Summary
Behavioral strategies that facilitate the maintenance of social bonds are critical for the preservation of high-quality social relationships. Central oxytocin (OT) activity modulates the behavioral features of socially monogamous relationships in a number of mammalian species (including marmoset monkeys), and plays a vital role in the behavioral maintenance of long-term social relationships. Two distinct variants of OT have been identified in some New World primates (including marmosets; Lee et al., 2011). The marmoset variant of the oxytocin ligand (Pro8-OT) is structurally distinct from the consensus mammalian variant of the oxytocin ligand (Leu8-OT), due to a proline substitution at the 8th amino-acid position. The goal of the present study was to determine if treating marmosets with Pro8-OT, relative to treatments with Leu8-OT, control saline, or an OT antagonist, had modulatory effects on the behavioral maintenance of long-term social relationships in marmosets. Treatment with the Pro8 variant, but not the Leu8 variant, of OT facilitated fidelity with a long-term partner by reducing time spent in close proximity with an opposite-sex stranger. However, this facilitative effect of Pro8-OT on proximity behavior manifested itself differently in male and female marmosets, such that females preferred to interact socially with their partner rather than a stranger when treated with Pro8-OT, while males spent less time in close proximity with both their partner and a stranger when treated with Pro8-OT. Furthermore, treatment with Pro8-OT, but not Leu8-OT, significantly delayed the expression of sexual solicitation behavior toward an opposite-sex stranger in both male and female marmosets, but had no effect on sociosexual behavior directed toward a long-term partner. These results suggest that the OT system is highly involved in reducing fidelity-threatening behaviors in well-established marmoset pairs, and that the effects were only produced by species-specific OT ligands.
Keywords: Oxytocin, Pro8-OT, pair-bond relationships, social behavior, partner-preference, fidelity
1. Introduction
Behavioral strategies that facilitate the maintenance of social bonds are critical for the preservation of high-quality social relationships between adult mates. The well-known functions of oxytocin (OT) in parturition (Blanks and Thornton, 2003) and lactation (Caruolo, 1971) have been recognized for decades; there is continuing interest in the neuromodulatory role of OT in social relationships (Insel et al., 2001). OT has been implicated in a suite of prosocial behaviors (MacDonald and MacDonald, 2010), particularly for its facilitative role in social bonding (Young and Wang, 2004). OT not only facilitates maternal bonding behaviors (Kendrick, 2007), but also promotes affiliative behavior and a partner-preference in adult sociosexual relationships (Lim and Young, 2006).
Monogamous social relationships are defined by a pervasive and reciprocal sociosexual preference for a long-term partner over an opposite-sex stranger, as well as high levels of sociosexual behavior between pair-mates (Hawkes, 2004). Accordingly, the differential behavioral response (i.e., prosocial, sexual, and/or aggressive) to an opposite-sex stranger is a good measure of attachment and fidelity to a pair-mate (Gubernick and Nordby, 1993). Fidelity to a long-term mate is threatened when one, or both, member(s) of a long-term pair spend time in close proximity, and engage in sociosexual behavior, with an opposite-sex stranger. OT has been shown to regulate fundamental mechanisms underlying mate fidelity. Human males in a monogamous relationship, but not single men, who received intranasal OT kept a greater distance on approach of an attractive woman (Scheele et al., 2012). Furthermore, central infusion of OT reduced the duration required to form a stable pair-bond in the socially monogamous prairie vole (Microtus ochrogaster; Williams et al., 1994). Blocking endogenous OT activity diminished a partner-preference and inhibited pair-bond formation in prairie voles (Cho et al., 1999). Thus, the OT system plays a critical role in the formation and maintenance of long-term monogamous relationships.
Marmosets (genus Callithrix) belong to a clade of New World primates (marmosets and tamarins) with a diverse set of socially monogamous mating strategies, including high levels of sociality with a pair-mate (Schaffner et al., 1995), avoidance of a potential mate in the presence of a pair-mate (Evans, 1983; Inglett et al., 1990), and aggressive responses to potential same-sex rivals (Ross et al., 2004). Like the socially monogamous prairie vole, marmosets form and maintain sociosexual relationships between males and females (Digby, 1995; Schaffner et al., 1995). However, unlike prairie voles, which display consistently strong sociosexual preferences for a long-term partner over an opposite-sex stranger (Wang and Aragona, 2004), marmosets display a flexible pattern of sociosexual preferences, similar to those displayed by humans (Buss and Schmitt, 1993), including a willingness to interact with a potential mate in the absence of a pair-mate (Evans, 1983; Inglett et al., 1990). For these reasons, the marmoset is an excellent translational primate model for the study of social-bond maintenance.
There is evidence from both correlational and experimental studies that OT plays a role in modulating social interactions in marmosets and tamarins. Urinary levels of OT co-vary with social behavior in male and female cotton-top tamarins (Saguinus oedipus), with variance in female OT levels associated with grooming behavior and mutual physical contact with a partner, and variance in male OT levels associated with sexual behavior (Snowdon et al., 2010). Thus, greater expression of affiliative and sexual behavior, which contributes to the quality of long-term social relationships, is associated with higher OT levels. In marmosets, paternal food sharing behavior was influenced by experimental treatment with an OT agonist. Marmoset fathers treated with OT displayed an increase in food sharing behavior with offspring (Saito and Nakamura, 2011). The development of sociosexual relationships in marmosets was also altered by pharmacological manipulation of the OT system in the first three weeks of cohabitation. Blocking endogenous OT activity in both males and females reduced measures of social proximity and food sharing with a new pair-mate (Smith et al., 2010). However, treatment with an OT agonist had a limited effect, and only subtly augmented marmoset sociality during the early period of cohabitation. To date, however, no studies have systematically manipulated the OT system in well-established pairs.
OT amino-acid sequences are highly conserved among placental mammals (Acher, 1980). However, two distinct variants of OT have been identified in some New World primates (including marmosets; Lee et al., 2011). The marmoset variant of the OT ligand (Pro8-OT) is structurally distinct from than the consensus mammalian variant of the OT ligand (Leu8-OT), due to a proline substitution at the 8th amino-acid position. This amino-acid substitution results in a substantially altered structural geometry of the OT ligand relative to the consensus mammalian variant; changes in structure that may affect OT-OT receptor (OTR) binding and downstream signaling. Therefore, the limited effect of the consensus mammalian OT agonist on marmoset sociality (Smith et al., 2010) may be a consequence of the distinct amino-acid structure of the OT ligand in marmosets.
The goal of the present study was to evaluate the impact of pharmacological manipulations of the OT system on patterns of sociosexual behavior and partner/stranger preferences in well-established marmoset pairs. Further, we tested OT ligand-specificity in modulating social behavior by administering both Pro8-OT and Leu8-OT. If OT promotes mate fidelity, then treatment with OT agonists should increase preferential associations with a partner over a stranger, and decrease sexual solicitation toward an opposite-sex stranger, while treatment with an OT antagonist (OTA) should decrease preferential associations with a partner, and increase sexual solicitation toward an opposite-sex stranger. Furthermore, if structural changes in the OT ligand alter OT signaling properties, then treating marmosets with Pro8-OT, but not Leu8-OT, should increase preferential associations with a partner and decrease sexual solicitation behavior directed toward an opposite-sex stranger. Thus, the goal of the study was to determine if treatment with Pro8-OT, relative to treatments with Leu8-OT, and an OTA, had modulatory effects on sociosexual behavioral responses to an opposite-sex stranger in the presence of a long-term partner in marmosets.
2. Method
2.1 Subjects
We tested six adult male and six adult female white-tufted ear marmosets (C. jacchus), housed at the Callitrichid Research Center (CRC) at the University of Nebraska – Omaha. Four unfamiliar marmosets of each sex were also required to test the subjects’ interactions with opposite-sex strangers in each of the four treatment periods. Animals were 3.2 ± 0.2 (mean ± SEM) years of age at the start of the study, and were kept in large indoor wire-mesh enclosures (1.0 × 2.5 × 2.0 m), equipped with a sleeping hammock, natural branches for climbing and various enrichment materials. Visual access was restricted between enclosures, but auditory and olfactory cues were not. Colony rooms at the CRC were maintained on a 12 h: 12h light: dark cycle and at a temperature range between 19 °C and 22 °C. For all dietary and husbandry protocols please refer to (Schaffner et al., 1995).
All males were surgically vasectomized a minimum of seven months prior to the initiation of the study. We vasectomized males to avoid pregnancy and the subsequent presence of young offspring, which could potentially complicate male-female relationships. All females received a 0.15 ml intra-muscular injection of cloprostenol (Estrumate®), a synthetic prostaglandin analogue, three days prior to each treatment period to synchronize females’ ovarian cycles throughout the duration of the experiment by inducing luteolysis (i.e., the structural and functional degradation of the corpus luteum; Summers et al., 1985). The University of Nebraska – Omaha/University of Nebraska Medical Center Institutional Animal Care and Use Committee evaluated and approved all procedures: Protocol #: 12-099-12-FC.
2.2 Partner-Preference Testing
After eight weeks of cohabitation, which has been shown to be a more than adequate duration of time to allow for significant establishment of a stable social bond in marmosets (Ågmo et al., 2012), male and female marmosets underwent partner-preference testing. Proximity behavior, as well as sociosexual, aggressive/territorial, and communicative behaviors were observed during the partner-preference test. Partner-preference testing took place 30 minutes post-treatment between the hours of 0900h–1030h. The treated marmoset was placed at one end of a T-shaped partner-preference apparatus, and was allowed to interact with either an opposite-sex stranger or long-term partner (through 2 × 2 cm wire mesh barrier), or no social preference, for 20 minutes. A different opposite-sex stranger was used for each treatment period. The long-term partner and opposite-sex stranger were placed on opposite ends of the T-shaped apparatus in a counterbalanced order, and visual contact between the partner and stranger was obscured via an opaque barrier. Social preference for either a long-term partner or an opposite-sex stranger was determined by measuring close proximity (within 30 cm), as well as sociosexual behavior (i.e., open-mouth displays). Open-mouth displays are expressed as an invitational behavior (i.e., proceptivity) and during copulation (i.e., receptivity; Kendrick and Dixson, 1983). Grooming and mating behaviors between the subject and partner/stranger were precluded due to the wire-mesh barrier. Observers were trained to achieve a level of proficiency (κ> 0.90) on scoring proximity, sociosexual, aggressive/territorial, and communicative behaviors. All focal animal observations were recorded using Stopwatch+ software (Emory University) by directly observing marmoset behavior during the partner-preference test. Marmosets were habituated to the partner-preference apparatus by allowing them to explore the apparatus without any social stimuli over a series of three 30-min sessions that took place in the two-week period prior to the first treatment period. This partner-preference testing procedure was repeated for each of the treatment periods over a period of five months (refer to Table 1 for experimental design).
Table 1.
Experimental Design
| Condition | Males | Females |
|---|---|---|
| Marmoset OT Agonist | Pro8-OT | – |
| Consensus OT Agonist | Leu8-OT | – |
| OT Antagonist | OTA | – |
| Placebo Control | Vehicle | – |
| Marmoset OT Agonist | – | Pro8-OT |
| Consensus OT Agonist | – | Leu8-OT |
| OT Antagonist | – | OTA |
| Placebo Control | – | Vehicle |
Note. Diagram identifying treatment conditions for males and females including: marmoset oxytocin variant agonist (Pro8-OT), consensus mammalian oxytocin variant agonist (Leu8-OT), oxytocin antagonist (OTA), placebo control (Vehicle), and untreated partners (−).
2.3 Drug Treatments
2.3.1 Intranasal administration of OT agonists
Marmosets were administered 25 IU (50μg/100μL saline solution) of an OT agonist or vehicle, which yielded a dose of 150 μg/kg. The dose was determined based on previous primate literature (Parker et al., 2005; Smith et al., 2010). Marmosets were administered either Pro8-OT (Anaspec Corp, California), Leu8-OT (synthesized by Maurice Manning, University of Toledo), or vehicle, 30 minutes prior to the partner-preference test, via intranasal administration during a brief (~3 min.) restraint. Intranasal administration was conducted using a 100-μL Eppendorf pipette to administer 50-μL of solution to each nostril drop-wise (30 sec between each nostril), and is a relatively well-tolerated, non-invasive method of administration.
Peptides administered intranasally are quickly absorbed into the bloodstream via the nasal passage, and appear to bypass the blood-brain barrier (BBB) to access the central nervous system (CNS) via the olfactory bulb and the maxillary branch of the trigeminal nerve (MacDonald and MacDonald, 2010; Bethlehem et al., 2013). The neuropeptides OT and arginine-vasopressin (AVP) are transported to the CNS and accumulate in the cerebrospinal fluid (CSF) in humans (Born et al., 2002; Striepens et al., 2013) and rhesus macaques (Chang et al., 2012) In rats and mice, OT levels were increased in microdialysates from the hippocampus and amygdala, and plasma, 30–60 minutes after intranasal administration (Neumann et al., 2013). Circulating levels of OT persisted for up to, but no more than 7-hr in humans (van IJzendoorn et al., 2012). These results suggest that intranasal administration rapidly upregulates OT levels in the brain and plasma during the timeframe of behavioral testing, and that OT clears the system several hours after testing.
2.3.2 Oral administration of OT antagonist
Marmosets were treated with 20 mg/kg OTA, or vehicle, 90 minutes prior to the partner-preference test, via oral administration in a preferred food treat. The OTA (L368, 899; provided by Dr. Peter Williams, Merck & Co., Inc.) is readily absorbed by the bloodstream after passage through the digestive system (Thompson et al., 1997), penetrates the CNS after peripheral administration, and accumulates in areas of the limbic system (Boccia et al., 2007).
Each member of the pair was treated individually during each treatment period in a counterbalanced order. Furthermore, the sequence of treatments among each individual was counterbalanced over the length of the study. Marmosets were treated every other day for ten days (Days 1, 3, 5, 7, 9). Social behavior between pair-mates was observed in their home environment on days 1, 3, 5, and 7 (data not shown). Partner-preference testing took place on Day 9. There was an 11–13 day washout period between each treatments period.
2.4 Data Analysis
To assess possible social preferences for interacting with a long-term partner, an opposite-sex stranger, or spending time alone, we calculated several derived variables. The proportion of time spent in proximity (average duration/visit) with a stranger, relative to the time spent in proximity to both the partner and stranger, was subtracted from the proportion of time spent in proximity (average duration/visit) with the partner, relative to the time spent in proximity to both the partner and the stranger [i.e., ((Partner)/(Partner + Stranger)) –((Stranger)/(Partner + Stranger))]. This measure yields a score from −1.0 to +1.0, where +1.0 indicates a strong social preference for a long-term partner. We used similar calculations to create variables for the preference to spend time in proximity with a partner vs. alone and for the preference to spend time in proximity with a stranger vs. alone. The effect of OT treatment on behavior exhibited during the partner-preference test was evaluated using several mixed-model ANOVAs, with OT treatment condition and sex as factors. If main effects or interactions were significant, post-hoc comparisons were made using Fisher’s least significant difference. All alpha levels were set at p < 0.05.
3. Results
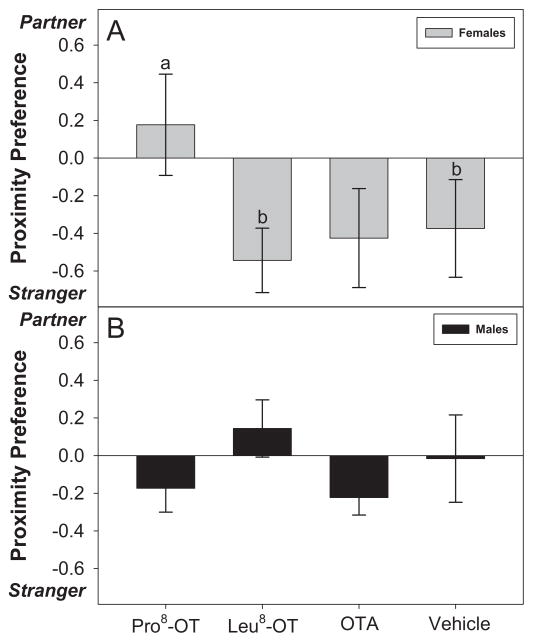
OT treatment influenced the proportion of time spent in close proximity to a long-term partner or an opposite-sex stranger differentially for male and female marmosets, as indicated by the significant interaction between OT treatment and sex [F(3, 30) = 3.13, p = 0.04, η2 = .24]. Treatment with Pro8-OT profoundly reduced female marmosets’ preference to spend time in close proximity with a stranger rather than their partner. Female marmosets treated with Pro8-OT spent proportionately less time in close proximity with an opposite-sex stranger vs. a long-term partner, relative to when they were treated with vehicle [t(5) = 2.74, p = .04] or Leu8-OT [t(5) = 4.36, p = .007] (Fig. 1A). OT treatment did not significantly alter proximity preferences for a partner vs. a stranger in male marmosets, as they spent roughly equivalent amounts of time with both an opposite-sex stranger and their long-term partner in all treatment conditions (Fig. 1B).
Figure 1. Preference: Partner vs. Stranger.
Preference scores (± SEM) for spending time in close proximity with a long-term partner or an opposite-sex stranger, expressed as a scale from −1 to +1. A positive score indicates a greater proportion of time (per bout) spent in close proximity with a partner vs. a stranger, while a negative score indicates a greater proportion of time (per bout) spent in close proximity with a stranger vs. a partner. Data are expressed as a function of OT treatment: marmoset oxytocin variant agonist (Pro8-OT), consensus mammalian oxytocin variant agonist (Leu8-OT), oxytocin antagonist (OTA: L368,899), and vehicle in (A) treated females and (B) males. Letters indicate significant differences (a > b at p <0.05).
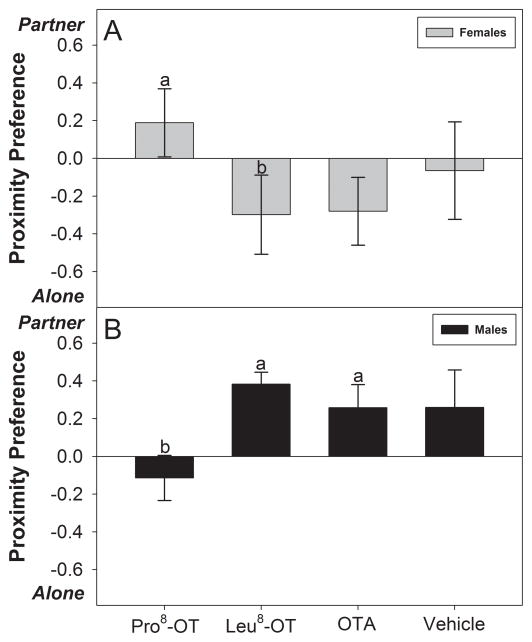
OT treatment also influenced the proportion of time spent in close proximity to a long-term partner or alone differentially for male and female marmosets, as indicated by the significant interaction between OT treatment and sex [F(3, 30) = 3.50, p = 0.027, η2 = .26]. Female marmosets treated with Pro8-OT spent proportionately less time alone vs. in close proximity with a partner, relative to when they were treated with Leu8-OT [t(5) = 4.10, p = .009], but not relative to treatments with vehicle or OTA [p > 0.05] (Fig. 2A). Interestingly, male marmosets treated with Pro8-OT spent proportionately less time in close proximity with their partner vs. alone, relative to when they were treated with Leu8-OT [t(5) = 4.65, p = 0.006] or OTA [t(5) = 4.19, p = 0.009], but not relative to vehicle [p > 0.05] (Fig. 2B).
Figure 2. Preference: Partner vs. Alone.
Preference scores (± SEM) for spending time in close proximity with a long-term partner or alone, expressed as a scale from −1 to +1. A positive score indicates a greater proportion of time (per bout) spent in close proximity with a partner vs. alone, while a negative score indicates a greater proportion of time (per bout) spent alone vs. with a partner. Data are expressed as a function of OT treatment (as indicated in Figure 1) in (A) treated females and (B) treated males. Letters indicate significant differences (a > b at p <0.05).
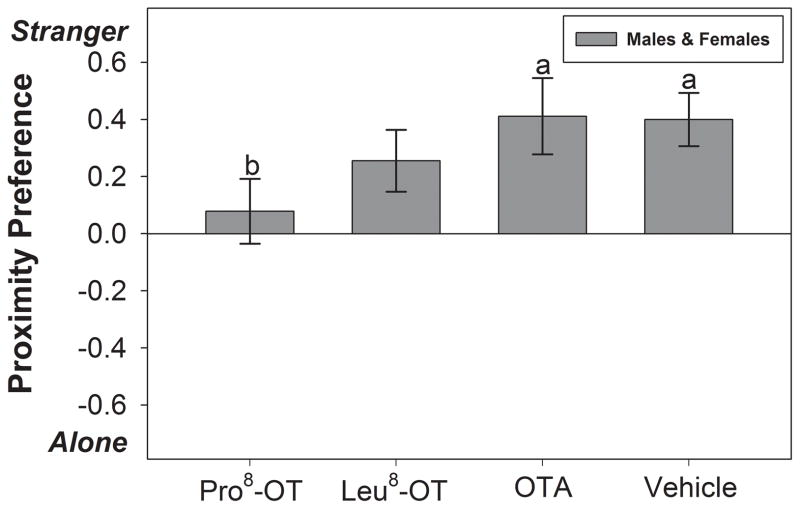
OT treatment also influenced the proportion of time spent in close proximity to an opposite-sex stranger or alone in marmosets, as indicated by the significant main effect of OT treatment [F(3, 30) = 2.99, p = 0.046, η2 = .23]. There was no significant effect of sex, or an interaction between OT treatment and sex, on the preference to spend time alone or with a stranger. Vehicle-treated marmosets spent a greater proportion of time in close proximity with a stranger vs. alone [t(11) = 4.29, p = 0.001]. Marmosets treated with Leu8-OT [t(11) = 2.36, p = 0.038] or OTA [t (11) = 3.08, p = .01] also spent a greater proportion of time in close proximity with a stranger vs. alone. However, marmosets treated with Pro8-OT spent proportionately less time in close proximity with an opposite-sex stranger vs. alone, relative to when they were treated with vehicle [t(11) = 2.59, p = 0.025] or OTA [t(11) = 3.54, p = 0.005] (Fig. 3). Overall, males engaged in significantly more locomotion (i.e., total frequency of proximity to partner/stranger/alone) than females, as indicated by the significant main effect of sex [F(1, 10) = 8.61, p = 0.015].
Figure 3. Preference: Stranger vs. Alone.
Preference scores (± SEM) for spending time in close proximity with an opposite-sex stranger or alone, expressed as a scale from −1 to +1. A positive score indicates a greater proportion of time (per bout) spent in close proximity with a stranger vs. alone, while a negative score indicates a greater proportion of time (per bout) spent alone vs. with a stranger. Data are expressed as a function of OT treatment (as indicated in Figure 1). Letters indicate significant differences (a > b at p <0.05).
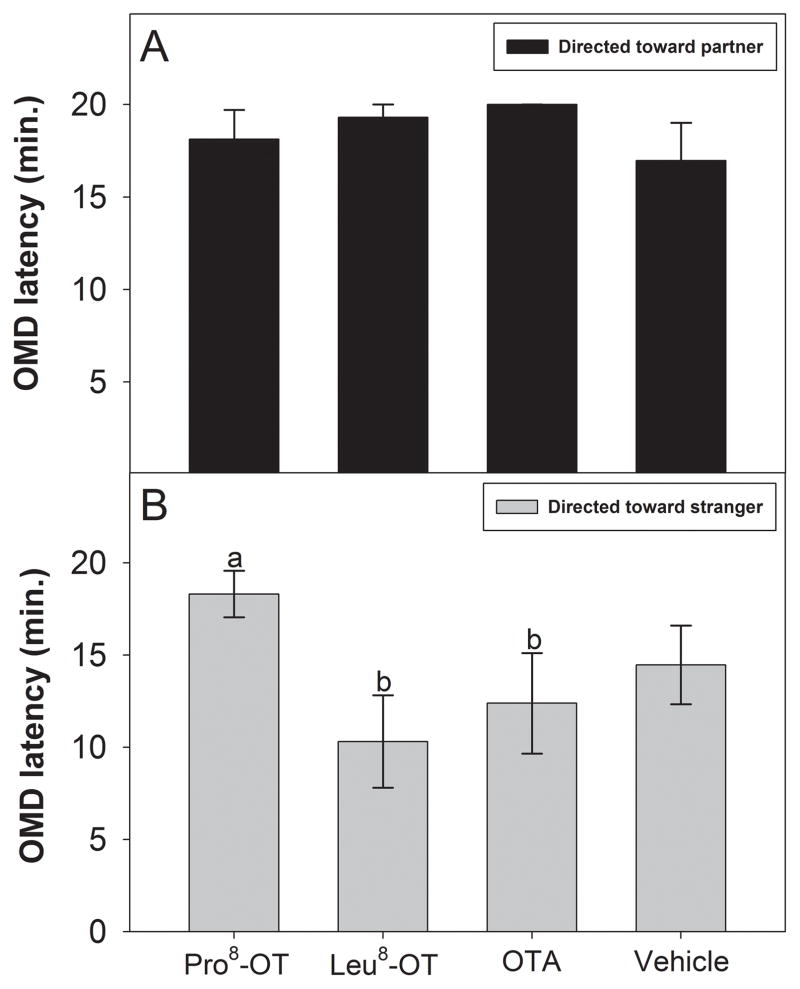
Marmosets engaged in sexual-solicitation behavior toward their partner or an opposite-sex stranger differentially due to OT treatment, as indicated by the main effect of treatment [F(3, 30) = 4.19, p = 0.014, η2 = .30]. There was no significant effect of sex, or an interaction between OT treatment and sex, on the expression sociosexual behavior. OT treatment did not have a significant effect on the latency to open-mouth display toward a long-term partner (Fig. 4A). However, OT manipulations markedly altered the latency to open-mouth display toward an opposite-sex stranger. Marmosets treated with Pro8-OT took significantly longer to open-mouth display toward an opposite-sex stranger, relative to when they were treated Leu8-OT [t(11) = 3.05, p = 0.011] or OTA, [t(11) = 2.21, p = 0.049] (Fig. 4B). Marmosets treated with Pro8-OT tended to take longer to open-mouth display to a stranger, relative to when they were treated with vehicle [t(11) = 1.89, p = 0.08]. Aggressive/territorial and communicative behaviors were not influenced by OT treatment or sex.
Figure 4. Sexual-Solicitation Behavior: Latency.
Latency (± SEM) to open-mouth display (OMD) at either (A) a long-term partner or (B) an opposite-sex stranger, expressed as a function of OT treatment (as indicated in Figure 1). Letters indicate significant differences (a > b at p <0.05).
4. Discussion
Central oxytocin activity appears to modulate behavioral strategies that facilitate the preservation of long-term social relationships by reducing the expression of sociosexual behavior toward opposite-sex strangers. Treatment with the Pro8 variant, but not the consensus mammalian variant, of OT facilitated fidelity with a long-term partner by decreasing time spent in close proximity with, and increasing the latency to exhibit sexual-solicitation behavior toward, a stranger. However, this facilitative effect of OT on fidelity manifested itself differently in male and female marmosets, such that females preferred to interact socially with their long-term partner rather than an opposite-sex stranger when treated with Pro8-OT, while males spent less time in close proximity with both their partner and a stranger when treated with Pro8-OT. Accordingly, increased levels of centrally available species-specific OT agonists significantly reduced fidelity-threatening behaviors in marmosets by enhancing a preferential association with a partner and simultaneously decreasing associations with a stranger in females, but not males.
Preference for a partner in marmosets and tamarins differs from the prairie vole model (Wang and Aragona, 2004) in the sense that it is labile, both within and among species, and tends to be strongly influenced by social context. Male saddle-back tamarins (Saguinus fuscicocollis) typically interact with a partner more than a stranger, while females display no preference (Epple, 1990). Male and female and golden-lion tamarins (Leontopithecus rosalia) typically interact with a stranger more than a partner under conditions when the partner’s visual access to the stranger is blocked or the partner is absent, but not when the partner has visual access to the stranger (Inglett et al., 1990). Male, but not female, marmosets (C. jacchus) typically display sexual solicitation behavior to a stranger in the absence of their mate. Furthermore, in the presence of their long-term partner, both male and female marmosets reduced the expression of sexual behavior and engaged in more aggressive behavior toward an unfamiliar conspecific (Evans, 1983). Male and female marmosets (C. penicillata) interacted more with a stranger than a newly paired partner after 24 hours of cohabitation. However, after three weeks of cohabitation, male and female marmosets interacted equally with both a partner and a stranger (Smith et al., 2010). The results of these studies suggest that social context plays a crucial role in partner/stranger preferences in marmosets and tamarins, and that length of cohabitation strongly influences partner/stranger preferences by reducing interactions with an opposite-sex stranger.
The current study showed that in well-established marmoset pairs, females spent proportionately more time in close proximity with a stranger over their partner under control conditions. This pattern is consistent with the finding that when an individual’s partner is present, but visual access between the partner and stranger is occluded, marmosets will interact with an opposite-sex stranger, as noted above. However, female marmosets treated with the Pro8 variant, but not the consensus mammalian variant, of OT had a reduced preference to interact with an unfamiliar potential mate. This suggests that one effect of increased OT may be to make it less likely for individuals in long-term social relationships to form social bonds with opposite-sex strangers.
Female marmosets treated with Pro8-OT spent proportionately less time in close proximity with a stranger. This finding could be interpreted in two subtly distinct ways related to social recognition. Social recognition (i.e., ability to differentiate a familiar individual from a stranger, and remember a previously encountered conspecific) is an essential skill for an individual living within a social group (Ross and Young, 2009). OT is highly involved in the regulation of social recognition, by facilitating social memory and selective attention to socially-relevant information through action in the medial amygdala (Petrovic et al., 2008; Samuelsen and Meredith, 2011). Furthermore, social recognition and familiarity strongly influence partner/stranger preferences (Cheetham et al., 2008). Thus, OT may either be modulating (1) the specific social distinction between a long-term partner and an opposite-sex stranger, or (2) the less sophisticated social distinction between a familiar “in-group” member and an unfamiliar “out-group” member. Thus, our finding that Pro8-OT reduced female marmosets’ preference for spending time in close proximity with a stranger may be interpreted as a shift away from a socially unfamiliar stranger and toward a socially familiar partner.
In addition to the facilitative effect of Pro8-OT on a partner-preference, female marmosets also displayed altered preferences to either spend time alone or interact socially with either their partner or a stranger under OT treatment. Female marmosets treated with Pro8-OT increased preferential associations with their partner over spending time alone and simultaneously decreased their preference for a stranger over spending time alone. This behavioral pattern is consistent with findings in prairie voles that OT increases preferential associations with a pair-mate (Williams et al., 1994; Cho et al., 1999). Thus, treatment with the Pro8 variant, but not the consensus mammalian variant, of OT facilitated a behavioral strategy to increase preferential associations with a partner over a stranger, and to reduce time spent in close proximity with a stranger in favor of spending more time alone.
Interestingly, males spent an equal amount of time in close proximity with their partner and a stranger in all treatment conditions, which is in contrast to the reduction in stranger-preference under Pro8-OT treatment in females. This sex difference suggests that the OT system in males may be differentially insensitive to an OT intervention in the context of modulating social distance between a partner and a stranger. In prairie voles, intracerebroventricular injections of OT induced a partner-preference in both males and females (Cho et al., 1999), whereas subcutaneous injections of OT only induced a partner-preferences in females (Cushing & Carter, 2000), suggesting that route of administration may have an effect on the differential behavioral response between males and females. Despite the similar phylogeny and socially monogamous mating structure of marmosets and humans, each appears to have different behavioral responses to OT interventions. In humans, males treated with OT perceived their pair-mates face as more attractive than the face of an opposite-sex stranger, and experienced increased neural activity in the branches of the brain’s reward system (i.e., nucleus accumbens, ventral tegmental area; Scheele et al., 2013). Unlike the positive effect of OT on perceived partner attractiveness in human males, OT appears to decrease the attractiveness of opposite-sex strangers in female marmosets, but does not appear to have a strong effect on male marmosets’ perceptions of their pair-mate or an opposite-sex stranger. Alternatively, male and female marmosets may have different behavioral responses to their partner and a stranger during OT interventions due to sex-specific consequences of extra-pair sexual encounters (i.e., increased potential for offspring vs. potential deleterious effects on current social relationship), or levels of anxiety (male marmosets exhibited greater levels of locomotion during partner-preference testing).
Treatment with Pro8-OT did not increase male marmosets’ preference for their partner over a stranger. The limited effect of OT treatment on partner/stranger preferences in male marmosets may indicate that the OT system simply influences male sociosexual behavior less so than female behavior. This may be explained by the potential influence of the related neuropeptide, AVP, on partner/stranger preferences in males. In addition to the critical role of AVP in partner-preference formation (Winslow et al., 1993), it is also highly involved in other male-typic behavior, including aggression and courtship (Goodson and Bass, 2001). Numerous studies in prairie voles have implicated the OT system as potentially more important for sociosexual bond development in females, and the AVP system as more important for sociosexual bond development in males (Young and Wang, 2004). More likely, both the OT system and the AVP system are highly involved in modulating partner/stranger preferences and the regulation of sociosexual behavior between pair-mates in both males and females. In the current study both male and female sexual solicitation behavior was strongly influenced by OT treatment, but OT only affected female’s partner/stranger preferences. Thus, one potential avenue for future research is to examine these context-specific behavioral responses during a social preference paradigm under AVP treatment conditions.
Multiple neuroendocrine systems may be interacting to modulate sociosexual preferences, which may help explain the sex-specific behavioral responses to an opposite-sex stranger in marmosets. The OT system may be interacting with the hypothalamic-pituitary-adrenal (HPA) axis to modify sex-specific behavior responses. Novel environments and distinct social contexts generate anxiety-like behavior and concurrent activation of the HPA axis in marmosets (Smith and French, 1997), and since OT is a well-known anxiolytic agent (Smith and Wang, 2012), an alternative interpretation of the differential behavioral responses during the partner-preference test is that treatment with Pro8-OT may be acting on the HPA axis to modify anxiety-like responses, as opposed to selectively acting on social neural circuits. Although, the results of the current study do not fall in line with the hypothesis that OT is anxiolytic. Instead, treatment with Pro8-OT reduced a preference for interacting with a potentially anxiety-inducing stimulus (i.e., opposite-sex stranger) in favor of a socially familiar partner in female marmosets. Thus, future investigations may find it prudent to use anxiolytic agents as controls to determine if OT manipulations are acting specifically on social neural circuits or on more generalized anxiety circuits (Churchland and Winkielman, 2012).
Additionally, the steroid/peptide theory of social bonds (van Anders et al., 2011) provides a series of predictions for the integration of the OT system and the hypothalamic-pituitary-gonadal (HPG) axis, which is known to play a role in the formation of sociosexual relationships (Burnham et al., 2003; van Anders and Goldey, 2010). To the extent that high levels of testosterone are associated with sociosexual competition, and low levels of testosterone are associated with nurturing physical contact with a partner (van Anders et al., 2011), we might expect OT responses to be antagonistic with testosterone responses in some behavioral contexts and facilitative in other contexts. Marmosets (particularly males) treated with OT that experience increases in testosterone, when exposed to an opposite-sex stranger, might be receiving two opposing biological signals that influence their sociosexual preferences. Thus, an examination of the interaction between the OT system with the HPA and HPG axes may provide valuable insight into sex-specific behavioral responses to an opposite-sex stranger in well-established social relationships.
Not only did Pro8-OT modulate the duration of time spent in proximity with a partner or a stranger, but it also altered sociosexual interactions with a stranger. Vehicle-treated marmosets engaged in sexual-solicitation behavior significantly more quickly toward a stranger than their partner. While treating marmosets with the consensus mammalian variant of OT did not alter these patterns, treating marmosets with Pro8-OT significantly increased the latency to engage in proceptive sexual displays toward a stranger. In at least one wild population of marmosets, extra-pair sexual behavior has been reported during intergroup interactions (Digby, 1999), potentially as a way to avoid inbreeding or as a way females migrate to another group (Bicca-Marques, 2003); yet there is no evidence to suggest that these extra-pair encounters diminish the quality of the bond with their long-term partner (Digby, 1999). Since infidelity appears to occur under normative conditions, one mechanism that may lead to the persistence of the long-term bond between pair-mates is via endogenous OT release upon reunion with a pair-mate. In chimpanzees, excreted OT levels were higher following grooming bouts with a bonded partner (regardless of genetic relatedness) than a non-bonded partner (Crockford et al., 2013). Thus, socio-physical interactions with a pair-mate following extra-pair encounters may facilitate the preservation of long-term bonds via OT system activation.
In the current study, partner-directed sexual behavior with a long-term partner was virtually absent; yet, stranger-directed sexual behavior occurred relatively quickly under control conditions. It is quite interesting that treatment with OT did not increase partner-directed sexual behavior, as the OT system has been implicated in female sexual receptivity (Cushing and Carter, 2000), as well as sexual arousal and orgasm (Behnia et al., 2014) during interactions between pair-mates. There did appear to be a ceiling effect in sexual solicitation latency toward the partner, suggesting that, relative to vehicle, it would be nearly impossible to observe an increase in latency to open-mouth display under OTA treatment. The results of the current study suggest that treatment with Pro8-OT reduced the likelihood of engaging in a sexual encounter by reducing stranger-directed sexual-solicitation behavior. Consequently, one effect of increased central OT activity is the pronounced reduction of fidelity-threatening behaviors, by reducing both the time spent in close proximity with a stranger and reducing sexual solicitations toward a stranger.
Pro8-OT had clear modulatory effects on social preferences for a partner vs. a stranger. However, treatment with an OTA did not significantly alter partner-stranger preferences. This finding is surprising because treatment with an OTA has been shown previously to reduce proximity, huddling, and food sharing behavior with a recently formed partner in marmosets. Furthermore treatment with an OTA reduced the frequency, and increased the latency, to establish contact with a partner before engaging with a stranger, after three weeks of cohabitation and continuous treatment (Smith et al., 2010). One possible explanation for the limited influence of an OTA in the current study is that endogenous OT release may not have been sufficiently stimulated due to the lack of socio-physical interactions with the partner or stranger. Another more likely explanation for the limited influence of an OTA is that well-established social relationships are less sensitive to OTA interventions, relative to the more dynamic sociosexual preferences of newly formed social bonds. Alternatively, acute treatment of an OTA in the current study may not have been sufficient to elicit the same reductions in sociality seen previously and modulate partner/stranger preferences in well-established social relationships.
5. Concluding Remarks
Female marmosets treated with the Pro8 variant of OT spent significantly more time away from a stranger, than vehicle-treated females. This suggests that in the presence of a pair-mate, female marmosets find novel social stimuli less attractive when treated with centrally available OT, and therefore display a reduced preference for a stranger. Treatment with the Pro8 variant, but not the consensus mammalian variant, of OT led to a decrease in more salient behaviors, such as sexual-solicitation behavior toward a stranger, in both males and females. Therefore, there seems to be a distinct sexual dichotomy in the effect of the OT system on the expression of sociosexual behavior in marmosets, which suggests there may be some underlying differences in physiology of the social and/or anxiety neural circuits.
This was the first study to determine if treatment with the Pro8 variant of OT has a functionally significant effect on fidelity and sociosexual preferences. We were able to elucidate critical underlying neuroendocrine mechanisms that influence maintenance of social relationships. Treatment with the Pro8 variant, but not the consensus mammalian variant, of OT reduced time spent in close proximity, and the expression of sociosexual behavior, with an opposite-sex stranger. Thus, treatment with centrally available OT significantly reduced fidelity-threatening behaviors in well-established adult sociosexual relationships as a means to maintain a vital social bond with a long-term pair-mate.
Acknowledgments
Role of the funding source
This work was supported in part by funds from the National Institutes of Health (HD 042882) awarded to Jeffrey A. French, and by funds from University of Nebraska – Omaha’s Graduate Research and Creative Activity Committee awarded to Jon Cavanaugh.
We would like to thank Rose Strasser and Alan Kolok for their exceptional recommendations on the development of the project as well as their thorough and thoughtful comments on previous drafts of the manuscript. Thanks also to April Harnisch, Rachel Stein, Victoria Wageman, and Michelle Huffman who assisted in the data collection process. We also give thanks to Heather Jensen and Liz Gunkelman for providing excellent care of the marmoset colony. We would also like to thank Maurice Manning (University of Toledo) and Peter Williams (Merck & Co., Inc.) for their professional courtesies offering material support. All procedures were approved by the University of Nebraska – Omaha/ Medical Center Institutional Animal Care and Use Committee and adhered to all local, state, and national laws regulating research on nonhuman primates.
Footnotes
Conflict of interest
In submitting this manuscript, all authors declare no conflict of interest, monetary or otherwise.
Contributors
Jon Cavanaugh designed the study, wrote the protocol, collected the data, conducted the statistical analysis, and wrote the first draft of the manuscript. Aaryn Mustoe assisted in data collection and provided comments/edits on previous drafts of the manuscript. Jack Taylor assisted in data collection and provided comments/edits on previous drafts of the manuscript. Jeff French provided comments/edits on previous drafts of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acher R. Molecular evolution of biologically active polypeptides. Proc R Soc Lond B Biol Sci. 1980;210:21–43. doi: 10.1098/rspb.1980.0116. [DOI] [PubMed] [Google Scholar]
- Ågmo A, Smith AS, Birnie AK, French JA. Behavioral characteristics of pair bonding in the black tufted-ear marmoset (Callithrix penicillata) Behaviour. 2012;149:407–440. doi: 10.1163/156853912X638454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia B, Heinrichs M, Bergmann W, Jung S, Germann J, Schedlowski M, Hartmann U, Kruger THC. Differential effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Horm Behav. 2014;65:308–318. doi: 10.1016/j.yhbeh.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Bicca-Marques JC. Sexual selection and foraging behavior in male and female tamarins and marmosets. In: Jones CB, editor. Sexual Selection and Reproductive Competition in Primates: New Perspectives and Directions. American Society of Primatologists; Norman: 2003. pp. 455–475. [Google Scholar]
- Blanks A, Thornton S. The role of oxytocin in parturition. BJOG Int J Obstet Gynaecol. 2003;110:46–51. doi: 10.1016/S1470-0328(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Goursaud A-PS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899®, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Horm Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Burnham T, Chapman JF, Gray P, McIntyre M, Lipson S, Ellison P. Men in committed, romantic relationships have lower testosterone. Horm Behav. 2003;44:119–122. doi: 10.1016/S0018-506X(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Buss DM, Schmitt DP. Sexual strategies theory: An evolutionary perspective on human mating. Psychol Rev. 1993;100:204–232. doi: 10.1037/0033-295x.100.2.204. [DOI] [PubMed] [Google Scholar]
- Caruolo EV. Exogenous oxytocin and lactation in the mouse. J Dairy Sci. 1971;54:1207–1211. doi: 10.3168/jds.S0022-0302(71)86001-0. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham SA, Thorn MD, Beynon RJ, Hurst JL. The effect of familiarity on mate choice. Chem Signals Vertebr. 2008;11:271–280. [Google Scholar]
- Cho MM, De Vries C, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preference in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbuhler K, Deschner T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc R Soc B Biol Sci. 2013;280:20122765–20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Digby LJ. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995;36:361–375. [Google Scholar]
- Digby LJ. Sexual behavior and extragroup copulations in a wild population of common marmosets (Callithrix jacchus) Folia Primatol (Basel) 1999;70:136–145. doi: 10.1159/000021686. [DOI] [PubMed] [Google Scholar]
- Epple G. Sex differences in partner preference in mated pairs of saddle-back tamarins (Saguinus fuscicollis) Behav Ecol Sociobiol. 1990;27:455–459. [Google Scholar]
- Evans S. The pair-bond of the common marmoset, Callithrix jacchus jacchus: An experimental investigation. Anim Behav. 1983;31:651–658. [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Gubernick Nordby JC. Mechanisms of sexual fidelity in the monogamous California mouse, Peromyscus californicus. Behav Ecol Sociobiol. 1993;32:211–219. [Google Scholar]
- Hawkes K. Mating, parenting, and the evolution of human pair bonds. In: Chapais B, Berman C, editors. Kinship and Behavior in Primates. Oxford University Press; Oxford: 2004. [Google Scholar]
- Inglett BJ, French JA, Dethlefs TM. Patterns of social preference across different contexts in golden lion tamarins (Leontopithecus rosalia) J Comp Psychol. 1990;104:131–139. doi: 10.1037/0735-7036.104.2.131. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ, et al. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–135. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Oxytocin, motherhood and bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Dixson AF. The effect of the ovarian cycle on the sexual behavior of the common marmoset (Callithrix jacchus) Physiol Behav. 1983;30:735–742. doi: 10.1016/0031-9384(83)90171-3. [DOI] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC, Neal DE, Buckmaster CL, Cheng MY, Hyde SA, Lyons DM, Parker KJ. A novel form of oxytocin in New World monkeys. Biol Lett. 2011;7:584–587. doi: 10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH., II Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151:66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Saito A, Nakamura K. Oxytocin changes primate paternal tolerance to offspring in food transfer. J Comp Physiol A. 2011;197:329–337. doi: 10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience. 2011;180:96–104. doi: 10.1016/j.neuroscience.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied’s black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Gunturkun O, Deutschlander S, Maier W, Kendrick KM, Hurlemann R. Oxytocin modulates social distance between males and females. J Neurosci. 2012;32:16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, French JA. Social and reproductive conditions modulate urinary cortisol excretion in black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1997;42:253–267. doi: 10.1002/(SICI)1098-2345(1997)42:4<253::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm Behav. 2010;58:614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3 doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers PM, Wennink CJ, Hodges JK. Cloprostenal-induced luteolysis in the marmoset monkey (Callithrix jacchus) J Reprod Fertil. 1985;73:133–138. doi: 10.1530/jrf.0.0730133. [DOI] [PubMed] [Google Scholar]
- Thompson KL, Vincent SH, Miller RR, Colletti AE, Alvaro RF, Wallace MA, Feeney WP, Chiu SHL. Pharmacokinetics and disposition of the oxytocin receptor antagonist L-368,899 in rats and dogs. Drug Metab Dispos. 1997;25:1113–1118. [PubMed] [Google Scholar]
- Van Anders SM, Goldey KL. Testosterone and partnering are linked via relationship status for women and “relationship orientation” for men. Horm Behav. 2010;58:820–826. doi: 10.1016/j.yhbeh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Van Anders SM, Goldey KL, Kuo PX. The Steroid/Peptide Theory of Social Bonds: Integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36:1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bhandari R, van der Veen R, Grewen KM, Bakermans-Kranenburg MJ. Elevated salivary levels of oxytocin persist more than 7 h after intranasal administration. Front Neurosci. 2012;6 doi: 10.3389/fnins.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. Ilar J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]