Abstract
Objectives
Head and neck cancer patients and their caregivers report high rates of psychological distress. High rates of physical symptom burden and the increased need for family members to provide caregiving during treatment may contribute to this distress.
Methods
This study examined trajectories of patient and caregiver distress over the course of radiotherapy for head and neck cancer as well how patients and caregivers influence each other's distress. Forty-nine head and neck cancer patient-caregiver dyads completed 6 weekly assessments of physical symptoms (MDASI-HN) and distress (e.g., NCCN distress thermometer) over the course of radiotherapy.
Results
Patient and caregiver distress increased steadily over the course of treatment, peaking at week 5; patients (82% male; 69% Stage 4) and caregivers (90% female) reported significant distress in 77% of cases. Linear mixed models with the patient-caregiver dyad as the unit of analysis showed that increases in patient-rated head and neck specific physical symptoms had a significant (p<.05) time-varying effect on both patient and caregiver distress. Increases in one dyad member's distress levels were also significantly associated with decreases in the other's distress levels over time.
Conclusion
Findings highlight the adverse impact of patient physical symptoms during radiotherapy on both patient and caregiver distress. They also support a dyadic coping model whereby patients and caregivers manage their distress together as a unit. Overall, dyadic supportive care interventions that focus on controlling patient physical symptoms and that address both patient and caregiver distress are sorely needed in head and neck cancer.
Keywords: head and neck cancer, depression, anxiety, radiotherapy, distress thermometer, caregivers
Introduction
Psychological distress is the “sixth vital sign” in cancer care, and is a critical component of quality of life (QOL) [1]. Head and neck cancer (HNC) patients report some of the highest distress levels of all cancer patients; the incidence of suicide in HNC is more than four times higher than the general population [2], and 22-57% of patients experience depression and/or anxiety symptoms [3-6]. Although few studies have explored causative factors [7], it has long been hypothesized that the significant physical symptom burden associated with existing treatments contributes to patient distress [5]. HNC patients often receive radiation therapy (RT), either alone or in combination with surgery or chemotherapy. RT causes physical (e.g., xerostomia, mucositis) and functional challenges (e.g., difficulty swallowing, loss of taste) that increase in severity over the course of treatment and often persist long after treatment ends [8-10]. Although clear linkages between specific symptoms/functional challenges and distress have yet to be established in HNC, research has shown that distress increases significantly during RT [11]. Thus, there is a great need to develop supportive care interventions to alleviate distress in this population.
The diagnosis of cancer in one family member has practical and emotional repercussions for the entire family. Supporting this idea in HNC, studies have shown that the rates of distress and psychiatric disorders among family caregivers are similar to those of patients [12-14]. Very little is known about the factors driving caregiver distress in HNC; however, the patient's functional decline and increasing distress coupled with the increased need for the caregiver to assist with symptom management and provide support are likely contributing factors [15, 16]. Dyadic studies in cancer have also shown that patient and caregiver distress levels often covary [17, 18]. Thus, it is possible that an increase in one dyad member's distress may “spill over” and exacerbate distress for the other dyad member [19].
Unfortunately, most studies in HNC have focused on either patients or caregivers in isolation. Such studies miss key information about the adjustment process because both the stress of the cancer experience and the factors that affect each person's distress occur within a larger relationship context. Family researchers have highlighted the importance of a dyadic coping paradigm which considers the role of individual factors as well as how each partner affects the other's distress and coping efforts [20]. From this perspective, patient and caregiver distress could be driven by both person-level factors (e.g., patient functional decline may exacerbate patient distress, caregiver burden may exacerbate caregiver distress) and cross-partner effects (e.g., patient functional decline may exacerbate caregiver distress). However, this has yet to be examined in HNC.
The consequences of untreated distress in the HNC setting can be severe, affecting patient and caregiver QOL, treatment compliance, and even survival [11, 21-23]. Because HNC patients receiving RT are seen on a daily basis by medical personnel and are frequently accompanied to clinic appointments by their caregivers, unique opportunities exist in the clinical setting for the both the diagnosis and management of patient and caregiver distress [11]. However, before supportive care programs can be developed, we need to first identify the person-level factors that are associated with changes in HNC patient and caregiver distress over the course of RT and clarify the role that cross-partner effects play in this process.
Method
This study examined whether there was a time trend in individual patient-caregiver dyad member's distress levels and whether this was related to changes in: the severity of the patient's physical symptoms, the degree of burden experienced by the caregiver, and each person's partner's distress levels. We also examined whether changes in distress and the factors associated with distress differed depending on role (i.e., whether a person was a patient or caregiver). Forty-nine patient-caregiver dyads completed 6 weekly assessments over the course of RT for HNC. First, we examined patterns of distress and hypothesized that patients/caregivers would have similar patterns of change in their distress levels over time. Next, we examined over-time associations and hypothesized that increases in patient distress would be associated with increases in patient physical symptom severity, caregiver burden, and caregiver distress. We further hypothesized that increases in caregiver distress would be associated with increases in patient physical symptom severity, caregiver burden, and patient distress. Finally, because we acknowledge that, in a real world context, the variables we are examining are not likely to occur independently, we conducted an exploratory analysis whereby we simultaneously examined the effects of all the predictors so we could identify the variables that were the most strongly related to changes in HNC patient and caregiver distress over time.
Procedure
This study was approved by the Mount Sinai Institutional Review Board. Patients were identified through medical chart review and approached to participate during their routine clinic visits. Patients and caregivers were eligible if the: 1) patient was initiating RT for HNC; 2) patient had a Karnofsky [24] performance status score of 60 or more (i.e., requires occasional assistance but able to care for most personal needs); 3) patient resided with a family member who was his/her primary caregiver; 4) patient and caregiver were 18 years of age or older; 5) patient and caregiver could provide informed consent; and, 6) patient and caregiver could read and understand English.
All patients who were approached completed a brief, anonymous questionnaire that asked about their health and socio-demographics. Research staff met patients and caregivers in the clinic for 6 consecutive weeks while the patient was undergoing RT and asked them to separately complete surveys. If time did not allow this or if the caregiver was not present during a particular week, participants could complete surveys at home or by telephone. Participants received gift cards worth $5 for completing each survey.
Measures
Patient physical symptom severity
The MD Anderson Symptom Inventory – Head and Neck (MDASI-HN) measures 13 core symptoms (MDASI-CS) common to cancer patients and 9 head and neck specific symptoms (MDASI-HNS) on a 0 (=not present) to 10 (=as bad as you can imagine) scale [25]. One of the MDASI-CS items refers to feeling upset and was removed so we could have a measure that assessed physical symptoms separately from distress. Internal reliability (Cronbach's alpha) was .94 for the MDASI-CS and .93 for the MDASI-HNS.
Caregiver burden
Four items developed by Pearlin [26] were used to assess how overwhelmed family members felt with caregiving on a 4-point Likert scale, from 1=not at all to 4=completely. Cronbach's alpha was .76.
Patient and caregiver distress
The National Comprehensive Cancer Network's (NCCN) distress thermometer [27] measures distress over the past week on a 10-point scale (0=no distress to 10=extreme distress). Scores ≥ 5 suggest significant distress and the need for further psychological evaluation. In addition, the tool includes a checklist of 35 practical, physical, family, emotional, and spiritual concerns in the past week that are perceived causes of distress.
Other measures
Patients provided demographic information (e.g., age, gender, relationship to the caregiver). Medical variables (e.g., stage, treatment type, time since diagnosis) were abstracted from patients' medical records.
Data Analysis
For descriptive purposes, the average level of each of the major study variables was calculated across the available time points. Given that multiple measurements were taken on the same individuals over six weeks, we also constructed a series of linear repeated-measures multilevel models that estimated the degree to which the major study variables were associated with one another. This approach is preferable to ordinary correlational analysis because the latter ignores the fact that reports are nested within persons, which, in turn, can bias inferential tests and limit the generalizability of the results [28].
Overall, multilevel models are well-suited to repeated measures designs like the present one because they can handle missing data due to sample attrition and maximize the utility of existing data [29]. They can also estimate latent trajectories for each member of the dyad, control for interdependency of dyad-level data and the autocorrelation among repeated assessments, and permit the examination of cross-partner effects [29, 30]. To test our hypotheses, data from dyad members were treated as nested scores within the same group (i.e., patient-caregiver dyad) [29]. Using this framework, we first ran an unconditional base model of distress in dyads over time. The model examined differences in trajectories of distress over the course of RT for HNC as a function of time, role (1=patients, -1=caregivers), and the interaction between time and role. Next, we ran four separate linear mixed models to estimate changes in a person's distress as a function of time, role, and each of the predictors of interest (i.e., patient MDASI-CS and MDASI-HNS, caregiver burden, and each person's partner's NCCN distress level). In each model, all possible interactions were also examined. Finally, to test our exploratory aim, we ran a linear mixed model in which all of the predictor variables were simultaneously entered along with time, role, and their interactions. Figure 1 depicts this analysis.
Figure 1.
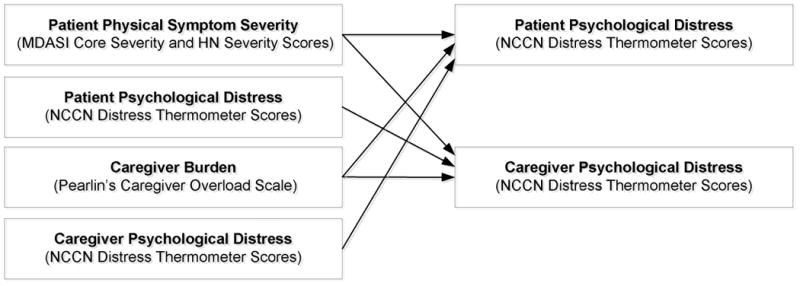
Depiction of the mixed models analysis conducted to simultaneously examine the effects of patient physical symptoms, caregiver burden, and one's partner's distress on both patient and caregiver distress over the course of radiotherapy for head and neck cancer.
All of the models that were tested included time varying predictors. There were a total of six assessment waves, so time was centered at the first week of treatment (baseline), and the Time variable was scored in weeks since baseline. Because time was centered at week 1, the intercept indicates distress at week 1, and the slope for Time represents the degree to which distress changes each week. All continuous predictors were grand mean centered across dyads and time [31]. Significant interactions were graphed using 1 SD above and below the grand mean as high and low values. Effect sizes were calculated using the formula [32].
Results
Recruitment and Participant Characteristics
Sixty-four consecutive HNC patients and their caregivers were approached. Of these, 15 (23%) declined (9 were too busy/felt too much was going on and 6 were not interested). Comparisons were made between participants and decliners based on available data for age, time since diagnosis, Karnofsky performance status, race, education, and distress thermometer scores at recruitment. The only significant difference was time since diagnosis (t=2.29, p=.03); participants had a longer timespan between initial diagnosis and time of recruitment (X̄=2 months) than those who declined (X̄=1 month). The final sample comprised 49 patient-caregiver dyads; patients completed 95%, and caregivers completed 90% of the weekly assessments. Table 1 details sample demographic and medical characteristics. All patients received Intensity-Modulated Radiation Therapy (IMRT); the majority received concurrent chemotherapy and radiotherapy (CRT; 55%) or surgery followed by CRT (26%).
Table 1. Sample Descriptives (N=49 patient-caregiver dyads).
| Patients | Caregivers | |||||
|---|---|---|---|---|---|---|
| Gender (%) | ||||||
| Male | 40 (82) | 5 (10) | ||||
| Female | 9 (18) | 44 (90) | ||||
| Age in years |
X̄=57.2, SD=11.9 (Range=35 to 82) |
X̄=53.1, SD=10.2 (Range=30 to 72) |
||||
| Race (%) | ||||||
| White (non-Hispanic) | 36 (74%) | |||||
| Caregiver Relationship to the Patient (%) | ||||||
| Spouse/Partner | 33 (68) | |||||
| Son/Daughter | 7 (14) | |||||
| Other Family Member | 9 (18) | |||||
| Married (%) | 37 (76%) | |||||
| Length of marriage in years | X̄=26.1, SD=10.7 (Range=6 to 43) | |||||
| Type of HNC (%) | ||||||
| Oral cavity | 12 (24) | |||||
| Laryngeal | 7 (14) | |||||
| Pharyngeal | ||||||
| Oropharyngeal | 24 (49) | |||||
| Nasopharyngeal | 2 (4) | |||||
| Hypopharyngeal | 1 (2) | |||||
| Nasal cavity/paranasal sinus | 3 (6) | |||||
| Stage of cancer (%) | ||||||
| 1 | 5 (10) | |||||
| 2 | 3 (6) | |||||
| 3 | 7 (15) | |||||
| 4 | 34 (69) | |||||
| Treatment received (%) | ||||||
| Radiotherapy (RT) only | 3 (6) | |||||
| Surgery followed by RT | 6 (13) | |||||
| Concurrent chemotherapy and radiotherapy (CRT) | 27 (55) | |||||
| Surgery followed by CRT | 13 (26) | |||||
| Raw means and (SDs) on the major study variables during RT | ||||||
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
| Patient Distress (0-10 scale) | 5.0 (2.4) | 5.5 (2.6) | 6.1 (2.4) | 6.1 (2.5) | 6.3 (2.2) | 5.9 (2.4) |
| Patient MDASI-CS (0-10 scale) | 3.4 (1.8) | 3.7 (1.9) | 4.2 (2.1) | 4.6 (2.0) | 5.4 (2.7) | 5.2 (2.8) |
| Patient MDASI-HNS (0-10 scale) | 3.6 (2.2) | 4.6 (2.4) | 5.2 (2.6) | 5.5 (2.8) | 6.0 (2.9) | 5.8 (3.0) |
| Caregiver Distress (0-10 scale) | 5.3 (2.4) | 6.6 (1.9) | 6.4 (1.9) | 7.2 (2.0) | 7.1 (2.6) | 6.3 (3.4) |
| Caregiver Overload (1 to 4 scale) | 2.6 ( .7) | 2.7 ( .7) | 2.7 ( .7) | 2.8 ( .7) | 2.6 ( .7) | 2.8 ( .7) |
X̄=Mean, SD=Standard Deviation
Descriptive Results
Across assessments, patients' average symptom severity scores were X̄=4.37 out of 10 (SD=2.22) on the MDASI-CS and X̄=5.11 (SD=2.75) on the MDASI-HNS. At time of recruitment, average NCCN distress scores were X̄=3.02 out of 10 (SD=.75) for patients and X̄=2.41 out of 10 (SD=.60) for caregivers. During RT, distress rose and was an average of X̄=5.80 (SD=2.40) for patients and X̄=6.46 (SD=2.43) for caregivers. In fact, patients and caregivers rated their distress at or above the NCCN cut-off score of 5 in 77% of assessments. For patients, the top 5 perceived causes of distress were sleep, pain, mouth sores, eating, and fatigue; for caregivers, they were worry, fatigue, sleep, dealing with the patient, and work.
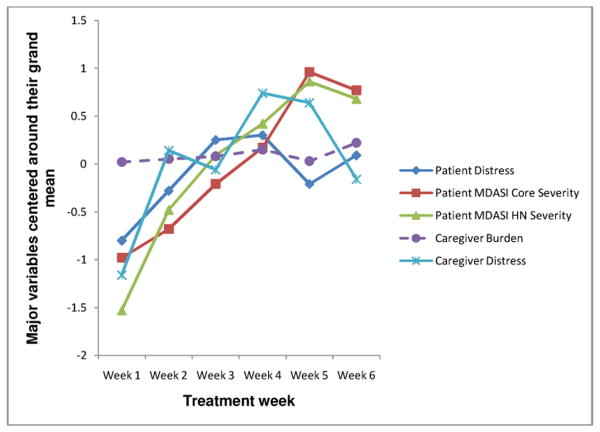
Figure 2 depicts the trajectory of symptoms, caregiver burden, and distress over the six week assessment period. Variables were grand mean centered for graphing purposes; raw means are in Table 1. Overall, physical symptom severity and distress increased steadily and peaked at week 5; caregiver burden remained relatively constant throughout radiotherapy.
Figure 2.
Trajectory of physical symptoms, caregiver burden, and patient and caregiver distress over six weeks during radiotherapy for head and neck cancer.
As Table 2 shows, associations between patient distress, patient MDASI-CS and MDASI-HNS severity, and caregiver distress ranged from moderate to high (B=0.37–0.64) over the 6 week assessment period. The only variable that was significantly associated with caregiver burden was patient MDASI-HNS severity (B=0.83).
Table 2. Results of Linear Mixed Models Analysis Showing Associations between the Major Study Variables over 6 Weeks over the Course of RT for HNC (N=49 patient-caregiver dyads).
| Major Study Variables | B1 | SE | t | Lower 95% Limit | Upper 95% Limit | |
|---|---|---|---|---|---|---|
| MDASI Core Severity | ||||||
| Intercept | 1.01 | .30 | ||||
| Time | .11 | .06 | 1.85 | -.01 | .24 | |
| MDASI HN Severity | .60 | .05 | 11.37*** | .49 | .70 | |
| Intercept | 1.48 | .47 | ||||
| Time | .26 | .08 | 3.15** | .09 | .43 | |
| Patient Distress | .37 | .07 | 5.14*** | .23 | .51 | |
| Intercept | 2.05 | .62 | ||||
| Time | .31 | .11 | 2.9** | .08 | .55 | |
| Caregiver Distress | .28 | .07 | 3.86*** | .13 | .42 | |
| Intercept | 2.24 | .94 | ||||
| Time | .33 | .13 | 2.61* | .06 | .60 | |
| Caregiver Burden | .55 | .31 | 1.76 | -.08 | 1.18 | |
| MDASI HN Severity | ||||||
| Intercept | .69 | .51 | ||||
| Time | .26 | .08 | 3.06** | .08 | .44 | |
| Patient Distress | .64 | .08 | 8.27*** | .48 | .79 | |
| Intercept | 2.04 | .79 | ||||
| Time | .32 | .11 | 2.85* | .07 | .56 | |
| Caregiver Distress | .39 | .08 | 5.00*** | .23 | .54 | |
| Intercept | 2.15 | 1.18 | ||||
| Time | .34 | .13 | 2.66* | .06 | .61 | |
| Caregiver Burden | .83 | .38 | 2.23* | .09 | 1.58 | |
| Patient Distress | ||||||
| Intercept | 8.96 | .87 | ||||
| Time | .34 | .18 | 1.92 | -.05 | .72 | |
| Caregiver Distress | -.56 | .07 | -8.24*** | -.69 | -.42 | |
| Intercept | 4.43 | 1.16 | ||||
| Time | .32 | .08 | 4.12*** | .15 | .50 | |
| Caregiver Burden | .36 | .34 | 1.06 | -.32 | 1.05 | |
| Caregiver Distress | ||||||
| Intercept | 5.27 | 1.22 | ||||
| Time | .18 | .19 | .93 | -.12 | .58 | |
| Caregiver Burden | .24 | .42 | .58 | -.60 | 1.09 |
Note.
With the exception of caregiver burden, all scales were 0-10. A one unit increase in the predictor is equal to the corresponding increase/decrease in the raw coefficient value for the outcome; B=raw coefficient, SE=standard error,
p<.05,
p<.01,
p<.001
Base Model
As Table 3 shows, there was a significant main effect for time, meaning that patients and caregivers experienced significant changes in distress over time. The role × time interaction was not significant, however, meaning that patients and caregivers had similar patterns of change in distress over time.
Table 3. Results of linear mixed models analyses showing changes in a person's distress as a function of time, role, and each of the major study variables.
| B | SE | t | Lower 95% Limit | Upper 95% Limit | Effect Size (r) | |
|---|---|---|---|---|---|---|
|
|
||||||
| Intercept | 5.42 | .47 | ||||
| Time | .22 | .09 | 2.31* | .03 | .41 | .32 |
| Role | -.14 | .28 | -.52 | -.70 | .41 | |
| Time × Role | .03 | .09 | .29 | -.15 | .21 | |
| Intercept | 6.04 | .31 | ||||
| Time | .07 | .08 | .85 | -.10 | .23 | |
| Role | -.11 | .16 | -.74 | -.42 | .19 | |
| MDASI-CS | .63 | .31 | 2.04∼ | -.02 | 1.27 | |
| Role × MDASI-CS | .55 | .16 | 3.48** | .24 | .86 | .30 |
| Intercept | 6.08 | .26 | ||||
| Time | -.02 | .06 | -.37 | -.14 | .10 | |
| Role | -.10 | .15 | -.66 | -.41 | .20 | |
| MDASI-HNS | .98 | .28 | 3.52* | .40 | 1.56 | .62 |
| Role × MDASI-HNS | .55 | .14 | 3.87** | .27 | .83 | .35 |
| Intercept | 5.73 | .56 | ||||
| Time | .26 | .09 | 2.92* | .07 | .46 | .64 |
| Role | -.04 | .17 | -.22 | -.38 | .31 | |
| Caregiver Burden | .42 | .34 | 1.23 | -.31 | 1.14 | |
| Role × Caregiver Burden | .22 | .17 | 1.26 | -.13 | .56 | |
| Intercept | 5.34 | .75 | ||||
| Time | .32 | .15 | 2.17∼ | -.02 | .65 | |
| Role | .13 | .13 | .99 | -.13 | .38 | |
| One's Partner's Distress | -.99 | .34 | -2.88* | -1.74 | -.24 | .64 |
| Role × One's Partner's Distress | .24 | .14 | 1.70 | -.04 | .52 | |
Note: MDASI-CS=Patient MDASI Core Symptom Severity Score; MDASI-HNS=Patient MDASI Head and Neck Symptom Severity Score;
p<.10,
p<.01,
p<.001
Hypothesis Testing
We ran four separate linear mixed models with repeated measures to estimate changes in a person's distress as a function of time, role, and each of our predictors of interest. Due to the small sample size, we were limited in the number of higher-order interactions that we could examine. In addition, there were no significant demographic or medical variables that were significant predictors of patient or caregiver distress, so these variables were not included in the final models.
Patient symptom severity
As Table 3 shows, the role X MDASI-CS and role X MDASI-HNS severity interactions were significant. As Figure 3 and tests of the simple slopes for the role X MDASI-CS interaction show, patient distress increased significantly as a function of symptom severity (t=3.93, p=.001), but caregiver distress started out high (over the NCCN cutoff of 5) and remained elevated, regardless of patient symptom severity (t=.19, p=.85). Figure 3 and tests of the simple slopes for the role X MDASI-HNS interaction also show that patient distress increased steadily as a function of HNS symptom severity (t=3.99, p=.001). Caregiver distress also started out high (≥ 5) and increased as patient HNS symptom burden increased (t=3.32, p=.005).
Figure 3.
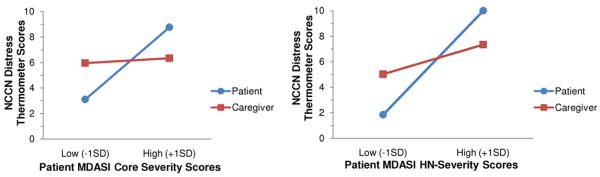
Linear mixed models analysis showing patient and caregiver distress as a function of patient physical symptom severity (MDASI core and head and neck severity subscales).
Caregiver burden
As Table 3 shows, there were no significant main effects or interactions for caregiver burden.
Cross-partner effects of distress
As Table 3 shows, the role X partner distress interaction was not significant, but there was a significant main effect suggesting that, on average, when one dyad member's distress increased, the other dyad member's distress decreased (B=-0.99, p=0.01).
Exploratory Analysis
As Table 4 shows, only patient MDASI-HNS scores (B=2.60, p<.001) and one's partners' distress levels (B=-1.65, p<.001) significantly predicted distress for patients and caregivers.
Table 4. Results of linear mixed models analysis showing changes in a person's distress as a function of time, role, and the major study variables.
| B | SE | t | Lower 95% Limit | Upper 95% Limit | Effect Size (r) | |
|---|---|---|---|---|---|---|
|
|
||||||
| Intercept | 6.27 | .49 | ||||
| Time | -.01 | .12 | -.12 | -.29 | .27 | |
| Role | -.03 | .10 | -.37 | -.23 | .16 | |
| MDASI-CS | .18 | .32 | .57 | -.45 | .82 | |
| MDASI-HNS | 2.60 | .35 | 7.34* | 1.90 | 3.30 | .53 |
| Caregiver Burden | -.17 | .21 | -.81 | -.56 | 014 | |
| Partner's Distress | -1.65 | .18 | -9.11* | -2.01 | -1.29 | .70 |
| Role × MDASI-CS | .01 | .21 | .06 | -.41 | .43 | |
| Role × MDASI-HNS | .02 | .20 | .12 | -.38 | .43 | |
| Role × Caregiver Burden | .06 | .10 | .32 | -.14 | .27 | |
| Role × Partner's Distress | .25 | .21 | 1.22 | -.16 | .67 | |
Note: MDASI-CS=Patient MDASI Core Symptom Severity Score; MDASI-HNS=Patient MDASI Head and Neck Symptom Severity Score;
p<.001
Discussion
This study examined the associations between weekly changes in physical symptom severity, caregiver burden, and distress in patient-caregiver dyads where the patient was undergoing RT for HNC. Consistent with our hypothesis as well as findings from other dyadic studies [33, 34], patients and caregivers had similar patterns of changes in distress over time. However, analysis of the raw means showed that caregivers were consistently more distressed than patients over the course of RT which underscores the need to address caregiver distress in the clinical setting.
Our findings highlight the idea that the process of undergoing RT for HNC is a dyadic stressor that has implications for both patients and caregivers. Specifically, increases in patient-rated head and neck specific physical symptoms had a significant time-varying effect on both patient and caregiver distress. Programs that teach strategies that patients and caregivers can use to control and manage the patient's physical symptoms may thus help to alleviate both dyad members' distress.
Our findings also highlight the importance of considering both the role that individual or person-level factors play in one's distress as well as how dyad members affect each other's distress [20]. We found that, regardless of role, increases in one dyad member's distress levels were associated with decreases in the other dyad member's distress levels on average. Unlike theories of emotional contagion which posit that when individuals in close relationships are faced with a common threat, the distress levels of one partner are likely to spill over and increase the other partner's distress [19, 35], our findings are more consistent with a dyadic coping perspective that posits when one partner senses and recognizes the other's distress, he/she also recognizes the need to assist him/her in coping to restore balance and alleviate that distress [20]. Toward that end, he/she may actively manage his/her own distress knowing that it is in the best interest of his/her own well-being as well as the well-being of his/her partner. This may explain why the vast majority of studies in cancer have found that even though high rates of distress exist for cancer patients and their spousal caregivers, the correlation between their distress levels is only low to moderate at best [36, 37]. An alternate explanation is that patients and caregivers cited different stressors as being the cause of their distress on the NCCN distress thermometer problem list and it is possible that these stressors come up at different times over the course of RT.
This study had some limitations. First, the sample size was small. Although, the number of repeated assessments increases power in a way that adding more dyads would not [38], replication of findings in a larger sample is needed. Indeed, it is possible that the small sample size and broad inclusion criteria with regard to stage and treatment contributed to the finding that these variables did not contribute to patient or caregiver distress. Second, we did not collect data regarding medical intervention for symptom management. It is possible that the high levels of physical symptoms experienced by patients at week 5 was sufficient to warrant medical intervention and that the achievement of better symptom control resulted in the slight drop in distress the following week. It is also possible that the decline in patient and caregiver distress at week 6 was caused by knowing that RT was nearly over and they were nearing the recovery phase. Third, participants had a longer amount of time pass between their diagnosis and treatment (2 months) than those who declined participation and 23% of those who were approached for participation declined, which may represent a potential selection bias. Although no other significant differences between participants and decliners were found, it is possible that having a longer amount of time to acclimate to a cancer diagnosis made some people more receptive to participating in a research study and that the lack of significant differences between the two groups was due to the small sample size. Finally, the distress thermometer is primarily a distress screening instrument. Although recent work in breast cancer has shown that moderately to severely distressed patients have significantly lower quality of life than those with mild distress and that the distress thermometer provides a quick and easy screening tool to alert the healthcare team to clinically relevant alterations in patients' quality of life [39], this has not yet been demonstrated in caregivers or in the context of HNC.
This study also had a number of strengths. Few studies have examined associations between physical symptoms and patient and caregiver distress using appropriate methodologies. We employed multilevel modeling to control for interdependencies in the data and we analyzed reciprocal interactions between patients and caregivers. Additionally, we went beyond existing research to examine both aggregate and time-varying effects of patient physical symptoms on distress using two different indicators of physical symptom severity (i.e., MDASI-CS and HNS). Participants were also recruited at a similar time in the illness trajectory, making changes over time more meaningful. Overall, our findings suggest similar patterns of prediction for patient and caregiver distress and further our understanding of the impact of increasing physical symptom severity on distress over the course of RT for HNC.
In conclusion, our findings suggest that worsening physical symptoms in the patient over the course of RT have important psychological implications for both patients and their caregivers. This has clinical implications and underscores the importance of screening for patient distress and extending distress screening to family caregivers. Finally, our findings underscore the need for supportive care interventions that take a dyadic approach to managing HNC symptoms and that address both patient and caregiver distress during RT.
Highlights.
We examined trajectories of patient/caregiver distress during head and neck cancer radiotherapy.
We also examined how patients and caregivers influence each other's distress.
Changes in patient symptoms had a time-varying effect on both patient and caregiver distress.
Increases in one person's distress levels were associated with decreases in the other's distress.
Acknowledgments
Special thanks to Turu Stadler, PhD, who provided feedback on an earlier draft of this manuscript.
Dr. Badr's work was supported by a career development award from the National Cancer Institute, K07 CA124668 and an ARRA career development award supplement K07 CA124668-S1.
Footnotes
Conflict of Interest Statement. None of the authors have any actual or potential conflicts of interest including any financial, personal, or other relationships with other people or organizations within that could inappropriately influence their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bultz BD, Carlson LE. Emotional Distress: The Sixth Vital Sign in Cancer Care. J Clin Oncol. 2005;23:6440–1. doi: 10.1200/JCO.2005.02.3259. [DOI] [PubMed] [Google Scholar]
- 2.Zeller J. High suicide risk found for patients with head and neck cancer. JAMA. 2006;296:1716–7. doi: 10.1001/jama.296.14.1716. [DOI] [PubMed] [Google Scholar]
- 3.Zabora J, Brintzenhofeszoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 5.Duffy SA, Ronis DL, Valenstein M, Lambert MT, Fowler KE, Gregory L, et al. A Tailored Smoking, Alcohol, and Depression Intervention for Head and Neck Cancer Patients. Cancer Epidemiol Biomarkers Prev. 2006;15:2203–8. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 6.Kangas M, Henry J, B R. Predictors of posttraumatic stress disorder following cancer. Health Psychol. 2005;24:579–85. doi: 10.1037/0278-6133.24.6.579. [DOI] [PubMed] [Google Scholar]
- 7.Lewis S, Salins N, Kadam A, Rao R. Distress screening using distress thermometer in head and neck cancer patients undergoing radiotherapy and evaluation of causal factors predicting occurrence of distress. Indian journal of palliative care. 2013;19:88. doi: 10.4103/0973-1075.116703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.List MA, Ritter-Sterr C, Lansky S. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–9. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.List MA, Stracks J. Evaluation of quality of life in patients definitively treated for squamous carcinoma of the head and neck. Curr Opin Oncol. 2000;12:215–20. doi: 10.1097/00001622-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Silverman S., Jr Oral cancer: complications of therapy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1999;88:122–6. doi: 10.1016/s1079-2104(99)70103-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen AM, Jennelle RL, Grady V, Tovar A, Bowen K, Simonin P, et al. Prospective study of psychosocial distress among patients undergoing radiotherapy for head and neck cancer. International Journal of Radiation Oncology* Biology* Physics. 2009;73:187–93. doi: 10.1016/j.ijrobp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Vickery L, Latchford G, Hewison J, Bellew M, Feber T. The impact of head and neck cancer and facial disfigurement on the quality of life of patients and their partners. Head Neck. 2003;25:289–96. doi: 10.1002/hed.10206. [DOI] [PubMed] [Google Scholar]
- 13.Drabe N, Zwahlen D, Buchi S, Moergeli H, Zwahlen RA, Jenewein J. Psychiatric morbidity and quality of life in wives of men with long-term head and neck cancer. Psychooncology. 2008;17:199–204. doi: 10.1002/pon.1199. [DOI] [PubMed] [Google Scholar]
- 14.Zwahlen RA, Dannemann C, Gratz KW, Studer G, Zwahlen D, Moergeli H, et al. Quality of life and psychiatric morbidity in patients successfully treated for oral cavity squamous cell cancer and their wives. Journal of Oral and Maxillofacial Surgery. 2008;66:1125–32. doi: 10.1016/j.joms.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Ross S, Mosher C, Ronis-Tobin V, Hermele S, Ostroff J. Psychosocial adjustment of family caregivers of head and neck cancer survivors. Support Care Cancer. 2010;18:171–8. doi: 10.1007/s00520-009-0641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babin E, Sigston E, Hitier M, Dehesdin D, Marie J, Choussy O. Quality of life in head and neck cancers patients: predictive factors, functional and psychosocial outcome. European Archives of Oto-Rhino-Laryngology. 2008;265:265–70. doi: 10.1007/s00405-007-0561-0. [DOI] [PubMed] [Google Scholar]
- 17.Hagedoorn M, Sanderman R, Bolks H, Tuinstra J, Coyne JC. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychol Bull. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Milbury K, Badr H, Carmack C. The role of blame in the psychosocial adjustment of couples coping with lung cancer. Ann Behav Med. 2012;44:331–40. doi: 10.1007/s12160-012-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatfield E, Cacioppo J, Rapson R. Emotional contagion. New York, NY: Cambridge University Press; 1994. [Google Scholar]
- 20.Bodenmann G. A systemic-transactional view of stress and coping in couples. Swiss Journal of Psychology. 1995;54:34–49. [Google Scholar]
- 21.Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen Hosp Psychiatry. 2006;28:396–402. doi: 10.1016/j.genhosppsych.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Graeff Ad, Rob J, Leeuw Jd, Ros WJG, Hordijk GJ, Blijham GH, et al. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head & Neck. 2000;22:398–407. doi: 10.1002/1097-0347(200007)22:4<398::aid-hed14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.McDonough EM, Boyd JH, Varvares MA, Maves MD. Relationship between psychological status and compliance in a sample of patients treated for cancer of the head and neck. Head & Neck. 1996;18:269–76. doi: 10.1002/(SICI)1097-0347(199605/06)18:3<269::AID-HED9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139–M44. doi: 10.1093/geronj/46.4.m139. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal DI, Chambers MS, Mendoza TR, Asper JA, Kies MS, Weber RS, et al. The reliability and validity of the M. D. Anderson Symptom Inventory (MDASI-HN) as a measure of symptom burden in the head and neck cancer (HNC) patient population. J Clin Oncol (Meeting Abstracts) 2005;23:8097. [Google Scholar]
- 26.Pearlin L, Mullan J, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist. 1990;30:583–91. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 27.National Comprensive Cancer Network (NCCN) Distress: Treatment guidelines for patients. American Cancer Society; 2005. [Google Scholar]
- 28.Jaccard J, Wan C. Statistical analysis of temporal data with many observations: Issues for behavioral medicine data. Ann Behav Med. 1990;15:41–50. [Google Scholar]
- 29.Kenny D, Kashy DA, Cook D. Dyadic data analysis. New York: Guilford; 2006. [Google Scholar]
- 30.Raudenbush SW, Bryk A, Congdon R. Hierarchical linear and nonlinear modeling. Lincolnwood, IL: SSI; 2004. [Google Scholar]
- 31.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. London: Sage; 1991. [Google Scholar]
- 32.Snijders T, Bosker R. Multilevel analysis. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- 33.Pruchno R, Wilson-Genderson M, Cartwright F. Self-rated health and depressive symptoms in patients with end-stage renal disease and their spouses: A longitudinal dyadic analysis of late-life marriages. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;64:212–21. doi: 10.1093/geronb/gbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Townsend AL, Miller B, Guo S. Depressive Symptomatology in Middle-Aged and Older Married Couples A Dyadic Analysis. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2001;56:S352–S64. doi: 10.1093/geronb/56.6.s352. [DOI] [PubMed] [Google Scholar]
- 35.Gump BB, Kulik JA. Stress, affiliation, and emotional contagion. J Pers Soc Psychol. 1997;72:305. doi: 10.1037//0022-3514.72.2.305. [DOI] [PubMed] [Google Scholar]
- 36.Carmack Taylor CL, Badr H, Lee L, Pisters K, Fossella F, Gritz ER, et al. Lung cancer patients and their spouses: Psychological and relationship functioning within 1 month of treatment initiation. Ann Behav Med. 2008;36:129–40. doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: a meta-analysis and critical review of role and gender effects. Psychol Bull. 2008;134:1. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Raudenbush SW, Xiao-Feng L. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychol Methods. 2001;6:387. [PubMed] [Google Scholar]
- 39.Head B, Schapmire T, Keeney C, Deck S, Studts J, Hermann C, et al. Use of the Distress Thermometer to discern clinically relevant quality of life differences in women with breast cancer. Quality of Life Research. 2012;21:215–23. doi: 10.1007/s11136-011-9934-3. [DOI] [PubMed] [Google Scholar]