Abstract
Nonmelanoma skin cancers (NMSCs), are among the most common human malignancies. Current methods for their prevention include avoidance of natural and artificial sources of UV radiation, photoprotective clothing and sunscreens. However, these methods have proven to be inadequate in stemming the rise in skin cancer incidence over the past several years. There is accumulating evidence that cyclooxygenase-2 (COX-2), an enzyme involved in prostaglandin synthesis, may be involved in the pathogenesis of NMSC. In preclinical studies, animals genetically deficient in the COX-2 enzyme or that have been treated with pharmacological inhibitors of COX-2 develop significantly fewer tumors when subjected to a UV-induced skin carcinogenesis protocol than control mice. Several epidemiological studies in humans support the concept that this enzyme is intimately involved in UV-induced skin cancer development, and UV radiation is known to augment COX-2 expression in human skin. Recent studies suggest that drugs that block COX-2 expression may prevent the development of NMSCs. Thus, pharmacologic agents that inhibit the enzyme cyclooxygenase-2 may be effective chemopreventive agents for NMSCs.
Basal cell and squamous cell carcinomas, grouped together under the term nonmelanoma skin cancer (NMSC), are a major dermatologic problem. In the United States alone, over 3.5 million new cases of this malignancy are diagnosed each year (Rogers et al., 2010). This far exceeds the 1.66 million cases of cancer in all other organs combined (Siegel et al., 2013). In contrast to most other malignancies in which the incidence has either stabilized or begun to decline, the likelihood of developing a NMSC continues to grow (Rogers et al., 2010). Moreover, NMSCs are developing in younger and younger age groups; it is not uncommon to see women in their 20s and 30s developing their first NMSC (Christenson et al., 2005). The epidemic of skin cancer represents a major public health issue and is a tremendous cost to healthcare systems in the United States and around the world (Rogers and Coldiron, 2013).
Because of the prevalence of the problem, there has been great interest in developing methods by which skin cancers can be prevented. The vast majority of skin cancers are caused by overexposure to ultraviolet radiation from the sun and artificial light sources. Thus, much of the effort to prevent skin cancer has centered on avoidance of excessive sun exposure, education about the deleterious effects of artificial tanning bed use, advice that outdoor activities should be conducted as much as possible in shaded areas, and recommendations that protective hats and long-sleeved clothing should be worn outside. But the mainstay of skin cancer prevention has focused on advising people to apply sunscreens regularly. While not to deny the importance of these topical agents, the few studies that have been conducted evaluating their efficacy for skin cancer prevention have shown only a modest reduction in actinic keratoses (AKs) (Thompson et al., 1993) and squamous cell carcinomas (SCCs) of the skin (Green et al., 1999) and no statistically significant reduction in the incidence of basal cell carcinomas (BCCs) (Green et al., 1999). In addition, there is inconsistent patient compliance with sunscreen use, even in organ transplant recipients who are at greatest risk for UV-induced NMSCs (Seukeran et al., 1998). Furthermore, large amounts of sunscreen are required to achieve the full sunburn protective factor (SPF) value on the product label, and patients only use about 25% of that amount when applying sunscreens (Faurschou and Wulf, 2007). Finally, there is no effect of sunscreens on prior UV damage to the skin. Thus, existing methods are inadequate and additional measures are required to retard the rising incidence of NMSC. Identification and implementation of chemopreventive agents against skin cancer represent one of the major unmet needs in photodermatology.
Cyclooxygenases and Chemoprevention
There is strong evidence from experiments in animal models and epidemiologic studies that cyclooxygenases are intimately involved in the promotion and progression stages of NMSCs, and therefore, may be excellent targets for the prevention of NMSCs (Rundhaug and Fischer, 2008). There are two major cyclooxygenase isoforms, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 is constitutively expressed in most cell types. COX-2 is not normally expressed in most tissues, but can be induced to do so by a variety of stimuli including growth factors, cytokines, and tumor promoters (Rundhaug and Fischer, 2008). Ultraviolet radiation is a known stimulus for COX-2 expression in the epidermis (see Figure) (An et al., 2002; Buckman et al., 1998; Fischer et al., 1999; Rodriguez-Burford et al., 2005). Cyclooxygenases are prostaglandin-endoperoxide synthases that catalyze the formation of prostaglandins from arachidonic acid (Brecher, 2002). UV-induced COX-2 expression increases PGE2, one of the major cyclooxygenase products implicated in NMSC development. PGE2 binds to four G-protein coupled receptors, EP1 - EP4, on the surface of cells, including keratinocytes (Rundhaug et al., 2011). Each receptor activates distinct signaling pathways, although there is extensive crosstalk between the pathways. EP1, EP2, and EP4 have all been linked to UV induced skin carcinogenesis in animal models. PGE2 has been shown to increase tumor cell proliferation, inhibit apoptosis, stimulate an inflammatory response, promote immunosuppression, and facilitate tumor invasion. All of these functional activities of PGE2 are important contributors to the development of UV-induced SCCs of the skin.
Figure.
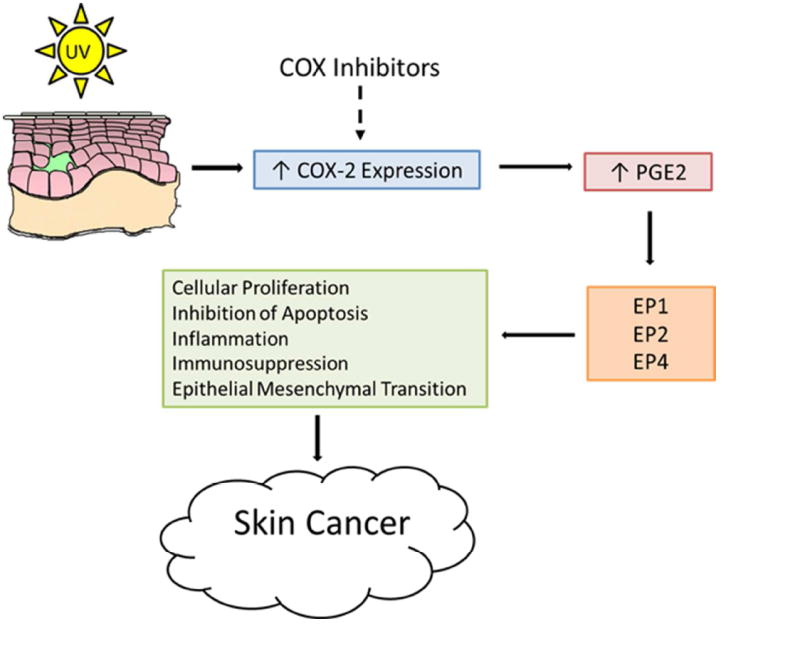
UV and Cyclooxygenases
Nonsteroidal anti-inflammatory drugs (NSAIDs) are medications that are widely used in clinical practice for the treatment of rheumatoid arthritis and osteoarthritis. These agents act by inhibiting the action of the COX-1 and COX-2 enzymes and thus impair production of PGE2. NSAIDs have been employed to investigate the role of cyclooxygenases in disease (Fischer et al., 2011; Ulrich et al., 2006). Examples of FDA approved agents that non-selectively inhibit both COX-1 and COX-2 include sulindac, naproxen, and indomethacin. Celecoxib, on the other hand, has a much greater effect on COX-2 than on COX-1 (Kawamori et al., 1998). When used on a chronic basis, COX-2 selective inhibitors have been associated with adverse cardiovascular events including heart attack and stroke (Kerr et al., 2007; Solomon et al., 2005; Solomon et al., 2008). Cardiovascular adverse events are also more common with some, but not all, non-selective NSAIDs that block both COX-1 and COX-2; naproxen may even have a slight protective effect (Fosbol et al., 2009; Ray et al., 2002). Other toxicities of NSAIDs include nausea, gastrointestinal pain and hemorrhage (Derry and Loke, 2000). Preclinical data has shown that nitric oxide (NO) releasing NSAIDs such as NO-naproxen and NO-sulindac have much less gastrointestinal toxicity compared to their non-NO releasing counterparts, i.e. naproxen and sulindac (Blackler et al., 2012; Steele et al., 2009). Furthermore, NO-releasing NSAIDs also augment the expression of antioxidant response element genes which may further augment their chemopreventive activity.
Animal Models
Convincing evidence to support the concept that cyclooxygenases play an essential role in UV-induced skin carcinogenesis has been obtained from experiments in animal models. In UV-induced skin tumorigenesis experiments in which wild type mice were compared to animals with a heterozygous mutation in either the COX-1 or COX-2 gene, COX-2 deficient mice had a significant reduction in SCCs compared to wild type mice, whereas those mice with a deficiency in COX-1 were unaffected by the deficiency and behaved exactly like wild type mice (Fischer et al., 2007). In contrast, both COX-1 and COX-2 appear to participate in the development of BCCs. Ptch+/- mice are known to develop large numbers of BCCs following exposure to ultraviolet radiation (Tang et al., 2010). When mutations in the COX-1 and COX-2 genes were backcrossed onto this strain and those mice were chronically exposed to UV-irradiation, both the COX-1 and COX-2 deficient mice, developed significantly fewer BCCs than Ptch+/- mice without cyclooxygenase deficiencies. The conclusion from these studies was that COX-2, but not COX-1, is important for UV-induced SCCs, whereas both COX-1 and COX-2 contribute to BCC development. Thus, cyclooxygenase participation differs depending on the type of malignancy. In other studies, it has been shown that COX-1 diminishes apoptosis in UV-induced squamous cell carcinomas, but does not inhibit tumor cell proliferation or tumor development (Pentland et al., 2004). Although it has not yet been investigated, this may be different in animal models of UV-induced basal cell carcinoma.
Experiments have also been conducted in animal models to determine whether selective COX-2 inhibitors and nonselective COX-1 and COX-2 inhibitors might be effective chemopreventive agents for UV induced NMSCs (Fischer et al., 1999; Pentland et al., 1999; Rundhaug et al., 2007; Tang et al., 2010). Those studies have shown that the COX-2 inhibitor celecoxib will block UV-induced SCC development in mice. The nonselective COX-1 and COX-2 inhibitors, naproxen, indomethacin, sulindac and the nitric oxide releasing derivative, NO-sulindac, have also been observed to dramatically reduce the number of UV-induced skin tumors (Athar, Unpublished; Chaudhary et al., 2013; Mikulec et al., 2013)
Over the past several years, a number of natural and dietary agents have been identified that are potent chemopreventive agents for UV-induced skin cancers. Many of these natural and dietary compounds contain polyphenols that have a variety of different activities. Recent studies have shown that some of these, such as grape seed proanthocyanidins, inhibit the expression of cyclooxygenase-2, and this effect is associated with a reduction in the number of UV-induced skin tumors in mice (Sharma and Katiyar, 2010).
Mechanistic Studies
The mechanism by which cyclooxygenases foster the development of UV induced skin cancers has been investigated in detail, primarily by evaluating the parameters that are affected by pharmacologic inhibition of these enzymes.
It is known that PGE2 stimulates the proliferation of malignant and premalignant keratinocytes (Ansari et al. 2008; Rundhaug et al. 2007). NSAIDs block this effect and also promote apoptosis. Consistent with this observation, sulindac is effective at attenuating expression of several markers of proliferation including c-fos, cyclins D1 and A and PCNA (Athar et al., 2004). Similarly, the reduction in UV-induced tumor formation with NO-sulindac is associated with an increase in the number of TUNEL-positive cells, increased expression of pro-apoptotic Bax and decreased expression of anti-apoptotic Bcl-2 (Chaudhary et al., 2013). In UV-irradiated skin, there is an increase in the phosphorylation of the MAP kinases, Erk1/2, p38 and JNK1/2, which are upstream signaling molecules of cellular proliferation and inflammation. NO-sulindac blocks this activity (Chaudhary et al., 2013).
COX-2 augments epithelial mesenchymal transition, the process by which malignant cells weaken intercellular adhesion and enhance motility, thus allowing them to penetrate into surrounding tissues (Lee et al. 2008). NO-sulindac inhibits epithelial mesenchymal transition to block the progression of UVB-induced tumors by decreasing the expression of mesenchymal markers fibronectin, N-cadherin, Snail, Slug and Twist and by increasing the epithelial cell polarity marker E-cadherin (Chaudhary et al., 2013).
In addition to promoting the proliferation of pre-neoplastic cells and facilitating epithelial mesenchymal transition, UV-induced PGE2 production stimulates inflammation (Wilgus et al., 2000), one consequence of which is to promote UV-induced skin tumorigenesis (Wilgus et al., 2003). Topical application of celecoxib or the EP1 specific inhibitor ONO-87713 blocks both UV-induced inflammation and tumor development (Tober et al., 2006; Wilgus et al., 2003).
In contrast to the non-specific inflammatory response which promotes UV-induced skin tumorigenesis, there is an effective cell-mediated anti-tumor immune response that inhibits UV-induced tumor development (Kripke, 1974). UV radiation suppresses that response (Gibbs and Norval, 2013; Krutmann et al., 2009; Schwarz, 2008). The nonselective COX-1 and COX-2 inhibitor, indomethacin, abrogates the immunosuppressive effects of UV radiation (Chung et al., 1986; Soontrapa et al., 2011). DNA hypermethylation has recently been shown to be a mediator of UVB induced immune suppression and skin tumorigenesis (Prasad and Katiyar, 2013). The effects of UV radiation on DNA hypermethylation can be reversed by the cyclooxygenase inhibitors indomethacin and celecoxib and by the EP2 antagonist AH6809. These agents mediate this effect by reversing the actions of PGE2 on DNA methyltransferase activity (Prasad and Katiyar, 2013).
Epidemiologic Studies
A number of epidemiologic studies support the concept that NSAIDs which inhibit cyclooxygenases have a positive effect in decreasing the risk of cutaneous NMSC (Butler et al., 2005; Clouser et al., 2009; Grau et al., 2006; Johannesdottir et al., 2012). A case-control study based in Australia with a cohort of 1621 individuals, captured NSAID use (Butler et al., 2005). The incidence of SCCs and BCCs was self-reported by patients and then confirmed by medical records. Participants were also examined for AKs on the face, ears, right hand and right forearm (Butler et al., 2005). People who used NSAIDs more than two times per week for at least a year had a statistically significant lower incidence of SCCs and lower AK counts than those who had never used them or used them infrequently. In another population base case-control study from Denmark, both NMSC and melanoma risk among NSAID users were evaluated (Johannesdottir et al., 2012). The incidence of BCCs, SCCs and melanomas was identified over a period of 18 years and compared to prescription data of aspirin, nonselective NSAIDs and selective COX-2 inhibitors. The use of aspirin, nonselective NSAIDs, and COX-2 inhibitors was associated with decreased risk of SCC and melanoma. Moreover, the reduction in risk increased as the frequency and duration of NSAID use increased. No association between NSAID use and BCC was found.
While several studies support the hypothesis that NSAIDs suppress the development of UV-induced skin cancers, other reports have not found a significant association between NSAIDs and skin cancer prevention or have found the results to be inconclusive (Asgari et al., 2010; Grau et al., 2006; Nunes et al., 2011). A retrospective case-control study assessing the association between NSAIDs and SCCs examined self-reported NSAID use in 415 patients with histopathologically confirmed SCC (Asgari et al., 2010). Study questionnaires collected information on over-the-counter and prescription NSAID use during the 10 years prior to SCC diagnosis. The results from this study showed no decrease in SCCs from NSAID use regardless of dose or duration. Another study examined data from the Skin Cancer Chemoprevention Study for an association between NSAID use with the risk of BCCs and SCCs. No significant protective effect of NSAIDs on BCCs was observed (Grau et al., 2006). Overall rates of SCC incidence were lower for NSAID users, although this may have been due to a chance association.
Translational Studies
The consequences of UV radiation on cyclooxygenase expression in animal models are similar to that which takes place in humans. When the skin of normal volunteers is exposed to a single 1-2 times the minimal erythema dose (MED) of ultraviolet radiation from a solar simulator, a substantial increase in COX-2 expression occurs, but there is no change in COX-1 expression (Buckman et al., 1998). In some individuals, this can be suppressed by pre-treatment with celecoxib (Rodriguez-Burford etal., 2005). Moreover, immunohistological studies have shown that while COX-2 is not found in normal skin, it is present in AKs and SCCs (An et al., 2002). COX-2 is also expressed in the parenchyma and/or the stroma surrounding BCCs (An et al., 2002; Tang et al., 2010).
Because of the abundance of data from animal experiments, epidemiologic studies suggesting that NSAIDs may suppress the development of UV-induced tumors, and the findings that NSAIDs exert a protective effect in colon chemoprevention trials (Meyskens et al., 2008), two clinical studies have been conducted to determine whether COX-2 inhibitors might be effective preventive agents for NMSCs (Elmets et al., 2010; Tang et al., 2010). One of these was a double blind, placebo-controlled trial conducted at eight U.S. academic centers (Elmets et al., 2010). Two hundred forty subjects with Fitzpatrick, sun reactive skin types I-III who had 10-40 AKs at baseline and a prior histological diagnosis of at least one AK or NMSC were randomized to receive celecoxib (200 mg b.i.d.), an oral selective inhibitor of COX-2 that is FDA approved for the treatment of rheumatoid arthritis, osteoarthritis, and the adjunct treatment of familial adenomatous polyposis, or placebo. A known photosensitivity disorder, topical medications other than sunscreens or emollients, recent treatment for AKs and NSAID use other than cardioprotective doses of aspirin were exclusion criteria. Participants that enrolled in the study were primarily males. The mean age was 65 years, and all had extensive actinic damage. The mean number of NMSCs prior to entry into the study was 2.3, and the mean number of AKs at baseline was 22.4. Participants were placed on celecoxib or placebo for 9 months and were followed for an additional 2 months off medication.
There was no effect of celecoxib on the incidence of AKs. However, there was a dramatic decrease in the incidence of NMSCs. At 11 months, there was a 58% reduction in NMSCs. The difference between the celecoxib and placebo treated groups first became apparent 3 months after initiation of therapy and became statistically significant at 9 months. It should be noted that there was no rebound in the incidence of skin cancer in the 2 months after completion of celecoxib treatment, although it should be noted that the two month duration was relatively short. When BCCs and SCCs were analyzed separately, celecoxib was protective for both. There was no significant difference in serious adverse events or cardiovascular adverse events between the two groups. However, it should be noted that the major cardiovascular toxicity from COX-2 inhibitors occurs after 12-18 months, so the absence of side effects after 9 months would be expected (Solomon et al., 2005).
Studies examining the chemopreventive effects of celecoxib have also been conducted in patients with basal cell nevus syndrome (BCNS) (Tang et al., 2010). Sixty BCNS patients were enrolled in a trial in which they received celecoxib or placebo for 2 years. In those individuals who had less than 15 BCCs at the initiation of study, the increase in new BCCs was only 22% compared to 48% in those who received placebo. The difference between the two groups was statistically significant.
From these studies, it is reasonable to conclude that: 1) inhibition of COX-2 is an effective means of limiting the development of cutaneous squamous cell and basal cell carcinomas; 2) that it acts at a late stage in skin tumor development based on the fact that actinic keratoses were not prevented by celecoxib treatment; and 3) celecoxib works rapidly and is highly effective.
The preclinical, epidemiologic and translational studies provide proof of principal that agents which inhibit cyclooxygenase-2 have the potential to limit the development of new NMSCs. Patients with extensive actinic damage often develop both BCC and SCC. A particularly attractive feature of NSAIDs and other agents that block COX-2 is their potential to block both types of NMSC. Whether alternatives to celecoxib, which include nonspecific COX-1 and COX-2 inhibitors such as naproxen or sulindac, topical application of cyclooxygenase inhibitors, or dietary chemopreventive agents which limit COX-2 activities can be employed on a long-term basis to stem the increase in nonmelanoma skin cancers remains to be determined.
Acknowledgments
This was supported by NIH grants and contracts NIH Grants and Contracts: P30 AR050948, P30 CA013148, N01 CN05014-69, R01 CA138998 and by VA Merit Review 18-103-02.
ABBREVIATIONS
- AK
actinic keratosis
- BCC
basal cell carcinoma
- BCNS
basal cell nevus syndrome
- COX
cyclooxygenase
- NMSC
non-melanoma skin cancer
- NO
nitric oxide
- PGE2
prostaglandin E2
- SCC
squamous cell carcinoma
- SPF
sunburn protection factor
Footnotes
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest.
References
- An KP, Athar M, Tang X, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Asgari MM, Chren M-M, Warton EM, et al. Association Between Nonsteroidal Anti-inflammatory Drug Use and Cutaneous Squamous Cell Carcinoma. Arch Dermatol. 2010;146:388–95. doi: 10.1001/archdermatol.2009.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar M. Unpublished. [Google Scholar]
- Athar M, An KP, Tang X, et al. Photoprotective effects of sulindac against ultraviolet B-induced phototoxicity in the skin of SKH-1 hairless mice. Toxicol Appl Pharmacol. 2004;195:370–8. doi: 10.1016/j.taap.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Blackler R, Syer S, Bolla M, et al. Gastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defence. PLoS One. 2012;7:e35196. doi: 10.1371/journal.pone.0035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher AR. The role of cyclooxygenase-2 in the pathogenesis of skin cancer. J Drugs Dermatol. 2002;1:44–7. [PubMed] [Google Scholar]
- Buckman SY, Gresham A, Hale P, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–9. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- Butler GJ, Neale R, Green AC, et al. Nonsteroidal anti-inflammatory drugs and the risk of actinic keratoses and squamous cell cancers of the skin. Journal of the American Academy of Dermatology. 2005;53:966–72. doi: 10.1016/j.jaad.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Chaudhary SC, Singh T, Kapur P, et al. Nitric oxide-releasing sulindac is a novel skin cancer chemopreventive agent for UVB-induced photocarcinogenesis. Toxicology and Applied Pharmacology. 2013;268:249–55. doi: 10.1016/j.taap.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–90. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- Chung HT, Burnham DK, Robertson B, et al. Involvement of prostaglandins in the immune alterations caused by the exposure of mice to ultraviolet radiation. J Immunol. 1986;137:2478–84. [PubMed] [Google Scholar]
- Clouser MC, Roe DJ, Foote JA, et al. Effect of non-steroidal anti-inflammatory drugs on non-melanoma skin cancer incidence in the SKICAP-AK trial. Pharmacoepidemiol Drug Saf. 2009;18:276–83. doi: 10.1002/pds.1718. [DOI] [PubMed] [Google Scholar]
- Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ. 2000;321:1183–7. doi: 10.1136/bmj.321.7270.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmets CA, Viner JL, Pentland AP, et al. Chemoprevention of Non-Melanoma Skin Cancer with Celecoxib: A Randomized, Double-Blind, Placebo-Controlled Trial. J Natl Cancer Inst. 2010 doi: 10.1093/jnci/djq442. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurschou A, Wulf HC. The relation between sun protection factor and amount of suncreen applied in vivo. British Journal of Dermatology. 2007;156:716–9. doi: 10.1111/j.1365-2133.2006.07684.x. [DOI] [PubMed] [Google Scholar]
- Fischer S, Lo H, Gordon G, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25 [PubMed] [Google Scholar]
- Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prevention Research. 2011;4:1728–35. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SM, Pavone A, Mikulec C, et al. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol Carcinog. 2007;46:363–71. doi: 10.1002/mc.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosbol EL, Gislason GH, Jacobsen S, et al. Risk of myocardial infarction and death associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85:190–7. doi: 10.1038/clpt.2008.204. [DOI] [PubMed] [Google Scholar]
- Gibbs NK, Norval M. Photoimmunosuppression: a brief overview. Photodermatol Photoimmunol Photomed. 2013;29:57–64. doi: 10.1111/phpp.12021. [DOI] [PubMed] [Google Scholar]
- Grau MV, Baron JA, Langholz B, et al. Effect of NSAIDs on the recurrence of nonmelanoma skin cancer. Int J Cancer. 2006;119:682–6. doi: 10.1002/ijc.21878. [DOI] [PubMed] [Google Scholar]
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723–9. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- Johannesdottir SA, Chang ET, Mehnert F, et al. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: A population-based case-control study. Cancer. 2012 doi: 10.1002/cncr.27406. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Rao CV, Seibert K, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–12. [PubMed] [Google Scholar]
- Kerr DJ, Dunn JA, Langman MJ, et al. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med. 2007;357:360–9. doi: 10.1056/NEJMoa071841. [DOI] [PubMed] [Google Scholar]
- Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–6. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- Krutmann J, Honigsmann H, Elmets C, editors. Dermatological Phototherapy and Photodiagnostic Methods. Springer; Berlin: 2009. [Google Scholar]
- Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine Plus Sulindac for the Prevention of Sporadic Colorectal Adenomas: A Randomized Placebo-Controlled, Double-Blind Trial. Cancer Prev Res. 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulec CD, Rundhaug JE, Simper MS, et al. The chemopreventive efficacies of nonsteroidal anti-inflammatory drugs: the relationship of short-term biomarkers to long-term skin tumor outcome. Cancer Prev Res (Phila) 2013;6:675–85. doi: 10.1158/1940-6207.CAPR-13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes AP, Lapane KL, Weinstock MA, et al. Association between non-steroidal anti-inflammatory drugs and keratinocyte carcinomas of the skin among participants in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Pharmacoepidemiol Drug Saf. 2011;20:922–9. doi: 10.1002/pds.2142. [DOI] [PubMed] [Google Scholar]
- Pentland A, Schoggins J, Scott G, et al. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–44. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- Pentland AP, Scott G, VanBuskirk J, et al. Cyclooxygenase-1 deletion enhances apoptosis but does not protect against ultraviolet light-induced tumors. Cancer Res. 2004;64:5587–91. doi: 10.1158/0008-5472.CAN-04-1045. [DOI] [PubMed] [Google Scholar]
- Prasad R, Katiyar SK. Prostaglandin E2 Promotes UV radiation-induced immune suppression through DNA hypermethylation. Neoplasia. 2013;15:795–804. doi: 10.1593/neo.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WA, Stein CM, Hall K, et al. Non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet. 2002;359:118–23. doi: 10.1016/S0140-6736(02)07370-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Burford C, Tu JH, Mercurio M, et al. Selective Cyclooxygenase-2 Inhibition Produces Heterogeneous Erythema Response to Ultraviolet Irradiation. J Investig Dermatol. 2005;125:1317. doi: 10.1111/j.0022-202X.2005.23960.x. [DOI] [PubMed] [Google Scholar]
- Rogers HW, Coldiron BM. Analysis of skin cancer treatment and costs in the United States Medicare population, 1996-2008. DermatolSurg. 2013;39:35–42. doi: 10.1111/dsu.12024. [DOI] [PubMed] [Google Scholar]
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 Plays a Critical Role in UV-induced Skin Carcinogenesis. Photochemistry and Photobiology. 2008;84:322–9. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE, Mikulec C, Pavone A, et al. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Molecular Carcinogenesis. 2007;46:692–8. doi: 10.1002/mc.20329. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE, Simper MS, Surh I, et al. The role of the EP receptors for prostaglandin E2 in skin and skin cancer. Cancer and Metastasis Reviews. 2011;30:465–80. doi: 10.1007/s10555-011-9317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–8. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- Seukeran D, Newstead C, Cunliffe W. The compliance of renal transplant recipients with advice about sun protection measures. Brit J Dermatol. 1998;138:301–3. doi: 10.1046/j.1365-2133.1998.02079.x. [DOI] [PubMed] [Google Scholar]
- Sharma SD, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm Res. 2010;27:1092–102. doi: 10.1007/s11095-010-0050-9. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJV, Pfeffer MA. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Wittes J, Finn PV, et al. Cardiovascular Risk of Celecoxib in 6 Randomized Placebo-Controlled Trials: The Cross Trial Safety Analysis. Circulation. 2008;117:2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontrapa K, Honda T, Sakata D, et al. Prostaglandin E2-prostoglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proceedings of the National Academy of Sciences. 2011;108:6668–73. doi: 10.1073/pnas.1018625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VE, Rao CV, Zhang Y, et al. Chemopreventive Efficacy of Naproxen and Nitric Oxide-naproxen in Rodent Models of Colon, Urinary Bladder, and Mammary Cancers. Cancer Prevention Research. 2009;2:951–6. doi: 10.1158/1940-6207.CAPR-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Aszterbaum M, Athar M, et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/- humans and mice. Cancer Prevention Research. 2010;3:25–34. doi: 10.1158/1940-6207.CAPR-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SC, Jolley D, Marks R. Reduction of Solar Keratoses by Regular Sunscreen Use. N Engl J Med. 1993;329:1147–51. doi: 10.1056/NEJM199310143291602. [DOI] [PubMed] [Google Scholar]
- Tober KL, Wilgus TA, Kusewitt DF, et al. Importance of the EP(1) receptor in cutaneous UVB-induced inflammation and tumor development. J Invest Dermatol. 2006;126:205–11. doi: 10.1038/sj.jid.5700014. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–40. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Koki AT, Zweifel BS, et al. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog. 2003;38:49–58. doi: 10.1002/mc.10141. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Ross MS, Parrett ML, et al. Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins & other lipid mediators. 2000;62:367–84. doi: 10.1016/s0090-6980(00)00089-7. [DOI] [PubMed] [Google Scholar]


