “medium-high”, and “high” diabetic care quality (Figure 1).
Figure 1.
Distribution of diabetic care quality across the U.S. population as indexed by quartiles of annual hemoglobin A1c and serum cholesterol testing rates among all hospital referral regions on the map. The dark red areas represent hospital referral regions within the quartile with the highest rates of annual testing (i.e. high-quality diabetic care), whereas the light areas are hospital referral regions with the lowest rates of annual testing (i.e. low-quality diabetic care).
Next, we linked regional diabetic care quality data by HRR quartiles to Medicare part A claims files for all patients with CLI undergoing LE revascularization between 2004 and 2007. All beneficiaries with CLI who underwent open and endovascular LE revascularization were assigned to one of 4 diabetic quality strata depending on where their procedure took place. This resulted in four equal patient cohorts stratified by level of diabetic quality (a.k.a frequency of hemoglobin A1c and serum lipid testing), which were used to compare outcomes for analyses.
Amputation Free Survival & Major Adverse Limb Events
The primary outcomes for the study were amputation-free survival (AFS) and major adverse limb events (MALE) following LE revascularization. We selected these outcomes given that they have been validated by the Society of Vascular Surgery (SVS) as specific objective performance goal measures for evaluating treatments of CLI.12,13 AFS was defined as any amputation or death that occurred within the 2 year period following an open or endovascular LE revascularization procedure. MALE was defined similarly for both open or endovascular procedures as any major amputation (above the ankle) or major re-intervention that occurred within the 2 year period following a LE revascularization procedure, which included placement of a new bypass graft, use of thrombectomy or thrombolysis, and/or major surgical revision such as jump or interposition graft. Both of these outcomes were evaluated at multiple follow-up time points including 30-days, 1-year, and 2-years following discharge from the index hospitalization for each beneficiary’s revascularization procedure. Analyses of both AFS and MALE outcomes were conducted after stratifying patients into cohorts of diabetic and non-diabetic patients with CLI.
30-Day Readmission
The secondary outcome for the study was 30-day readmission following LE revascularization for CLI. Readmission was defined as a readmission to any hospital within 30 days of discharge from index hospitalization for the vascular procedure. Only the first readmission during the first 30 days post-operatively was examined. Transfers to and from another hospital and admissions for rehabilitation were not counted as readmissions.
Statistical Analysis
We started by performing univariate analyses to examine whether any significant associations existing between patient characteristics, diabetic quality quartiles, and each of our outcome measures for diabetic and non-diabetic patients. Analyses of patient variables and outcome variables were performed using chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables that were normally distributed. The Wilcoxon signed-rank sum test was used to compare non-normally distributed data. We then used Cox proportional hazards models to estimate the effect of diabetic care quality on AFS and MALE outcomes while adjusting for patient-level and regional-level variables and potential confounders. We studied only index revascularization procedures occurring before January 1, 2008 to allow for 2-year follow-up of all patients in our cohort. Risk adjustment models were constructed for both diabetic and non-diabetic cohorts, and included baseline patient demographic variables for age (continuous and categorical variables), gender, race, procedure type (open versus endovascular) and comorbidity score using the Charlson comorbidity index. These models accounted for clustering of patient-level outcomes within hospital referral regions. Potential interactions between variables were also explored using multivariate analysis. P values less than 0.05 (two-sided) were considered to be significant for all statistical tests and models, and the Bonferroni correction was used to control for multiple comparisons. STATA 12.0 statistical software (College Station, TX) was used for all analyses.
RESULTS
Regional variation in outpatient diabetic care
The frequency of outpatient diabetic testing was found to vary significantly across U.S. hospital referral regions (Fig 1). Annual mean hemoglobin A1c and serum lipid testing ranged as low as 59% in low-quality regions to 87% in high-quality regions. As shown by the map, variation in diabetic testing appeared to follow geographical boundaries, with the highest quartiles clustered in the Northern New England regions, Great Lakes regions, and Pacific Northwest regions of the United States. The lowest quartiles appeared clustered in the Mountain West regions, Midwest regions, and Southeast regions.
Patient characteristics by regional diabetic care quality
We identified 172,134 beneficiaries from the MedPar dataset who underwent lower extremity revascularization for CLI using either endovascular intervention or open bypass during the period between 2004 and 2007. This cohort included 84,653 (49%) patients who were diabetic (diabetes mellitus type 1 or 2) and 87,481 patients with CLI who were non-diabetic. A total of 138,808 (80%) endovascular procedure were performed and 33,326 (20%) open surgical bypasses were performed within this cohort. After stratifying beneficiaries into quartiles of regional diabetic care quality by testing frequency, we identified 44,444 (25%) of all procedures were undertaken in regions defined as low quality, 45,473 (27%) procedures were undertaken in low-medium quality regions, 41,470 (24%) procedures were undertaken in medium-high quality regions, and 40,747 (24%) procedures undertaken in high quality regions.
Characteristics of our patient cohort are presented in Table 1, stratified by quartile of regional diabetic care quality where LE revascularization procedures were undertaken. Compared to patients in the lowest quartile of diabetic care, patients who underwent LE revascularization in regions with high quality diabetic care were older, but less likely to be female and had fewer comorbidities as determined by the Charlson Index (all P<0.05). Patients undergoing revascularization in high quality regions were also less likely to have diabetes, congestive heart failure, chronic pulmonary disease, and undergo an endovascular revascularization (all P<0.05). Most profoundly, regions with high-quality diabetic care had a 3-fold lower rate of undergoing secondary revascularization procedures (4.4% high quality vs. 17.0% low quality; P<0.001). Mean per capita income did not differ across diabetic care regions (P=0.15).
Table 1.
Characteristics of patients with critical limb ischemia undergoing lower extremity revascularization, by quartile of diabetic care quality
| Variable | Quality of Diabetic Care | P-value* | |||
|---|---|---|---|---|---|
| Low | Low-Med | Med-High | High | ||
| Number of patients | 44,444 | 45,473 | 41,470 | 40,747 | |
| Age, % | <0.001 | ||||
| 65–69 years | 20.1 | 19.4 | 19.1 | 17.5 | |
| 70–79 years | 50.5 | 50.6 | 50.3 | 51.0 | |
| ≥ 80 years | 29.4 | 30.0 | 30.6 | 31.5 | |
| Male gender, % | 49.3 | 49.6 | 50.2 | 51.5 | <0.05 |
| Black race, % | 17.0 | 11.6 | 10.4 | 4.4 | <0.001 |
| Charlson Comorbidity Score, mean | 4.73 | 4.79 | 4.76 | 4.63 | <0.05 |
| Diabetes Type 1 or 2, % | 50.2 | 49.6 | 49.6 | 47.1 | <0.05 |
| Cerebrovascular Disease, % | 45.6 | 45.7 | 46.6 | 45.4 | 0.71 |
| Congestive Heart Failure, % | 46.5 | 44.6 | 43.5 | 41.2 | <0.001 |
| Chronic Renal Insufficiency, % | 28.5 | 30.5 | 29.5 | 28.7 | 0.68 |
| Chronic Obstructive Pulmonary Disease, | 39.1 | 40.3 | 39.5 | 38.5 | <0.05 |
| Per-Capita Income, mean $ | 18,058 | 20,162 | 18,144 | 18,458 | 0.15 |
| Endovascular Procedures, % | 82.3 | 81.2 | 79.4 | 79.5 | <0.05 |
P-value calculated using analysis of variance
Association between regional diabetic care and major adverse limb events
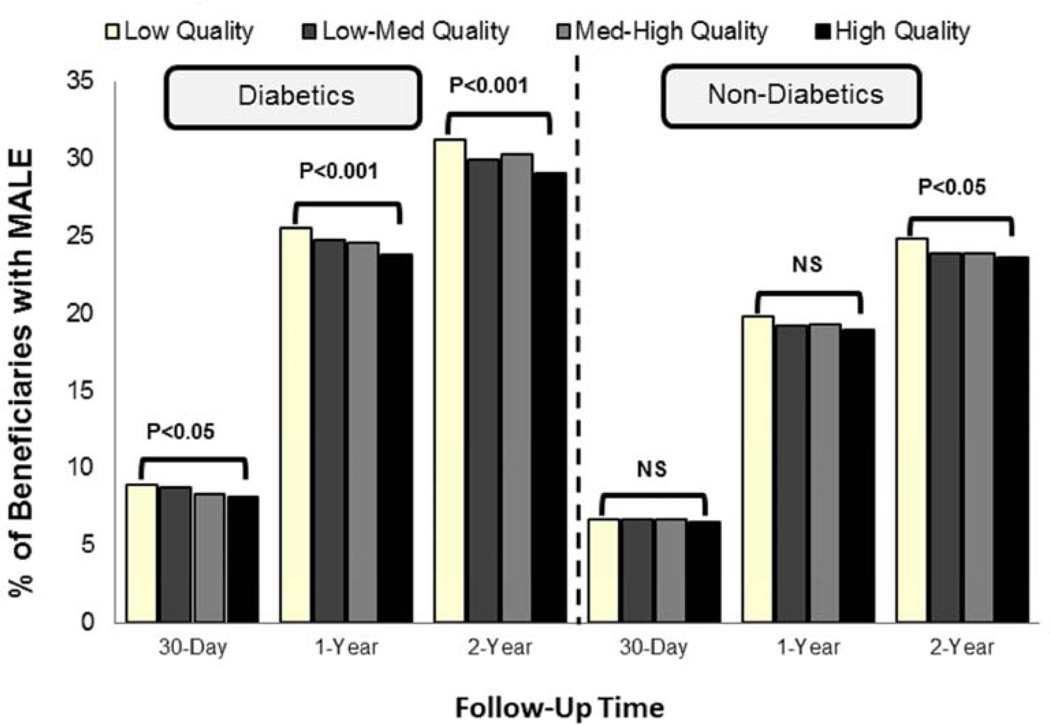
We found an inverse relationship between regional utilization of diabetic care quality measures and the rate of major adverse limb events (MALE) among diabetic patients with CLI undergoing open or endovascular LE revascularization (Figure 2). When stratified by procedure type, diabetic patients undergoing open LE revascularization in regions with high quality diabetic care had a significantly lower rate of MALE at 1-year (28.6% high vs. 31.3% low, P<0.05), and 2-years (34.3% high vs. 37.4% low, P<0.05) of follow-up when compared to regions with low quality diabetic care. Diabetic patients undergoing endovascular LE revascularizations in regions with high quality diabetic care also had significantly lower rate of 1-year (22.9% high vs. 24.1% low; P=0.05), and 2-years (27.9% vs. 29.6%; P<0.05) of follow-up when compared to regions with low quality diabetic care. In comparison, the beneficial association between high quality diabetic care and MALE outcomes was not consistently found among non-diabetic patients with CLI (Figure 2), irrespective of procedure type.
Figure 2.
Relationship between regional quality of diabetic care and major adverse limb events (MALE) at 30-days, 1-year, and 2-years following lower extremity revascularization among diabetic and non-diabetic patients with critical limb ischemia.
We found similar results when examining the association between regional utilization of diabetic care quality measures and MALE at all follow-up time points using Cox proportional hazard models, which controlled for patient, procedural and regional-level covariates. Similar to our unadjusted analyses, risk adjusted models demonstrated a significant lower likelihood of MALE outcomes for diabetic patients with CLI at 2-years following LE revascularization in regions with high quality diabetic care (Table 2). As with our crude analyses, non-diabetic patients were not found to have a significant improvement in MALE outcomes after controlling for confounders. Other variables associated with MALE outcomes in parsimonious regression models included age ≥ 70 years, male gender, black race, Charlson comorbidity scores ≥2, and whether revascularization procedure was endovascular vs. open.
Table 2.
Cox regression model identifying independent factors associated with major adverse limb events (MALE) in diabetics and non-diabetics with critical limb ischemia (CLI) two years following lower extremity revascularization.
| Diabetics with CLI | Non-Diabetics with CLI | ||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | P- value |
Variable | HR (95% CI) | P- value |
| Diabetic Care Quality | Diabetic Care Quality | ||||
| Low Quality | Ref | Low Quality | Ref | ||
| High Quality | 0.92 (0.89–0.96) | <0.01 | High Quality | 0.97 (0.93–1.01) | 0.185 |
| Male Gender | 1.13 (1.09–1.17) | <0.01 | Male Gender | 1.07 (1.03–1.11) | <0.01 |
| Age | Age | ||||
| 65–69 yrs | Ref | 65–69 yrs | Ref | ||
| 70–79 yrs | 1.04 (0.99–1.08) | 0.09 | 70–79 yrs | 1.00 (0.95–1.05) | 0.99 |
| ≥ 80 yrs | 1.17 (1.11–1.24) | <0.01 | ≥ 80 yrs | 1.10 (1.04–1.16) | <0.05 |
| Black Race | 1.25 (1.19–1.31) | <0.01 | Black Race | 1.26 (1.18–1.35) | <0.01 |
| Charlson Comorbidity | Charlson Comorbidity | ||||
| Score 0–2 | Ref | Score 0–2 | Ref | ||
| Score 2–4 | 1.47 (1.36–1.59) | <0.01 | Score 2–4 | 1.35 (1.28–1.42) | <0.01 |
| Score 4–6 | 1.90 (1.76–2.05) | <0.01 | Score 4–6 | 1.61 (1.53–1.70) | <0.01 |
| Score >6 | 2.96 (2.75–3.19) | <0.01 | Score >6 | 2.15(2.04–2.29) | <0.01 |
| Procedure Type | Procedure Type | ||||
| Open Bypass | Ref | Open Bypass | Ref | ||
| Endovascular | 0.90 (0.86–0.94) | <0.01 | Endovascular | 0.73 (0.70–0.77) | <0.01 |
Association between regional diabetic care and amputation free survival (AFS)
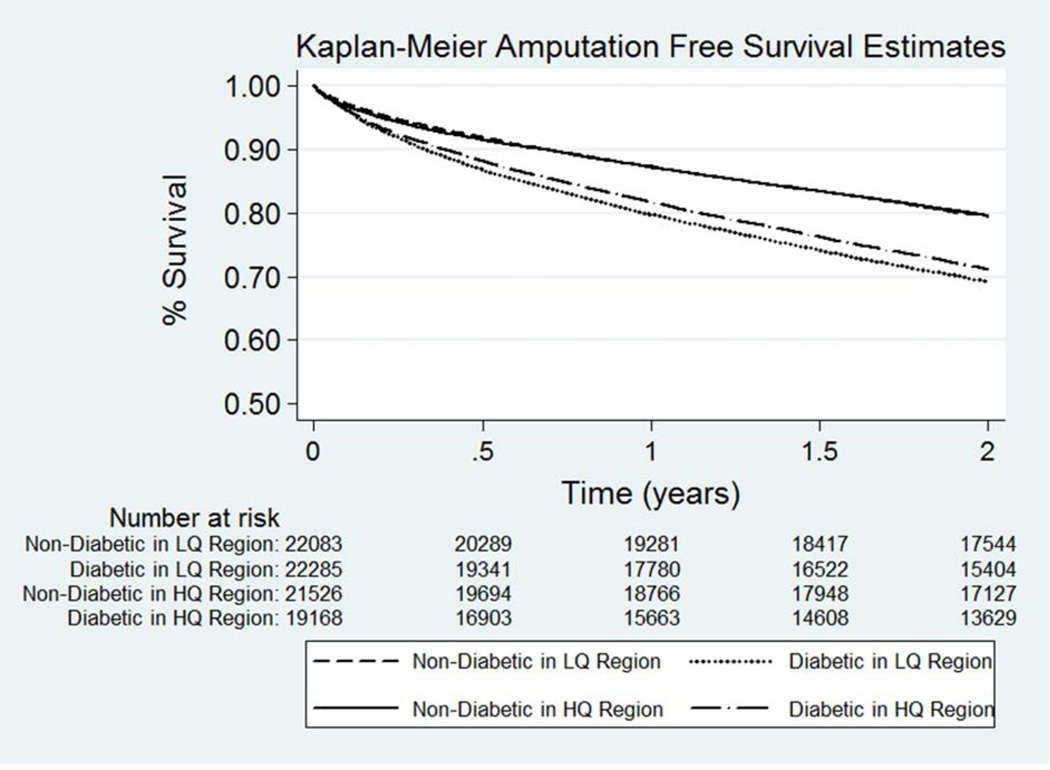
We compared AFS estimates among diabetic and non-diabetics beneficiaries following LE revascularization, stratified by regional level of diabetic care quality. As shown in Figure 3, there was a significant AFS benefit at 2-years for diabetic patients undergoing LE revascularization within regions with high versus low quality diabetic care (65.9% vs. 63.4%, P<0.001). In comparison, the regional level of diabetic care quality had no impact on AFS estimates at 2-years among non-diabetic patients with CLI (77.4% vs. 77.0%, P=0.19) (Fig. 3).
Figure 3.
Amputation-free survival estimates following lower extremity revascularization among diabetic and non-diabetic patients with critical limb ischemia, by quality of regional diabetic care. Abbreviations: LQ = low-quality; HQ = high-quality
We next compared 2-year AFS among diabetics and non-diabetics in different diabetic quality regions, stratified by whether they had undergone open versus endovascular revascularization. Among diabetics who underwent open revascularization, there was a significant improvement in AFS among patients in high versus low quality regions (59.6% high vs. 55.3% low; P<0.01). Similarly, diabetics who underwent endovascular revascularization in regions with high versus low quality diabetic care had a significant improvement in AFS at 2-years (67.0% high vs. 64.7% low; P<0.01). In comparison, non-diabetic patients undergoing either open or endovascular revascularization did not show any significant AFS benefits associated with high quality diabetic care
Using Cox proportional hazard models, we next assessed independent variables associated with the composite outcome of amputation and mortality among diabetics and non-diabetics with CLI. In risk adjusted models, diabetic patients undergoing LE revascularization in regions with high quality diabetic care were significantly less likely to require amputation or die at 2-years when compared to patients in low quality regions (Table 3). Additional variables associated with AFS included advanced age, male gender, black race, Charlson score > 2 and whether revascularization procedure was endovascular. However, regression models that evaluated non-diabetic patients with CLI found no difference in likelihood of amputation or mortality following LE revascularization between high versus low quality diabetic care regions.
Table 3.
Cox regression model identifying independent factors associated with composite outcome of amputation or mortality among diabetics and non-diabetics with critical limb ischemia (CLI) two years following lower extremity revascularization
| Diabetics with CLI | Non-Diabetics with CLI | ||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | P- value |
Variable | HR (95% CI) | P- value |
| Diabetic Care Quality | Diabetic Care Quality | ||||
| Low Quality | Ref | Low Quality | Ref | ||
| High Quality | 0.94 (0.90–0.97) | <0.01 | High Quality | 0.99 (0.95–1.03) | 0.59 |
| Male Gender | 1.05 (1.02–1.09) | <0.05 | Male Gender | 1.02 (0.98–1.06) | 0.37 |
| Age | Age | ||||
| 65–69 yrs | Ref | 65–69 yrs | Ref | ||
| 70–79 yrs | 1.22 (1.17–1.28) | <0.01 | 70–79 yrs | 1.25 (1.18–1.34) | <0.01 |
| ≥ 80 yrs | 1.91 (1.82–2.01) | <0.01 | ≥ 80 yrs | 2.34 (2.20–2.49) | <0.01 |
| Black Race | 1.21 (1.15–1.26) | <0.01 | Black Race | 1.40 (1.32–1.50) | <0.01 |
| Charlson Comorbidity | Charlson Comorbidity | ||||
| Score 0–2 | Ref | Score 0–2 | Ref | ||
| Score 2–4 | 1.76 (1.60–1.95) | <0.01 | Score 2–4 | 1.52 (1.42–1.62) | <0.01 |
| Score 4–6 | 2.84 (2.58–3.12) | <0.01 | Score 4–6 | 2.28 (2.13–2.44) | <0.01 |
| Score >6 | 5.53 (5.03–6.06) | <0.01 | Score >6 | 4.07 (3.83–4.33) | <0.01 |
| Procedure Type | Procedure Type | ||||
| Open Bypass | Ref | Open Bypass | Ref | ||
| Endovascular | 0.84 (0.81–0.88) | <0.01 | Endovascular | 0.66 (0.63–0.69) | <0.01 |
Association between regional diabetic care and risk of 30-day readmission
Similar to limb-salvage outcomes, we found an inverse relationship between regional utilization of diabetic care quality measures and the rate of readmission among diabetic patients at 30-days following LE revascularization. There was a significant decrease in readmission for diabetic patients within regions with high versus low quality diabetic care at 30-days (20.3% high vs. 22.2% low; P<0.001) following LE revascularization. The readmission benefit associated with high quality diabetic care was found for both patients undergoing open bypass (23.0% high vs. 27.3% low; P<0.001) as well as endovascular revascularization (19.4% high vs. 21.1% low; P<0.001). These results were confirmed in risk-adjusted regression models demonstrating a significant reduction in the risk of 30-day readmission for diabetic patients undergoing LE revascularization in regions with high versus low quality diabetic care (Table 4). Finally, non-diabetic patients who underwent revascularization in regions with high quality care were found to have reduction in readmission at 30-days, but this did not meet statistical significance in adjusted models (Table 4).
Table 4.
Logistic regression model identifying independent factors associated with risk of 30-day readmission among diabetics and non-diabetics with critical limb ischemia following lower extremity revascularization.
| Diabetics with CLI | Non-Diabetics with CLI | ||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | P- value |
Variable | HR (95% CI) | P- value |
| Diabetic Care Quality | Diabetic Care Quality | ||||
| Low Quality | Ref | Low Quality | Ref | ||
| High Quality | 0.91 (0.85–0.97) | <0.01 | High Quality | 0.94 (0.88–1.00) | 0.06 |
| Male Gender | 1.00 (0.95–1.05) | 0.96 | Male Gender | 0.97 (0.92–1.02) | 0.21 |
| Age | Age | ||||
| 65–69 yrs | Ref | 65–69 yrs | Ref | ||
| 70–79 yrs | 1.07 (1.00–1.13) | 0.05 | 70–79 yrs | 1.09 (1.02–1.19) | <0.05 |
| ≥ 80 yrs | 1.21 (1.13–1.30) | <0.01 | ≥ 80 yrs | 1.36 (1.27–1.47) | <0.01 |
| Black Race | 1.11 (1.03–1.19) | <0.01 | Black Race | 1.19 (1.10–1.31) | <0.01 |
| Charlson Comorbidity | Charlson Comorbidity | ||||
| Score 0–2 | Ref | Score 0–2 | Ref | ||
| Score 2–4 | 1.32 (1.17–1.48) | <0.01 | Score 2–4 | 1.20 (1.10–1.30) | <0.01 |
| Score 4–6 | 1.73 (1.52–1.96) | <0.01 | Score 4–6 | 1.50 (1.38–1.64) | <0.01 |
| Score >6 | 2.61 (2.32–2.94) | <0.01 | Score >6 | 2.11 (1.94–2.30) | <0.01 |
| Procedure Type | Procedure Type | ||||
| Open Bypass | Ref | Open Bypass | Ref | ||
| Endovascular | 0.82 (0.77–0.88) | <0.01 | Endovascular | 0.83 (0.77–0.88) | <0.01 |
DISCUSSION
Diabetic patients who develop critical limb ischemia have discouragingly poor outcomes and often suffer amputation, hospital readmissions, and death following LE revascularization procedures.3,4,10 To date, few interventions have been shown to improve amputation-free survival or reduce major adverse limb events within this high-risk population. However, our study demonstrates that diabetic patients with CLI who undergo open or endovascular LE revascularization in regions with a higher utilization of outpatient diabetic care quality measures have significant improvements in amputation-free survival and major adverse limb events when compared to diabetic patients undergoing these procedures in regions with a low utilization of diabetic care quality measures. These limb-salvage benefits of regional outpatient care are specific to diabetic patients in our analysis, are found following both open and endovascular LE revascularization, and appear to be durable, extending up to 2-years following LE revascularization. Furthermore, our data show that patients undergoing open or endovascular revascularization in regions with higher quality diabetic care also benefit from lower rates of 30-day readmission.
The treatment of diabetes requires a comprehensive approach to prevent complications of chronic disease.2,6 This involves an integrated approach from primary care providers, endocrinologists, vascular surgeons, ophthalmologists, podiatrists, as well as other members of the multidisciplinary team. But how well diabetic care services are being delivered to individual patients by different providers is largely unknown. For example, a vascular surgeon who is referred a diabetic patient with hyperglycemia, hyperlipidemia, or hypertension may not have a laboratory or clinical assessment of how well these conditions are being treated other than documentation showing they are on appropriate medications. Moreover, prior reports have documented low rates of compliance with multidisciplinary care pathways among diabetic patients with CLI.14 In the context of other studies, our results highlight the importance of annual hemoglobin A1c and serum lipid testing in diabetic patients with CLI undergoing revascularization procedures.1,2 Testing enables identification of patients who are poorly medically managed, as well as to ensure those patients on proper regimens are maintained. While surgeons traditionally have not focused on these aspects of outpatient patient care, nevertheless our results suggest that the non-surgical management of diabetic patients is critical in order to achieve the best long term surgical outcomes.
The traditional focus on improving outcomes in high-risk patients following surgical procedures has centered on implementing perioperative processes of care that occur within the hospital. Among diabetic patients, for instance, there have been numerous studies that have identified an association between glycemic control during the perioperative period and in-hospital outcomes.15,16 In contrast, there has been limited research on the impact of post-discharge care following surgical procedures that occurs in the outpatient setting. Our study provides the first line of evidence demonstrating that regions where diabetic patients receive more frequent testing of HgA1c and lipid levels in the outpatient setting have better limb-related outcomes following revascularization surgery. Moreover, non-diabetics in regions with more frequent testing did not receive the same benefits, supporting the specificity of these targeted measures. In theory and practice, maintaining tight chronic control of serum glucose and lipid levels in diabetics should help promote improved wound healing, help prevent infections, decelerate the progression of atherosclerosis, and reduce the overall number of complications that can lead to re-intervention, amputation, or mortality.
While our study is a good starting point, it does not delineate the factors’ responsible for variation in outpatient diabetic quality of care across the U.S. Race and poverty have previously been shown to be associated with disparities in access to vascular care and worse limb-related outcomes.17–19 In our study, a significant higher proportion of African Americans underwent LE revascularization in regions with low vs. high quality diabetic care, although per-capita income was not significantly different between low and high-quality regions (Table 1). More frequent lab testing may simply be a surrogate for greater access to outpatient health care resources such as wound care and/or the ability to afford treatment. Consequently, our future work will aim to use patient-level analyses to explore relationships between patient factors, racial or socioeconomic disparities in access to high quality outpatient diabetic care, and long-term outcomes after LE revascularization. These findings will have important health policy implications, as they may identify future quality improvement initiatives likely to result in better outcomes for diabetic patients undergoing endovascular and lower extremity bypass surgery.
While the proportional improvement in clinical outcomes associated with high-quality regional diabetic care is small, these findings are magnified when we consider the cost of amputations, re-interventions, and readmissions for diabetic patients with CLI on a population level. For example, the annual average cost of medical care and health care services for a diabetic patient that undergoes a major lower extremity amputation ranges upwards of $58,000 to $83,000.20,21 This includes acute care hospital costs as well as outpatient care services, which are significantly higher than healthcare costs associated with amputations for non-diabetic patients. Moreover, the projected lifetime health care costs for patients with amputations is over 3 times higher than those who undergo revascularization alone.22
Our study has several main limitations. First, the study was a retrospective analysis of administrative claims data, which has a limited ability to discriminate important patient variables, including the severity of diabetes, smoking status, severity of limb ischemia (anatomic or physiologic), or whether they had prior revascularization attempts. In addition, the study design is unable to assess the temporal relationship of diabetic care with outcome measures. However, this dataset is well suited to our study objective, which aimed to look at variation in the utilization of diabetic care quality measures and limb-related outcomes across the entire U.S. population. Second, the analysis of diabetic care quality was undertaken at the level of U.S. hospital referral regions, and information about preventive diabetic care measures applied to individual patients is unknown. Our future work will build on these findings, and use patient-level studies to validate the association between diabetic care quality measures and limb related outcomes. Third, differences in quality of regional diabetic care (i.e frequency of testing) may serve as proxies for disparities in socioeconomic status or access to medical care. However, the fact that our study demonstrated an effect specific mainly to diabetic patients suggests that our effect is real, and not simply confounding related to differences in race or socioeconomic status. Finally, our study used only hemoglobin A1c and serum lipid testing as evidence of high quality diabetic care, not the actual lab values themselves. While our future work aims to integrate linked clinical-claims analyses that incorporate actual clinical values, current quality measures in the U.S. and the U.K. recognize frequency of testing as a first step towards exploring the quality of diabetic care.7,9
CONCLUSION
Diabetic patients with CLI undergoing lower extremity open and endovascular revascularization in U.S. regions with more frequent utilization of outpatient diabetic care quality measures have significantly better long-term amputation-free survival, readmission, and limb salvage outcomes. While the effect size is modest, our data suggests that improving outpatient diabetic care may help impact limb salvage outcomes in high-risk patients that traditionally have been hard to improve. Promoting comprehensive care for high-risk surgical patients at transitions of care and in the outpatient setting is a good target for quality improvement. Our future work will focus on patient-level studies to evaluate the specific effects of diabetic quality measures and care pathways for diabetic patients undergoing vascular surgery.
ACKNOWLEDGMENTS
We thank the Dartmouth Atlas for Healthcare for providing U.S. nationwide hospital referral region data for annual hemoglobin A1c testing and blood lipid testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005 Jan 12;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Kalish J, Hamdan A. Management of diabetic foot problems. J Vasc Surg. 2010 Feb;51(2):476–486. doi: 10.1016/j.jvs.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Malmstedt J, Leander K, Wahlberg E, Karlstrom L, Alfredsson L, Swedenborg J. Outcome after leg bypass surgery for critical limb ischemia is poor in patients with diabetes: a population-based cohort study. Diabetes Care. 2008 May;31(5):887–892. doi: 10.2337/dc07-2424. [DOI] [PubMed] [Google Scholar]
- 4.Wallaert JB, Nolan BW, Adams J, et al. The impact of diabetes on postoperative outcomes following lower-extremity bypass surgery. J Vasc Surg. 2012 Nov;56(5):1317–1323. doi: 10.1016/j.jvs.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dick F, Diehm N, Galimanis A, Husmann M, Schmidli J, Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: influence of diabetes mellitus on clinical outcome. J Vasc Surg. 2007 Apr;45(4):751–761. doi: 10.1016/j.jvs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Sumpio BE, Armstrong DG, Lavery LA, Andros G. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the Society for Vascular Surgery and the American Podiatric Medical Association. J Vasc Surg. 2010 Jun;51(6):1504–1506. doi: 10.1016/j.jvs.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Quality Assurance. HEDIS and Quality Measurement. [Retrieved: November 10, 2012];2013 Available at: http://www.ncqa.org/HEDISQualityMeasurement/HEDISandQualityMeasureImprovement.aspx. [Google Scholar]
- 8.Harman JS, Scholle SH, Ng JH, et al. Association of Health Plans' Healthcare Effectiveness Data and Information Set (HEDIS) performance with outcomes of enrollees with diabetes. Med Care. 2010 Mar;48(3):217–223. doi: 10.1097/MLR.0b013e3181ca3fe6. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence. Diabetes in Adult Quality Standard. [Retrieved: August 10, 2013];2013 Available at: http://publications.nice.org.uk/diabetes-in-adults-quality-standard-qs6/quality-statement-8-complications#quality-statement-8. [Google Scholar]
- 10.Conte MS. Critical appraisal of surgical revascularization for critical limb ischemia. J Vasc Surg. 2013 Feb;57(2 Suppl):8S–13S. doi: 10.1016/j.jvs.2012.05.114. [DOI] [PubMed] [Google Scholar]
- 11.The Dartmouth Institute for Health Policy & Clinical Practice. Dartmouth Atlas for Healthcare. [Retrieved: June 20, 2012];2013 Available at: http://www.dartmouthatlas.org/ [Google Scholar]
- 12.Goodney PP, Schanzer A, Demartino RR, et al. Validation of the Society for Vascular Surgery's objective performance goals for critical limb ischemia in everyday vascular surgery practice. J Vasc Surg. 2011 Jul;54(1):100–108. doi: 10.1016/j.jvs.2010.11.107. e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte MS. Understanding objective performance goals for critical limb ischemia trials. Semin Vasc Surg. 2010 Sep;23(3):129–137. doi: 10.1053/j.semvascsurg.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Ellis E, Ballance K, Lunt H, Lewis D. Diabetes outpatient care before and after admission for diabetic foot complications. J Wound Care. 2010 Apr;19(4):150–152. doi: 10.12968/jowc.2010.19.4.150. [DOI] [PubMed] [Google Scholar]
- 15.van Kuijk JP, Schouten O, Flu WJ, den Uil CA, Bax JJ, Poldermans D. Perioperative blood glucose monitoring and control in major vascular surgery patients. Eur J Vasc Endovasc Surg. 2009 Nov;38(5):627–634. doi: 10.1016/j.ejvs.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Sathya B, Davis R, Taveira T, Whitlatch H, Wu WC. Intensity of peri-operative glycemic control and postoperative outcomes in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2013 Jun 6; doi: 10.1016/j.diabres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg JB, Goodney PP, Cronenwett JL, Baker F. The effect of risk and race on lower extremity amputations among Medicare diabetic patients. J Vasc Surg. 2012 Dec;56(6):1663–1668. doi: 10.1016/j.jvs.2012.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodney PP, Holman K, Henke PK, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg. 2013 Feb 1; doi: 10.1016/j.jvs.2012.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2011 Jan;5(1):94–102. doi: 10.1161/CIRCOUTCOMES.111.962233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Archives of physical medicine and rehabilitation. 2005 Mar;86(3):480–486. doi: 10.1016/j.apmr.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 21.Sargen MR, Hoffstad O, Margolis DJ. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. Journal of diabetes and its complications. 2013 Mar-Apr;27(2):128–133. doi: 10.1016/j.jdiacomp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. The Journal of bone and joint surgery. American volume. 2007 Aug;89(8):1685–1692. doi: 10.2106/JBJS.F.01350. [DOI] [PubMed] [Google Scholar]