Abstract
Although exposure to a recent major life event is one of the strongest known risk factors for depression, many people who experience such stress do not become depressed. Moreover, the biological mechanisms underlying differential emotional reactions to social adversity remain largely unknown. To investigate this issue, we examined whether the endogenous opioid system, which is known to influence sensitivity to physical pain, is also implicated in differential risk for depression following socially painful targeted rejection versus non-targeted rejection life events. Adolescents (n = 420) enrolled in a large longitudinal birth cohort study had their recent stress exposure and current mental health status assessed using self-report and interview-based methods. Participants were also genotyped for the A118G polymorphism in the μ-opioid receptor gene (OPRM1, rs1799971), which has been found to influence neural and psychological responses to rejection, likely by affecting opioid receptor expression and signaling efficiency. As hypothesized, G allele carriers, who are known to exhibit less opioid receptor expression and signaling efficiency, were more severely depressed and twice as likely to meet criteria for major depressive disorder following a recent targeted rejection major life event (e.g., being broken up with, getting fired) relative to A/A homozygotes who experienced such stress. However, A118G genotype did not moderate the effects of other similarly severe major life events on depression. These data thus elucidate a biological pathway that may specifically influence sensitivity to social pain and rejection, which in turn has implications for understanding differential risk for depression and several other social stress-related disorders.
Keywords: stress, pain, genetic, opioid, A118G, polymorphism, adolescence, risk, health, disease
Major life events involving interpersonal loss and social rejection are the strongest proximal risk factors for depression (Farmer & McGuffin, 2003; Slavich et al., 2010a). Stressors of this type, called targeted rejection life events, involve intentional social rejection and the severing of important social bonds. They thus include major life events such as being broken up with or getting fired (Slavich et al., 2009). Targeted rejection life events are associated with a 22-fold increase in risk for MDD and precipitate MDD three times faster than other life events of comparable severity (Kendler et al., 2003; Slavich et al., 2009). These stressors have also been found to activate molecular signaling pathways that upregulate inflammation (Murphy et al., 2013), which in turn has been implicated in the pathophysiology of depression, as well as several physical health conditions that frequently co-occur with depression, including diabetes, cardiovascular disease, chronic pain, certain cancers, and neurodegeneration (Miller et al., 2009b; Slavich & Irwin, 2014). Despite these findings, many people who experience targeted rejection and other types of severe social stress do not develop depression. Moreover, the biological mechanisms underlying differential risk for MDD following these social stressors remain largely unknown. Because depression often emerges early in life and presages the development of several serious medical illnesses over the lifespan, identifying factors that shape social stress-related depression risk, especially in adolescence, is critically important (Stroud et al., 2011; Auerbach et al., 2014).
One neurobiological system that may influence risk for depression following socially painful targeted rejection life events is the endogenous opioid system. Opioids have long been known to play a central role in regulating experiences of physical pain (Basbaum & Fields, 1984; Drolet et al., 2001; Millan, 2002). Indeed, endogenous opioid neurotransmission increases during physical pain and lessens the affective experience of pain (Zubieta et al., 2001, 2002). Given the critical importance of social connection for survival, it has been proposed that neural systems originally responsible for processing experiences of physical pain may have evolved to represent experiences of “social pain” or rejection, perhaps to reduce the likelihood of separation from primary caregivers (Panksepp, 1998; MacDonald & Leary, 2005). Consequently, endogenous opioids may influence not only experiences of physical pain, but experiences of social pain and rejection as well (Panksepp, 2003; Way, 2013).
Several findings are consistent with this effect, which we refer to here as the opioid rejection sensitivity hypothesis. First, evidence has accumulated suggesting that social and physical pain are represented by similar neural systems (Eisenberger et al., 2003; Kross et al., 2011). Second, experimental studies in non-human species have shown that the μ-opioid receptor regulates behavioral distress responses to caregiver separation (Panksepp et al., 1978; Kalin et al., 1988; Barr et al., 2008). Third, positron emission tomography studies in humans have revealed that central μ- opioid signaling is altered while experiencing or recalling interpersonal loss and rejection (Zubieta et al., 2003; Hsu et al., 2013). Finally, a central analgesic has been shown to blunt neural responses to social rejection in brain systems that have been implicated in processing experiences of physical and social pain (Dewall et al., 2010). Together, these data suggest that μ-opioid signaling may modulate the experienced intensity of social rejection in addition to physical pain.
One factor that greatly alters μ-opioid signaling involves a functional single nucleotide polymorphism (SNP) in the μ-opioid receptor gene (OPRM1, rs1799971). Specifically, an A/G transition (A118G) within OPRM1 leads to an amino acid change (N40D) that governs central OPRM1 expression (Mague et al., 2009; Ray et al., 2011), leading to differences in sensitivity to both physical pain and social rejection. As evidence of these effects, patients with at least one G allele experience greater pain intensity during surgery, and require larger doses of opiates to relieve post-surgical pain, compared to A/A homozygotes (Tan et al., 2009; Sia et al., 2013). In addition, G allele carriers exhibit greater neural responses to being socially rejected, more behavioral withdrawal to angry faces indicating social rejection, and higher levels of rejection sensitivity in daily life relative to A/A homozygotes (Way et al., 2009; Bertoletti et al., 2012). Finally, a recent study reported that A118G genotype moderates the effects of early maternal care on adult attachment style, with G allele carriers exhibiting high levels of fearful attachment regardless of the quality of their early maternal care (Troisi et al., 2012).
To examine the relevance of these findings for depression, we recruited 420 adolescents from a large longitudinal birth cohort study and followed them over time to assess their stress exposure and depression status. More specifically, we used a state-of-the-art, interview-based measure of life stress to identify whether participants experienced a major life event in the year prior to age 20, and standardized questionnaire and diagnostic interviewing methods to assess their levels of depression at ages 15 and 20. Finally, participants were genotyped for the A118G polymorphism in their early twenties. Consistent with the large body of research on life stress and depression (Hammen, 2005), we hypothesized that adolescents who experienced a major life event of any kind (i.e., targeted rejection or non-targeted rejection) in the year prior to age 20 would have more depressive symptoms, and a higher likelihood of meeting diagnostic criteria for MDD at age 20, compared to participants who did not experience such stress (i.e., controlling for relevant demographic and clinical factors). In addition, given prior research linking the G allele with heightened sensitivity to social rejection at the neural and psychological level (Way et al., 2009; Bertoletti et al., 2012), we hypothesized that these effects would be moderated by variation at the A118G locus, with G allele carriers exhibiting more stress-related depressive symptoms and higher rates of MDD following a recent major life event, relative to A/A homozygotes. Because endogenous opioids have been shown to specifically influence experiences of pain, however, we reasoned that moderation by A118G genotype would be unique to socially painful targeted rejection life events and not extend to other types of similarly severe major life stress (e.g., educational, financial, housing, health, crime events).
Method
Participants
Participants were 420 adolescents (250 females, 170 males) from Brisbane, Australia, who completed all assessments in a community-based, longitudinal study of children of depressed and never-depressed mothers. These adolescents were retained from an initial sample of participants who were intensively studied at ages 15 (n = 815) and 20 (n = 705), and who provided DNA samples between ages 22-25 (n = 444). Additional details of the original sampling ascertainment are reported elsewhere (see Hammen & Brennan, 2001).
The present sample was 92.9% Caucasian, 3.8% Asian, and 3.3% “other,” with participants being raised in largely working and lower middle class families. These adolescents did not differ from the larger sample on measures of depression history by age 15, depression severity at age 20, maternal depression status by age 15, or number of major life events experienced at age 20 (ps > .60). However, females were more likely to complete the genetic sampling protocol than males, χ2 (1, 815) = 36.44, p < .001.
Procedure
Participants and their mothers completed an extensive battery of interviews and questionnaires at several time points as part of the parent project. For the present study, we focused on the expert-rated assessments of recent life stress, which were based on semi-structured interviews that were conducted at age 20 and that covered all acute life events and chronic stressors occurring in the year prior to age 20; self-reported symptoms of the youths’ depression, which were assessed using standardized instruments at ages 15 and 20; interviewer-rated caseness of depression, which was assessed by an expert diagnostic interviewer at age 20; and genotyping data, which were derived from blood draws that occurred between ages 22-25 (see below). All of the life stress and diagnostic interviews that took place with the youth were conducted blind to the mothers’ history of depression. Written informed assent and consent was obtained prior to each study visit, and all procedures were approved by the institutional review boards of the University of Queensland, University of California, Los Angeles, Emory University, and the Queensland Institute of Medical Research Genetic Epidemiology Laboratory.
Assessment of Life Stress and Targeted Rejection
Major life events occurring over the past year and chronic stress occurring over the past six months were assessed at age 20 using the UCLA Life Stress Interview (LSI; Adrian & Hammen, 1993). Interviewers trained in the LSI used a semi-structured interview to obtain extensive factual information about the contextual features and precise timing of each reported stressor, in addition to relevant biographical details of each participant (i.e., “contextual” ratings; Brown & Harris, 1978). Detailed narratives of each life event were subsequently presented to an independent team of expert raters who were blind to participants’ emotional reactions to the stressors, as well as to all study outcomes. Raters judged the severity (i.e., “contextual threat”) of each life event on a 5-point scale, on which higher scores represent more severe impact (κ = .84). Only life events that preceded onset of depression (for adolescents who developed MDD) were included in analyses to ensure that the major life events could have contributed to the depressive episode and not vice versa. Participants experienced one major life event, on average, in the year prior to age 20 (M = 1.2; Range = 0-7), and all analyses controlled for total number of recent major life events so that overall life event exposure could not influence the results. These stress assessment procedures have been extensively validated in adolescent and young adult samples (e.g., Adrian & Hammen, 1993; Hammen & Brennan, 2001).
All major life events (i.e., severity of ≥ 2.5) in the relationship and work life domains were subsequently identified as possible targeted rejection life events. We focused on events in these life domains because they are generally stressful and evenly divided between targeted rejection and nontargeted rejection life events (Slavich et al., 2009). Targeted rejection ratings were conducted by two expert raters who, after reading the detailed narrative for each life event, made a judgment of targeted rejection or non-targeted rejection based on previously established criteria (see Slavich et al., 2009). These raters were blind to participants’ emotional responses and personal characteristics, and to the original severity ratings of the life events. Events were judged to be targeted rejection if they: (a) happened primarily to the participant, (b) involved the rejection of the participant by another person or group, (c) involved a clear intent to actively reject the participant, (d) directly affected the participant, and (e) resulted in the severing of a social bond between the participant and the other person or group. The team showed excellent inter-rater agreement (κ = .94). Of the 80 participants who experienced a major life event in the relationship or work domain, 31 (38.75%) experienced recent targeted rejection. Given the large body of research showing that the best predictor of depression-related outcomes is having experienced at least one recent major life event (e.g., Slavich et al., 2009; Murphy et al., 2013, 2014), work and relationship events were combined into a total indicator of presence versus absence of a recent targeted rejection major life event.
Although our hypotheses focused on acute life events, onset and severity of depression are also known to be influenced by levels of recent chronic stress (Hammen, 2005; Monroe et al., 2007, 2009). Consequently, we also assessed participants’ levels of chronic stress burden and impact over the past six months in several key domains (i.e., close friendships, social life, romantic relationships, family relations, work, school, finances, and the health of the participant and his or her family) and controlled for these levels in all analyses. To derive these chronic stress scores, interviewers obtained detailed, factual information about chronic stressors occurring in each life domain using the LSI. This information was then compared to behaviorally specific anchors to obtain a chronic stress severity score for each domain, which ranged from “1” (superior circumstances and functioning) to “5” (severely adverse conditions). Inter-rater agreement for these judgments was good (κ = .72-.88 across the domains). Total scores were computed by summing participants’ levels of chronic stress across all life domains (M = 22.59; Range: 12-35), and these total scores were then included in all models to ensure that recent chronic stress exposure could not influence the results.
Genotyping
Adolescents who agreed to participate in the genotyping protocol were mailed consent forms, a blood collection kit, and questionnaires, and were instructed to have their blood drawn at a local pathology lab. Samples were picked up by courier from the individual and transported to the Genetic Epidemiological Laboratory at the Queensland Institute of Medical Research for storage. Aliquots of DNA were subsequently shipped to the UCLA Inflammatory Biology Core Laboratory for processing. The A118G polymorphism (rs1799971) was genotyped using a 5′ nuclease assay (Taqman SNP Genotyping Assay, Applied Biosystems, Foster City, CA) to discriminate between the A and G allele at the A118G locus. Real-time polymerase chain reactions (PCR) were performed on an iCycler PCR instrument (BioRad, Hercules, CA), using 5-μL reaction volumes in 384-well plates with 5ng of DNA. The manufacturer’s specified protocol was followed. End point reads of fluorescence levels were obtained with an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Each sample was re-genotyped, and test-retest reliability of the duplicated specimens yielded a total genotyping error rate of < 1%. Allele frequencies at the A118G locus across the entire sample were examined using Haploview v4.2. The frequencies did not deviate from Hardy–Weinberg equilibrium, χ2(2, N = 420) = 3.68, p > .05, and were distributed as follows: GG, n = 2 (0.5%); AG, n = 98 (23.3%); and AA n = 320 (76.2%). Given the very low frequency of G/G homozygotes in this sample, and consistent with prior research on social rejection and the A118G polymorphism (e.g., Way et al., 2009), individuals were re-coded into two groups: G allele carriers (GG or AG; n = 100) and A/A homozygotes (AA, n = 320).
Assessment of Depression
Past depressive symptom severity (at age 15) and current depressive symptom severity (at age 20) were assessed using the Beck Depression Inventory-II (BDI; Beck et al., 1996). The BDI is a widely used, 21-item self-report instrument that measures cognitive, affective, behavioral, and physiologic symptoms of depression. The BDI has excellent psychometric properties (α = .91), and is highly sensitive for detecting symptoms that are influenced by stress and specific to depression (Beck et al., 1996; Muscatell et al., 2009). Presence of current MDD (at age 20), in turn, was assessed by expert diagnostic interviewers using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First et al., 2002). Diagnostic reliability for judgments of current MDD was good (κ = .83).
Results
Preliminary Analyses
Preliminary analyses were conducted to examine factors that could potentially confound the results. G allele carriers and A/A homozygotes did not differ with respect to the number of major life events they experienced in the year prior to age 20, number of targeted rejection major life events they experienced in the year prior to age 20, levels of chronic stress at age 20, BDI scores at age 15, BDI scores at age 20, or mothers’ depression status (i.e., never depressed, past MDD but no current MDD, current MDD) by age 20 (all ps > .20). The A118G genotypes were not differentially represented across genders (p = .730), but their frequencies did differ across ethnic groups. Consistent with prior research (http://www.hapmap.org: rs1799971), the G allele was more common in individuals of Asian descent (50.0%) than European-American descent (22.8%), χ2(1, N = 99) = 66.27, p < .001, or other ancestral background (12.5%), χ2(1, N = 10) = 6.4, p = .01. Finally, BDI scores at age 20 were strongly related to BDI scores at age 15 (r = .41), as well as number of major life events at age 20 (r = .20), levels of chronic stress at age 20 (r = .48), and gender (d = 0.22), with females having higher levels of depression (all ps < .038). Given these associations, we adopted a conservative analytic approach and adjusted for the following five factors in all of the primary analyses below: gender, ethnicity, BDI score at age 15, number of major life events in the year prior to age 20, and levels of chronic stress at age 20.
Targeted Rejection, A118G Genotype, and Severity of Depression
We hypothesized that adolescents who experienced a recent major life event of any kind (i.e., targeted rejection or non-targeted rejection) would have more depressive symptoms compared to their counterparts who did not experience such stress. We also hypothesized that A118G genotype would moderate the effects of targeted rejection (but not non-targeted rejection) major life events on depressive symptoms. To test these hypotheses, we first examined the effects of Targeted Rejection Major Life Event (Yes, No) and A118G Genotype (GG/AG, AA) on BDI scores at age 20 using generalized linear modeling, while controlling for the abovementioned demographic and clinical factors. This analysis revealed significant main effects of both targeted rejection and A118G genotype on depression severity, F(1, 376) = 5.53, p = .019, ηp2 = .014, and F(1, 376) = 8.42, p = .004, ηp2 = .022, respectively. As hypothesized, these effects were qualified by a significant Targeted Rejection × A118G Genotype interaction, F(1, 376) = 11.26, p = .001, ηp2 = .029. As illustrated in Figure 1a, G allele carriers who experienced a recent targeted rejection major life event had significantly higher BDI scores (M = 16.06, SE = 2.73) than G allele carriers who did not experience such stress (M = 6.84, SE = 0.71), F(1, 89) = 14.46, p < .001, ηp2 = .14. In contrast, exposure to targeted rejection was unrelated to depression severity for A/A homozygotes (Targeted rejection: M = 5.72, SE = 1.41; No targeted rejection: M = 7.53, SE = 0.41), F(1, 282) = 1.04, p = .308, ηp2 = .004. Most importantly, and consistent with hypotheses, the simple effects comparison of G allele carriers and A/A homozygotes exposed to targeted rejection was significant, F(1, 22) = 8.96, p = .007, ηp2 = .290, and indicated that G allele carriers who experienced a recent targeted rejection major life event had significantly higher BDI scores (M = 16.06, SE = 2.73) than A/A homozygotes who experienced such stress (M = 5.72, SE = 1.41). Interestingly, whereas G allele carriers who experienced targeted rejection had the highest overall levels of depression, in contrast, A/A homozygotes who experienced targeted rejection appeared to be relatively protected from the depressogenic effects of these socially painful stressors insofar as they exhibited the lowest overall levels of depression despite having experienced a recent targeted rejection major life event.
Figure 1.
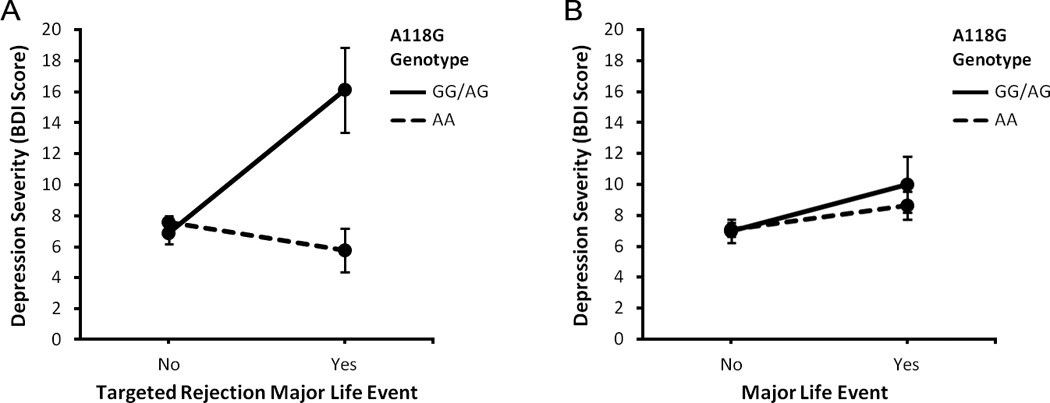
Relations of exposure to a recent major life event (Yes, No) and A118G genotype (GG/AG, AA) to severity of depression, expressed as mean ± SEM. (A) As hypothesized, G allele carriers who experienced a recent targeted rejection major life event had significantly higher Beck Depression Inventory (BDI) scores at age 20 than A/A homozygotes who experienced such stress. This effect was robust while controlling for several factors that are known to influence depression severity and risk, including adolescents’ gender, ethnicity, prior levels of depression (at age 15), number of recent major life events, and current levels of chronic stress. (B) In contrast, A118G genotype did not moderate the effects of other types of major life events on severity of depression. Taken together, these data suggest that variation in the μ-opioid receptor gene moderates the effects of socially painful targeted rejection life events on depression severity and, in addition, that these moderated effects by A118G genotype are specific to targeted rejection types of stress (n = 420).
To examine if this same pattern of effects on depression severity was present for other types of recent major life events (e.g., educational, financial, housing, crime events), we conducted parallel analyses using presence versus absence of a recent major life event as the main stress variable. Consistent with prior research on stress and depression, participants who experienced a recent major life event had significantly higher BDI scores (M = 9.32, SE = 1.02) than those who did not experience such stress (M = 7.02, SE = 0.44), F(1, 376) = 4.17, p = .042, ηp2 = .011 (see Figure 1b). However, there was no main effect of A118G genotype on depression severity, F(1, 376) = 0.14, p = .708, ηp2 = .001, and no Major Life Event × A118G Genotype interaction effect on depression severity, F(1, 376) = 0.19, p = .662, ηp2 = .001. Consequently, variation at the A118G locus of OPRM1 appears to specifically moderate the effects of targeted rejection on depression severity.
Targeted Rejection, A118G Genotype, and Major Depressive Disorder
To replicate and extend these results using an independent, expert-rated diagnostic assessment of depression (i.e., instead of a self-report measure of depression severity), we subsequently conducted a logistic regression analysis and examined whether A118G genotype moderated the effects of experiencing a recent major life event on developing a SCID-diagnosed episode of MDD. The model including the abovementioned demographic and clinical covariates and the Major Life Event (No Major Life Event, Non-Targeted Rejection Major Life Event, Targeted Rejection Major Life Event) × A118G Genotype (GG/AG, AA) interaction effect was significant, χ2(1, N = 420) = 41.57, p < .001, and correctly classified 92% of cases as non-depressed (386 cases) versus depressed (34 cases). The odds of having MDD were 4.4-times greater for G allele carriers who experienced a recent targeted rejection major life event (OR = 5.06, 95% C.I. = 1.09, 23.53) than for G allele carriers who experienced a recent non-targeted rejection major life event (OR = 1.14, 95% C.I. = 0.42, 3.07). Most importantly, and consistent with hypotheses, G allele carriers who experienced a recent targeted rejection major life event were twice as likely to have MDD (OR = 5.06, 95% C.I. = 1.09, 23.53) relative to A/A homozygotes who experienced such stress (OR = 2.66, 95% C.I. = 0.81, 8.72).
Alternative Explanations
We conducted follow-up analyses to examine several possible alternative explanations for the present findings. First, to examine whether the more depressogenic effects of targeted rejection could be attributed to these events being more severe in nature than non-targeted rejection life events, we tested for possible differences in the objective severity ratings of targeted rejection versus non-targeted rejection major life events. As described above, these severity judgments were made by expert raters who were blind to each life event’s targeted rejection status. We found no evidence that targeted rejection major life events were rated more severely (M = 2.8, SE = 0.05) than non-targeted rejection major life events (M = 2.78, SE = 0.04), F(1,133) = 0.16, p = .687, ηp2 = .001. Consequently, the relatively greater depressogenic impact observed for targeted rejection relative to non-targeted rejection major life events is likely due to differences in the underlying social-psychological characteristics of these stressors as opposed to differences in their severity.
Second, to test whether the sensitizing effects of having a G allele at the A118G locus were driven by adolescents with a history of MDD (24.4%) who might be especially sensitive to stress, we reran the stress and depression severity models after restricting the sample to participants with no prior lifetime history of MDD. However, this did not alter the results. There was still a Targeted Rejection × A118G Genotype interaction effect on depression severity, F(1, 348) = 9.90, p = .002, ηp2 = .028, and no Major Life Event × A118G Genotype interaction effect on depression severity, F(1, 348) = 1.15, p = .220. Third, we reran these models without controlling for the two main demographic factors (i.e., gender and ethnicity), but again, this did not alter the pattern of results [Targeted Rejection × A118G Genotype interaction effect on depression severity: F(1, 378) = 11.19, p = .001, ηp2 = .029; Major Life Event × A118G Genotype interaction effect on depression severity: F(1, 378) = 0.30, p = .582, ηp2 = .001). Fourth, we reran these models after restricting the sample to the largest ethnic subsample (i.e., Caucasian), and the Targeted Rejection × A118G Genotype interaction effect on depression severity held under these conditions, F(1, 352) = 7.67, p = .006, ηp2 = .021; however, there was still no Major Life Event × A118G Genotype interaction effect on depression severity, F(1, 352) = 0.01, p = .990, ηp2 < .001. Finally, we corrected for multiple comparisons, and all of the main findings survived this more stringent statistical threshold.
Discussion
Recent research has shown that the endogenous opioid system influences neural and psychological responses to social rejection, and that these effects are governed in part by commonly occurring variation in the μ-opioid receptor gene (OPRM1). The present data replicate and extend this work by demonstrating for the first time that a functional SNP at the A118G locus of OPRM1 moderates the effects of socially painful life events on depression. Specifically, in a large community sample of adolescents who were followed longitudinally and well-characterized with respect to their recent stress exposure and depression status, we found that adolescents with the variant G allele at the A118G locus were more severely depressed than A/A homozygotes following a recent targeted rejection major life event. A/A homozygotes, in contrast, were relatively protected from the depressogenic effects of targeted rejection insofar as they exhibited the lowest overall levels of depression despite having experienced a recent targeted rejection major life event. These effects were robust while controlling for several demographic and clinical factors that are known to influence depression severity and risk, including gender, ethnicity, prior levels of depression, number of recent major life events, and current levels of chronic stress. In addition, as hypothesized, these moderated effects by A118G genotype were specific to targeted rejection life events and were not present for other similarly severe stressors, such as educational, financial, housing, crime, or health life events. Finally, when we examined the influence of major life events and A118G genotype on the odds of meeting DSM-IV criteria for MDD, the same pattern of results emerged, with G allele carriers being twice as likely to develop MDD following targeted rejection relative to A/A homozygotes who experienced such stress. Taken together, these data indicate that variation in the μ-opioid receptor gene moderates the effects of targeted rejection on depression, thus revealing a biological mechanism that may help explain the substantial variability that is evident in risk for depression following recent major life stress.
In addition to shedding light on neurobiological factors that may underlie differential risk for depression, these findings add to the growing literature showing that the endogenous opioid system influences the experienced intensity of social rejection as well as physical pain (Kalin et al., 1988; Zubieta et al., 2001, 2002, 2003; Liberzon et al., 2002; Mague et al., 2009; Way et al., 2009; Ray et al., 2011; Hsu et al., 2013; Sia et al., 2013). Consequently, the results may have implications for our conceptual understanding of depression, particularly in relation to chronic pain. Pain is a key feature of many depressive episodes (Poole et al., 2009), and stressful life events are known to promote symptoms of both physical pain and depression (Hammen, 2005; Van Houdenhove et al., 2009). To date, though, it has been unclear if interrelations between stress, physical pain, and depression could be due to a common neurobiological process. The present findings are consistent with this possibility insofar as they show that the endogenous opioid system is implicated in social stress-related depression risk in addition to physical pain sensitivity. This discovery adds to the mounting empirical evidence demonstrating that social and physical pain are influenced by similar mechanisms at several levels of analysis, including psychological, neural, and genetic. Perhaps more importantly, the findings suggest that targeting opioids may be an effective method for reducing the enormous disease burden caused by the co-occurrence of debilitating physical pain and depressive symptoms.
An important question that arises from these findings involves how variation at the A118G locus affects risk for depression following targeted rejection. Recent research related to this issue indicates that social rejection increases μ-opioid signaling in several brain regions that are relevant for depression, including the anterior cingulate cortex (ACC), amygdala, ventral striatum, periaqueductal gray, and thalamus; increased μ-opioid signaling in the ACC in turn predicts feeling less sad and rejected during social rejection (Hsu et al., 2013). Along related lines, μ-opioid receptor agonists that increase central opioid signaling have been shown to have antidepressant effects (Berrocoso et al., 2009; see also Saitoh & Yamada, 2012).
Considered together, these data suggest that μ-opioid neurotransmission may be important for blunting the negative affective responses that typically accompany targeted rejection. In this context, however, G allele carriers may be at a disadvantage for at least three reasons. First, opioid receptor expression has been found to be 10-fold lower for the G versus A allele (Zhang et al., 2005; see also Huang et al., 2012; Wang et al., 2012). Second, although G allele carriers have receptors that bind opioids normally, at least one study has found that opioid signaling efficiency is 58% lower in human brain tissue sampled postmortem from the secondary somatosensory area of G allele carriers relative to A/A homozygotes (Oertel et al., 2009). Finally, consistent with the notion that these differences have functional implications, G allele carriers have been found to exhibit both more self-reported sensitivity to social rejection and greater neural responses to rejection in brain regions that have been implicated in MDD, including the ACC and insula (Way et al., 2009; Masten et al., 2011). As a result of these differences, G allele carriers may experience rejection as particularly distressing or painful, which in turn leads to a relatively greater risk for depression following targeted rejection.
Although we did not assess physical health outcomes in the present study, it is possible that opioid-induced differences in responsivity to socially painful life events may have implications for several somatic disease conditions. Specifically, targeted rejection has been shown to upregulate proinflammatory gene expression (Murphy et al., 2013) and down-regulate anti-inflammatory gene expression (Murphy et al., 2014), and inflammation, in turn, is a key component of several major disorders including asthma, cardiovascular disease, ovarian and breast cancer, obesity, diabetes, and Alzheimer’s disease (Couzin-Frankel, 2010; Slavich & Irwin, 2014). Differential reactions to social stressors, governed by μ-opioid signaling, may thus contribute to individuals’ susceptibility to a range of diseases that cause substantial morbidity and mortality.
This study has several strengths, including a priori, biologically and psychologically plausible hypotheses regarding interplay between a specific type of life stress and a specific genetic locus. Second, the sample of adolescents was well-characterized using a state-of-the-art system for assessing stress, which involved detailed contextual information and an independent team of expert stress raters, and an interview-based measure of depression, which provided independent judgments regarding the presence versus absence of MDD. Third, we controlled for several known risk factors for depression that could have confounded results, including gender, ethnicity, prior levels of depression, number of recent major life events, and current levels of chronic stress, and conducted follow-up tests to rule out possible alternative explanations. Finally, we utilized a longitudinal study design that enabled us to verify that the life events that were reported preceded onset of depression and thus could have contributed to the disorder, and not vice versa.
Several limitations should also be noted. First, the sample size was not large enough to allow us to conduct stratified analyses within each ethnic subgroup. All of the results held while controlling for ethnicity and while restricting the sample to the largest ethnic subsample (i.e., Caucasian), but additional research with a more diverse sample is needed to examine these effects in each of the main ethnic groups. Second, consistent with prior research, analyses compared A/A homozygotes to a combined group of heterozygotes and G allele homozygotes, given that the latter is rare. Additional research with larger samples is thus needed to explore differences among all three A118G genotypes. Third, this investigation focused on negative life events and did not address whether A118G genotype moderates the effects of positive social experiences on mental health. There is some evidence that G allele carriers are more sensitive to both positive and negative social interactions (Troisi et al., 2011, 2012). Additional research on this topic is thus warranted.
Finally, this study was not designed to assess possible mechanisms linking targeted rejection and A118G genotype with depression. However, a variety of psychological and biological processes could potentially mediate the effects of targeted rejection and A118G genotype on depression. These processes include stress-induced increases in negative thoughts (e.g., “I’m unlovable,” “Other people don’t like me”), self-conscious emotions (e.g., shame, humiliation), and physical and social pain sensitivity, as well as disruptions in emotion regulation, coping behaviors, sleep-wake rhythms, social support, and innate immune system dynamics that affect health. Elsewhere we have hypothesized that targeted rejection evokes depressive symptoms in part by activating negative self-schemas and self-conscious emotions that undermine feelings of social worth (Slavich et al., 2010a), and by triggering neural, physiologic, molecular, and genomic processes that upregulate components of the innate immune system involved in inflammation (Slavich et al., 2010b; Slavich & Cole, 2013; Slavich & Irwin, 2014). Although studies have implicated these processes in the pathogenesis of depression, additional research is needed to examine whether these mechanisms operate differently as a function of variation at the A118G locus of OPRM1.
Notwithstanding these questions, a growing body of research now exists showing that opioids shape not only experiences of internal physical pain, but experiences of the external social world as well. The present data are novel in this context as they are the first to show that the endogenous opioid system is implicated in differential reactions to socially painful targeted rejection life events, an effect we have called the opioid rejection sensitivity hypothesis. Given that social stress is known to be involved in a variety of disease conditions other than depression, including anxiety disorders, cardiovascular disease, certain cancers, and neurodegeneration (Miller et al., 2009a), differences in the experienced intensity of social stress as influenced by activity of the endogenous opioid system may have implications not just for depression, but for human health and wellbeing more generally.
Acknowledgements
We thank the principal investigators of the Mater-University Study of Pregnancy, the Brisbane, Australia staff coordinators, the National Health and Medical Research Council, and the Mater Misericordiae Mother’s Hospital in Queensland, Australia, for supporting this study. We also thank Keely Muscatell and Tristen Inagaki for their very helpful comments on a previous version of this report.
Role of Funding Source
This research was supported by National Institutes of Health (NIH) grant R01 CA140933 and by a Society in Science – Branco Weiss Fellowship to George Slavich; by NIH grant R01 MH52239 to Constance Hammen and Patricia Brennan; by NIH grant T32 MH017140 to Molly Tartter; and by a seed grant from the UCLA Cousins Center for Psychoneuroimmunology to Constance Hammen. We are also grateful for the assistance of the UCLA Inflammatory Biology Core Laboratory, which is supported by NIH grant P30 AG028748. These organizations had no role in designing the study; in collecting, analyzing, or interpreting the data; in writing this report; or in deciding to submit this report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no conflicts of interest with respect to their authorship or the publication of this article.
Author Contributions
The idea for this study was developed by George Slavich, based on data originally collected by Patricia Brennan and Constance Hammen. George Slavich and Molly Tartter conducted the analyses and wrote the initial draft of the article, which was subsequently edited by Patricia Brennan and Constance Hammen. All authors read and approved the final version of the manuscript.
References
- Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. J. Consult. Clin. Psychol. 1993;61:354–359. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Ho MH, Kim JC. Identifying cognitive and interpersonal predictors of adolescent depression. J. Abnorm. Child Psychol. 2014:1–12. doi: 10.1007/s10802-013-9845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc. Natl. Acad. Sci. USA. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, T.X.: Psychological Corporation; 1996. [Google Scholar]
- Berrocoso E, Sánchez-Blázquez P, Garzón J, Mico JA. Opiates as antidepressants. Curr. Pharm. Des. 2009;15:1612–1622. doi: 10.2174/138161209788168100. [DOI] [PubMed] [Google Scholar]
- Bertoletti E, Zanoni A, Giorda R, Battaglia M. Influence of the OPRM1 gene polymorphism upon children's degree of withdrawal and brain activation in response to facial expressions. Dev. Cogn. Neurosci. 2012;2:103–109. doi: 10.1016/j.dcn.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. New York: Free Press; 1978. [Google Scholar]
- Couzin-Frankel J. Inflammation bares a dark side. Science. 2010;330:1621. doi: 10.1126/science.330.6011.1621. [DOI] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychol. Sci. 2010;21:931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuropsychopharmacol. 2001;4:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Farmer AE, McGuffin P. Humiliation, loss and other types of life events and difficulties: A comparison of depressed subjects, healthy controls and their siblings. Psychol. Med. 2003;33:1169–1175. doi: 10.1017/s0033291703008419. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer MB, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders, research version, non-patient edition (SCID-I/NP) New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- Hammen C. Stress and depression. Annu. Rev. Clin. Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA. Depressed adolescents of depressed and nondepressed mothers: Tests of an interpersonal impairment hypothesis. J. Consult. Clin. Psychol. 2001;69:284–294. doi: 10.1037//0022-006x.69.2.284. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, Ni L, Walker SJ, Mickey BJ, Korycinski ST, Koeppe RA, Crocker JK, Langenecker SA, Zubieta JK. Response of the μ-opioid system to social rejection and acceptance. J. Mol. Psychiatry. 2013;18:1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Chen C, Mague SD, Blendy JA, Liu-Chen LY. A common single nucleotide polymorphism A118G of the μ opioid receptor alters its N-glycosylation and protein stability. Biochem. J. 2012;441:379–386. doi: 10.1042/BJ20111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. JAMA Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc. Natl. Acad. Sci. USA. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Zubieta JK, Fig LM, Phan KL, Koeppe RA, Taylor SF. Mu-opioid receptors and limbic responses to aversive emotional stimuli. Proc. Natl. Acad. Sci. USA. 2002;10:7084–7089. doi: 10.1073/pnas.102174799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol. Bull. 2005;131:202–223. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc. Natl. Acad. Sci. USA. 2009;106:10847–10852. doi: 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Dev. Psychopathol. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu. Rev. Psychol. 2009a;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009b;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Georgiades K. The social environment and life stress in depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Second Edition. New York: Guilford Press; 2009. pp. 340–360. [Google Scholar]
- Monroe SM, Slavich GM, Torres LD, Gotlib IH. Major life events and major chronic difficulties are differentially associated with history of major depressive episodes. J. Abnorm. Psychol. 2007;116:116–124. doi: 10.1037/0021-843X.116.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MLM, Slavich GM, Chen E, Miller GE. Targeted rejection predicts decreased anti-inflammatory gene expression and increased symptom severity in youth with asthma. Under invited resubmission. Psychol. Sci. 2014 doi: 10.1177/0956797614556320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MLM, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clin. Psychol. Sci. 2013;1:30–40. doi: 10.1177/2167702612455743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Slavich GM, Monroe SM, Gotlib IH. Stressful life events, chronic difficulties, and the symptoms of clinical depression. J. Nerv. Ment. Dis. 2009;197:154–160. doi: 10.1097/NMD.0b013e318199f77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel BG, Kettner M, Scholich K, Renné C, Roskam B, Geisslinger G, Schmidt PH, Lötsch J. A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J. Biol. Chem. 2009;284:6530–6535. doi: 10.1074/jbc.M807030200. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Panksepp J. Feeling the pain of social loss. Science. 2003;302:237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: Opiates alleviate separation distress. Biol. Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Poole H, White S, Blake C, Murphy P, Bramwell R. Depression in chronic pain patients: Prevalence and measurement. Pain Pract. 2009;9:173–180. doi: 10.1111/j.1533-2500.2009.00274.x. [DOI] [PubMed] [Google Scholar]
- Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, Blendy JA, Logan J, Zubieta JK, Lerman C. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc. Natl. Acad. Sci. USA. 2011;108:9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Yamada M. Antidepressant-like effects of δ opioid receptor agonists in animal models. Curr. Neuropharmacol. 2012;10:231–238. doi: 10.2174/157015912803217314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia AT, Lim Y, Lim EC, Ocampo CE, Lim WY, Cheong P, Tan EC. Influence of mu-opioid receptor variant on morphine use and self-rated pain following abdominal hysterectomy. J. Pain. 2013;14:1045–1052. doi: 10.1016/j.jpain.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clin. Psychol. Sci. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neurosci. Biobehav. R. 2010a;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH. Targeted rejection predicts hastened onset of major depression. J. Soc. Clin. Psychol. 2009;28:223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. USA. 2010b;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Hammen C, Vrshek-Schallhorn S. Severe and nonsevere events in first onsets versus recurrences of depression: Evidence for stress sensitization. J. Abnorm. Psychol. 2011;120:142–154. doi: 10.1037/a0021659. [DOI] [PubMed] [Google Scholar]
- Tan EC, Lim EC, Teo YY, Lim Y, Law HY, Sia AT. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for postoperative pain. Mol. Pain. 2009;5:32–38. doi: 10.1186/1744-8069-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D’Amato FR, Moles A, Siracusano A, Gross C. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc. Neurosci. 2011;6:88–97. doi: 10.1080/17470919.2010.482786. [DOI] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, Siracusano A, Gross C. Variation in the μ-opioid receptor gene (OPRM1) moderates the influence of early maternal care on fearful attachment. Soc. Cogn. Affect. Neurosci. 2012;7:542–547. doi: 10.1093/scan/nsr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdenhove B, Luyten P, Tiber Egle U. Stress as a key concept in chronic widespread pain and fatigue disorders. J. Musculoskelet. Pain. 2009;17:390–399. [Google Scholar]
- Wang YJ, Huang P, Ung A, Blendy JA, Liu-Chen LY. Reduced expression of the μ opioid receptor in some, but not all, brain regions in mice with OPRM1 A112G. Neuroscience. 2012;205:178–184. doi: 10.1016/j.neuroscience.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM. Social relationships and public health: A social neuroscience perspective focusing on the opioid system. In: Hall PA, editor. Social neuroscience and public health: Foundations for the science of chronic disease prevention. New York: Springer; 2013. [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc. Natl. Acad. Sci. USA. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J. Biol. Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. JAMA Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu-opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci. 2002;12:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


