Abstract
Failure of the hypothalamus-pituitary-adrenal (HPA) axis to habituate to repeated stress exposure is related with adverse health outcomes, but our knowledge of predictors of non-habituation is limited. Rumination, defined as repetitive and unwanted past-centered negative thinking, is related with exaggerated HPA axis stress responses and poor health outcomes. The aim of this study was to test whether post-stress rumination was related with non-habituation of cortisol to repeated stress exposure. Twenty-seven participants (n=13 females) were exposed to the Trier Social Stress Test (TSST) twice on consecutive afternoons. Post-stress rumination was measured after the first TSST, and HPA axis responses were assessed by measuring salivary cortisol 1 minute before, and 1, 10, 20, 60, and 120 minutes after both TSSTs. Stress exposure induced HPA axis activation on both days, and this activation showed habituation indicated by lower responses to the second TSST (F= 3.7, p=.015). Post-stress rumination after the first TSST was associated with greater cortisol reactivity after the initial stress test (r = 0.45, p < 0.05) and with increased cortisol responses to the second TSST (r = 0.51, p < 0.01), indicating non-habituation, independently of age, sex, depressive symptoms, perceived life stress, and trait rumination. In summary, results showed that rumination after stress predicted non-habituation of HPA axis responses. This finding implicates rumination as one possible mechanism mediating maladaptive stress response patterns, and it might also offer a pathway through which rumination might lead to negative health outcomes.
Keywords: acute stress, habituation, rumination, hypothalamic-pituitary-adrenal axis, cortisol, perseverative cognition
3 Introduction
Psychosocial stress has been shown to be predictive of negative health outcomes over time (Cohen, Janicki-Deverts, & Miller, 2007). In addition to being exposed to long-term, chronic stress, or to single traumatic experiences, individuals are exposed to daily occurrences of potentially stressful experiences. Acute stress causes biological responses, such as hypothalamic-pituitary adrenal (HPA) axis and sympathetic nervous system (SNS) activation (Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012). While most investigations of stress responses involve exposing participants to novel, one-time stressors, people in everyday life experience similar or identical stressors many times over. This makes it important to examine responses to repeated stressors. In particular, the extent to which individuals are able to habituate (i.e. show decreased physiological activation) in response to repeated stressors may have implications for long term health (e.g., (McEwen & Lasley, 2003). Therefore, understanding determinants of habituation vs. non-habituation is necessary to understand interindividual differences in the health effects of everyday stress. One factor that might explain non-habituation of stress responses is rumination. Rumination, defined here as negative and unwanted past-centered repetitive thoughts, may be particularly likely to exacerbate the physiological stress response because it could interfere with healthy post-stress emotional and cognitive processing of a situation (Takano & Tanno, 2009; Teasdale, 1999) and thereby inhibit habituation across repeated stressors. Although rumination has been studied in relation to initial cortisol responses to stress, no studies have examined rumination and cortisol responses to repeated stress.
Acute psychosocial stress stimulates the two major stress response systems, the SNS and the HPA axis, resulting in increases of stress hormones epinephrine and norepinephrine as well as cortisol. When an acute stressor is repeated, the majority of individuals show habituation of the HPA axis (Epel et al., 2000; Kirschbaum et al., 1995; Kudielka et al., 2006; Schommer, Hellhammer, & Kirschbaum, 2003; Wüst, Federenko, van Rossum, Koper, & Hellhammer, 2005). Of note, habituation is not seen in the SNS and other stress responsive systems (Schommer et al., 2003; von Kanel, Kudielka, Preckel, Hanebuth, & Fischer, 2006).
HPA axis habituation is thought to generally be adaptive (McEwen, 2003, 2004; McEwen & Lasley, 2003). The allostatic load model puts forth the idea that activation of stress systems that is either sustained or not balanced or counter-acted by another system can put wear and tear on dependent systems. This has been shown to lead to negative health consequences (McEwen & Lasley, 2003). For example, an allostatic load index has been shown to be predictive of long-term health (Juster, McEwen, & Lupien, 2010).
In line with this theory, inter-individual differences in habituation have been found to be related cross-sectionally with reduced psychosocial and biomedical health. Kirschbaum et al. (1995) found lower self-esteem and higher depressed mood in a subgroup of male participants who did not show HPA axis habituation to repeated laboratory stress (Kirschbaum et al., 1995). Furthermore, self-report measures of vital exhaustion have also been related to reduced habituation to stress over time (Kudielka et al., 2006). Additionally, women with greater central fat showed sensitization, rather than habituation, of the cortisol response to repeated acute stress (Epel et al., 2000). Finally, while genetic factors were not found to be related with cortisol response patterns to repeated stress (Wüst et al., 2005), individuals in an active episode of major depression displayed non-habituation, while healthy controls habituated (Morris & Rao, 2014).
One factor that could potentially explain why some individuals do not habituate might be rumination. Rumination is a well-studied psychological construct that is predictive of future depression occurrence (Nolen-Hoeksema, 2000; Robinson & Alloy, 2003), and has been correlated with altered stress responses (Brosschot, Gerin, & Thayer, 2006). Broadly defined, rumination consists of ‘past-centered negative, unwanted and persistent thoughts’, and has components of emotional upset, anger, and depression (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008; Segerstrom, Tsao, Alden, & Craske, 2000). Rumination is distinct from worry, as it is past-oriented while worry is considered to be future-centric. Rumination can be viewed as a ‘response-style’; that is, when presented with a stressful experience, people may tend to ruminate about it, as opposed to engaging in another coping style such as distracting themselves from the stressor (Nolen-Hoeksema, Morrow, & Fredrickson, 1993). Generally, rumination is considered to be a maladaptive response to stress, and it has been related to many negative psychological outcomes. For example, rumination has been shown to be a precursor of future depression (Nolen-Hoeksema, 2000; Robinson & Alloy, 2003). It has often been shown that women ruminate more than men, a finding that may help to explain sex discrepancies in depression rates (Strauss, Muday, McNall, & Wong, 1997). According to the perseverative cognition theory, rumination can have prolonged physiological consequences, such as post-stress heart rate elevations (Brosschot et al., 2006). Recent work has shown that higher levels of rumination are related to non-adaptation of heart rate responses to repeated stress (Johnson, Lavoie, Bacon, Carlson, & Campbell, 2012). A recent review paper indicated that both trait and state-based rumination measures are related to heightened cortisol responses to a variety of different stressors (Zoccola & Dickerson, 2012). Trait-rumination measures have been found to be correlated with both increases in cortisol stress responses in some studies (Zoccola, Quas, & Yim, 2010) and decreases in others (Zoccola, Dickerson, & Zaldivar, 2008).Other work has failed to find any relationship between trait rumination and acute stress responses (Young & Nolen-Hoeksema, 2001), suggesting a complex relationship between trait-based rumination and acute stress responses that may be very dependent on how trait rumination is conceptualized and measured.
Post-stress state rumination measures have been generally related to increased cortisol responses to acute stress (Zoccola et al., 2008; Zoccola et al., 2010), and as shown by a meta-analyses of more than 60 acute stress studies, cortisol responses were significantly higher in context that were related with repetitive thoughts and brooding (Denson et al., 2009). However, it should be noted that some factors, such as physical activity levels, have been shown to moderate this relationship (Puterman et al., 2011). It has also been shown that experimentally inducing rumination increases cortisol responses (Byrd-Craven, Granger, & Auer, 2011; Zoccola, Figueroa, Rabideau, Woody, & Benencia, 2014). The relationship between rumination and cortisol adaptation to repeated stress is so far unknown.
In summary, an individual's response to repeated acute stress, in particular the ability to show HPA axis habituation, might have important implications for long-term health outcomes. Several psychosocial and biomedical factors have been found related with HPA axis non-habituation, for example major depressive disorder. Since rumination is a cognitive response style that has been shown to be an antecedent of depression, we hypothesized that post-stress rumination might be a predictor of HPA axis non-habituation. However, no studies have been published relating post-stress rumination with cortisol response patterns across multiple stressors. Therefore, the main aim of the present study was to test the hypothesis that post-stress rumination would predict increased cortisol reactivity to a repeated psychosocial stressor. To test this, participants were exposed to a laboratory stress task on two consecutive days. State rumination, as well as psychological trait variables and basic demographic data were assessed using self-reports. HPA axis responses to both stress exposures were assessed by measuring salivary cortisol repeatedly before and after stress
4 Methods
4.1 Participants
The study sample included healthy adults from the Greater Boston area and the Brandeis University campus who were recruited via newspaper, magazine, and Facebook advertisements. Data for this article were collected as part of a larger research project conducted over two years. All participants underwent a brief medical and psychological screening by telephone before testing and were invited to participate only if they met a specific selection criteria: a) body mass index (BMI) within the reference range between 18 and 35 kg/m2; b) luteal phase of menstrual cycle at time of participation, for women; c) absence of psychiatric, endocrine, or cardiovascular diseases, or other specific chronic diseases; d) no intake of psychoactive drugs, beta-blockers, gonadal steroids (hormonal contraceptives), GCs; e) non-smoker, and e) no previous experience with the stress protocol.
The final sample consisted of 27 participants (48% female: BMI range = 18-35 kg/m2, M=24.1 kg/m2) in two age groups (10 younger adults, ages 18-22; 17 older adults, ages 50-65). Because salivary cortisol reactivity differs across different phases of the menstrual cycle all female participants were contraceptive-free, reported normal and consistent menstrual cycles, and were tested during the luteal phase of their cycle. Sixty-seven percent of participants identified as White or Caucasian American, 11% as Asian or Asian American, 7.4% as Black or African American, and 14.6% as Other/More than one race.
4.2 Procedures
Eligible participants were invited to the laboratory on two consecutive afternoons, between 13:30 and 18:30 to control for circadian variation of stress hormones. Upon arrival to the first session, procedures were described in detail and written informed consent was obtained. Afterwards, participants were seated comfortably in a private testing room for 45 minutes while providing basic demographic and health information. At the end of this period, the first saliva sample was collected (described below). Then, participants were exposed to the standardized psychosocial stress situation Trier Social Stress Test (TSST). The TSST is a psychosocial stress procedure that mainly consists of a simulated job interview and mental arithmetic task while standing in front of an evaluative panel, microphone, and video camera, lasting approximately 15 minutes. After the conclusion of the TSST, participants returned to their private testing room and filled out state measures (described below). Additional saliva samples were taken immediately after the TSST, as well as 10, 30, 60, and 120 minutes post-stress. Participants returned to the laboratory at the same time on the following day and underwent similar procedures. The repeated TSST was modified to include a different job interview and the math task was modified to included different numbers. Identical state-based questionnaires were completed on both the initial and subsequent days of testing.
4.3 Measures
State rumination
Post stress rumination was assessed 10 minutes after stress on both study days using an adapted post-event rumination scale (Abbott & Rapee, 2004; Edwards, Rapee, & Franklin, 2003). In this questionnaire participants rated how often since their speech they had experienced eighteen different negative thoughts such as “I made a fool of myself” or “I should have chosen a different topic” on a 5-point Likert scale (from “never” to “very often”). Nine positive items, such as “My speech was good” were reverse-coded and a sum score was computed (Dannahy & Stopa, 2007). Higher scores on this questionnaire reflected increased post-stress emotional rumination specific to the TSST. The questionnaire showed good internal consistency (day 1: α = .88, day 2: α = .90).
Trait rumination
During the initial resting period all participants completed self-report measures of trait rumination via the Rehearsal subscale of the Revised Emotion Control Questionnaire (ECQ2-R; (Roger & Najarian, 1989). The ECQ-R measured participants' tendency to rehearse emotionally distressing events, specifically in regards to anger, fear, and general distress rumination. In this questionnaire, participants are asked to indicate how often they tend to ruminate using 14 True/False statements. Nine items assessed ruminative tendencies, such as ‘I often find myself thinking over and over about things that have made me angry’ or ‘I never forget people making me angry or upset, even about small things’ (Nolen-Hoeksema et al., 1993). Five reverse coded items were included (e.g.,‘Thinking about upsetting things just seems to keep them going, so I try to put them out of my mind’). The items were summed to create a total trait rumination score. The trait rumination measure had good infternal consistency (α = .90).
Chronic stress and depressive symptoms
Additional measures were obtained in order to assess the general psychological well-being of the participants. To assess chronic stress over the past month we used the 10-item Perceived Stress Scale (PSS)(Cohen, Kamarck, & Mermelstein, 1983). Items on this scale included direct questions about how often a person has experienced stress in the past month, for example, ‘In the last month, how often have you found that you cannot cope with all the things you have to do?.’ This scale had good internal consistency (α =.91). To assess depressive symptoms, the 20-item Center for Epidemiological Studies Depression scale (CES-D; (Radloff, 1977) was used. The CES-D is designed to measure depressive symptoms in the general healthy population by asking participants how often in the past week they experienced symptoms such as ‘I had crying spells’ and ‘I felt sad’ (α =.94).
4.4 HPA Axis Stress Responses
HPA axis responses to stress were assessed by measuring salivary cortisol. Saliva was collected using the Salivette collection system (Sarstedt, Newton, NC, USA). Samples were kept at room temperature until the conclusion of the session and then stored at -30°C until later analysis. Prior to analysis, salivettes were thawed and centrifuged at 2000g and 4°C for ten minutes. Free cortisol concentrations in saliva were measured using commercial chemiluminescence immunoassay (CLIA; IBL-International, Toronto, Canada). All samples were assayed in duplicate. Intra- and inter-assay CVs were below 10%.
4.5 Statistical Analyses
Data were analyzed using SPSS 21 Statistical Software (IBM, Chicago IL). All reported results were considered to be significant at the p ≤ 0.05 level, and were considered a trend at the p ≤ 0.10 level. All data were tested for normality prior to analyses using Kolmogorov-Smirnov and Shapiro-Wilks tests in addition to Q-Q plots. No transformations of the data were required. In the case of a violation of the sphericity assumption, corrections by the Greenhouse-Geisser procedure were applied (Greenhouse & Junker, 1992; Vasey & Thayer, 1987). To analyze HPA axis responses and habituation, we computed analyses of variance (ANOVA) for repeated measures with the within-subjects factors day (day 1 vs. day 2) and time (six time points for cortisol sampling, -1, +1, 10, 20, 60, and 120 minutes relative to the TSST), and we also computed separate repeated measures ANOVAs to individually test for cortisol responses on the two study days separately. To control for effects of participants' age and sex these variables were added as covariates to the repeated measures ANCOVAs. We further computed response indices as delta scores using increases from baseline to peak on both study days. T-tests were used to determine differences between peak responses on the two testing sessions. Pearson correlations were computed to analyze the relationship between state rumination measures and cortisol responses. Furthermore, linear regression was used to control for the effects of variables such as depressive symptoms on the relationship between state rumination and cortisol responses.
5 Results
5.1 Preliminary Analyses
We first tested if post stress rumination on either of the two days of testing was related with trait rumination, and with general measures of psychological well-being. Post-stress rumination after initial stress exposure was related with more self-reported depressive symptoms (r = 0.57, p = 0.003), and more perceived stress (r = 0.49, p=0.01). State rumination on day one was further positively related with trait rumination (r = 0.55, p = 0.003). None of the trait measures were significantly related with cortisol responses to the first stress exposure (highest r = 0.30, lowest p = 0.14), or the second stress exposure (highest r = 0.33, lowest p = 0.10). We further found that post stress rumination after second-time stress exposure was related with more self-reported depressive symptoms (r = 0.63, p = 0.003), more perceived stress (r = 0.43, p = 0.049), and greater trait rumination (r = 0.55, p = 0.012). Additionally, state rumination after initial stress exposure was related with state rumination after the second stressor (r = 0.81, p <.001). There were no significant sex differences in any of the trait measures and in post-stress rumination either of the two days of testing (all p > 0.29).
5.2 HPA Axis Responses to Repeated Stress
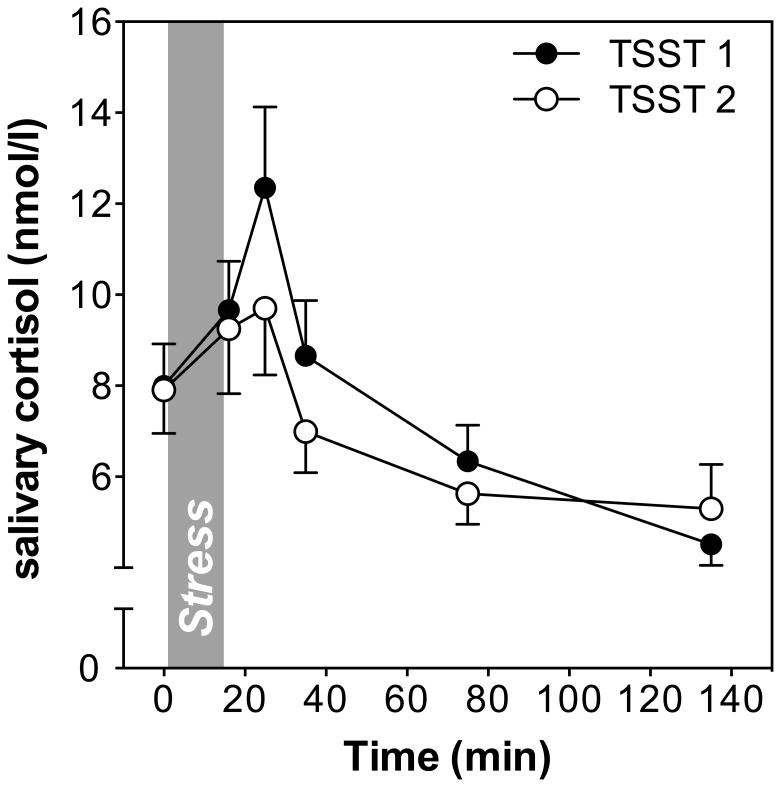
Exposure to the TSST elicited a robust salivary cortisol response to initial stress exposure (time effect on first study day: F1.6,39.8 = 17.4, p<0.001), and to repeated stress exposure on the second study day (time effect: F2.5, 65.9 = 8.8, p<0.001; see Figure 1). Time effects did not change on either of the days when controlling for age and sex. There was a marginally significant time by sex interaction on day 1 (F1.6, 36.8 = 2.59; p<0.10), but not on day 2 (F2.5, 59.6 = 1.35; p=0.27). Age was not related with cortisol responses to stress on either day (both F < 1.6; p > 0.20).
Figure 1.
Salivary cortisol responses to the Trier Social Stress Test (TSST) on two consecutive days. Mean cortisol concentrations (± SEM) are shown at baseline, as well as 1, 10, 30, 60, and 120 min after stress.
To test for habituation of HPA axis responses to repeated stress, we computed a repeated measures ANOVA including cortisol values on both study days. Results revealed a significant day by time interaction indicating differences in cortisol responses to the first and second stress exposure (F3.0,75.5 = 3.7, p=0.015). Adding sex and age as covariates did not change the significance of this day by time interaction, but we found a significant day by time by age interaction (F3, 69 = 3.3; p=0.027), indicating age differences in responses to repeated stress. More specifically, younger participants showed higher stress responses than older participants to the first TSST, while responses to the second TSST were similar. No effects of or interactions with sex were found. The overall pattern of habituation was further supported by a marginally significant t-test comparing delta scores as response indices (cortisol change from baseline to peak) computed for the first vs. the second study day (t26= 1.9, p= 0.056). On average, participants showed lower cortisol increases on the second day of testing, indicating habituation (increase in response to initial exposure = 4.45 ± 7.47 nmol/l, increase in response to repeated exposure = 2.34 ± 6.3 nmol/l).
5.3 Post-Stress Rumination and HPA Axis Responses
To address whether post-stress rumination would be related with cortisol baselines, we computed correlations between mean cortisol values immediately prior to the TSST and post-stress state rumination on both days. State rumination on day 1 correlated with day 1 baseline cortisol (r=.38. p=.046). However, state rumination on day 1 did not predict cortisol baselines on day 2 (r=-.13, p=n.s.). State rumination on day 2 was not correlated with either baseline (highest r=.03, lowest p=.14).
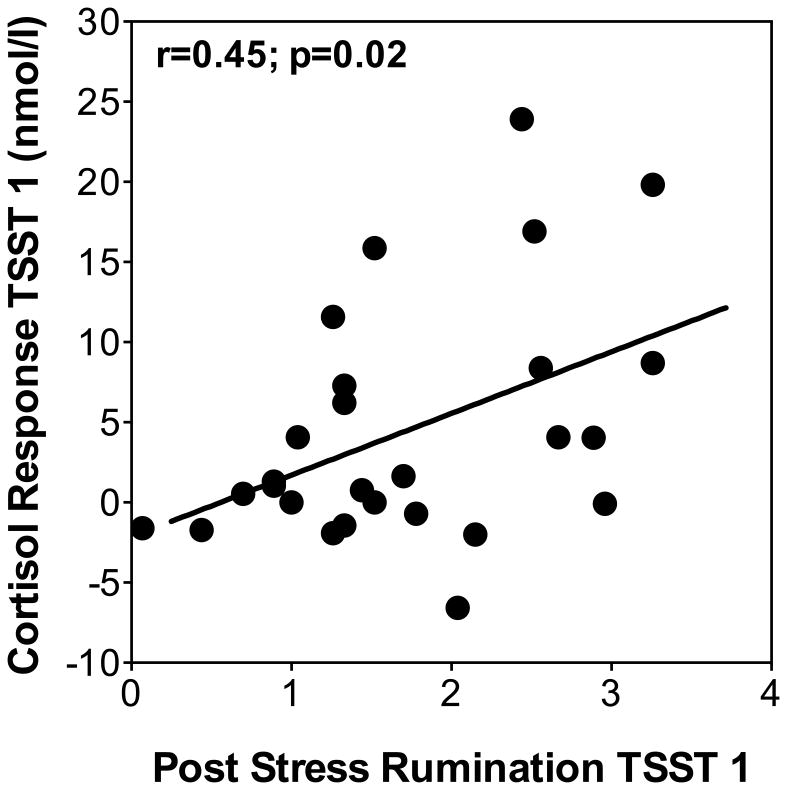
To address the hypotheses that post-stress rumination would be related with cortisol reactivity to initial stress exposure, we first computed a Pearson correlation. We found that post-stress rumination after the first TSST was related with higher cortisol increases (from baseline to peak) on the same day (r = 0.45, p = 0.02; see Figure 2). In order to control for trait rumination and other psychological factors, we computed separate linear regressions predicting cortisol responses from baseline to peak to the first TSST by state rumination. We controlled for depressive symptoms, perceived stress, and trait rumination. Post-stress rumination remained significant when controlling for sex and age (state rumination: beta = 0.45; p=0.029), depressive symptoms (state rumination: beta = 0.44; p = 0.07), and perceived stress (state rumination: beta = 0.50; p = 0.025) and marginally significant when controlling for trait rumination (state rumination: beta = 0.47; p = 0.051). None of the control variables except for sex explained additional variance of day 1 cortisol responses, with female sex predicting lower responses (sex: beta = –0.45; p = 0.021). We also found that state rumination positively correlated with mean cortisol levels at each time point after the rumination measure was taken, indicating a relationship between post-stress rumination and cortisol levels 20, 60, and 120 minutes after the TSST (lowest r = .56, highest p = .005).
Figure 2.
Scatterplot showing the relationship between post-stress rumination measured after TSST 1 with cortisol response (max. increase) to initial stress exposure (TSST 1).
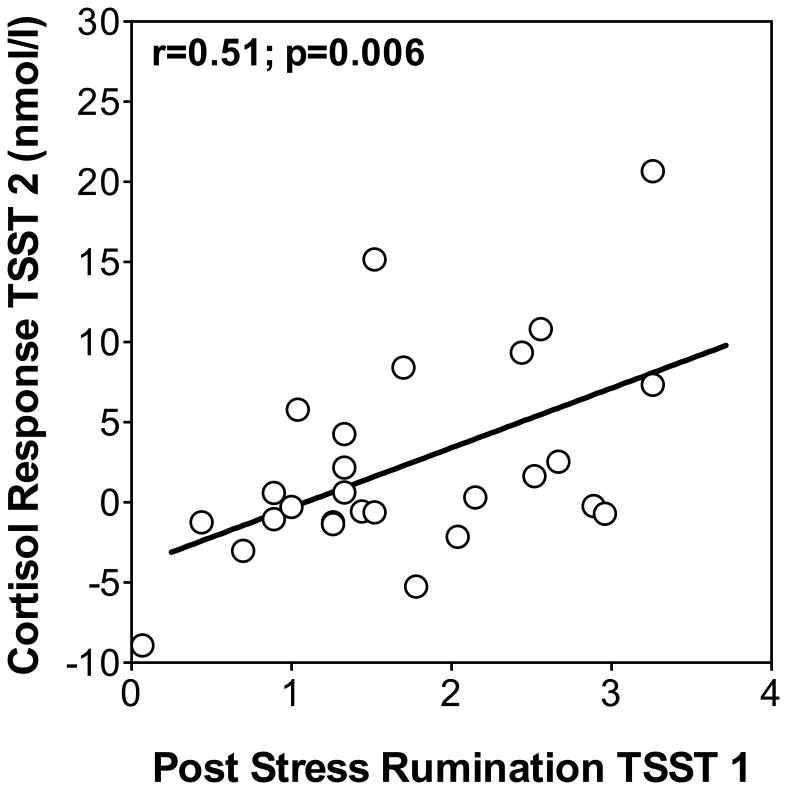
To test our main hypothesis that state rumination after initial stress exposure would predict cortisol responses to a subsequent stress test one day later, we first computed Pearson correlations and found that rumination measured after the first TSST was positively related with cortisol responses from baseline to peak to the second TSST (r = 0.51, p = 0.006; see Figure 3). We again computed a linear regression to control for trait rumination and other variables. Post-stress rumination on day 1 remained a significant predictor of day 2 cortisol responses when controlling for sex and age (state rumination: beta = 0.50; p=0.018), trait rumination (state rumination: beta = 0.49; p = 0.029), depressive symptoms (state rumination: beta = 0.53; p = 0.026), and perceived stress (state rumination: beta = 0.53; p = 0.014), while none of the control variables explained additional variance of day 2 cortisol responses from baseline to peak. Post-stress rumination on day 1 was also correlated with increased mean cortisol levels at time points after the stressor on day 2 (time points +20, +60, and +120; lowest r=.39, highest p=.04).
Figure 3.
Scatterplot showing the relationship between post-stress rumination measured after TSST 1 with cortisol response (max. increase) to repeated stress exposure (TSST 2).
Measures of post-stress rumination after the second stressor were marginally related to cortisol increases from baseline to peak on both study days (day 1 r = .40, p = .08; day 2 r = .39, p = .08). Additionally, post-stress rumination after the second TSST was not related to mean cortisol values at time points +20, +60, and +120 mins after the second stressor (highest r=.24, lowest p=.29). Finally, an index of habituation computed by subtracting cortisol responses to the first TSST from cortisol responses to the second TSST was not related with state rumination on either of the days (both r's < 0.10, n.s.).
6 Discussion
The present study aimed to elucidate the relationship between post-stress rumination and cortisol responses to repeated acute stress. In particular, we set out to test whether ruminating about an acute stress experience would predict how an individual would respond if exposed to a similar stressor again. In our study, participants were exposed to near-identical psychosocial stressors on two consecutive days, and most showed robust HPA axis activation in response to a first-time stressor. When this stressor was repeated, we found habituation of the HPA axis response in the overall group of participants. A post-stress state rumination measure was completed after the first time stress exposure. Specifically, this questionnaire measured post-stress ruminative thoughts, primarily anxiety, anger, and fear-based that were related to the TSST. This measure of state rumination was related to cortisol responses to that preceding stressor. Higher post-stress rumination was associated with both cortisol increases from baseline to peak, as well as sustained high cortisol levels at time points afterwards. This indicates a relationship between post-stress rumination and sustained cortisol response. Furthermore, state rumination after initial stress exposure was related with higher cortisol responses to a repetition of the same stressor one day later, as well as with higher cortisol levels after the peak. State rumination after a second stress exposure was not related to cortisol responses on either day. In other words, increased rumination after experiencing a novel stressor was predictive of increased cortisol reactivity to second-time stress. Rumination, therefore, was associated with reduced ability to adapt to repeated stress. This relationship was independent of age, sex, perceived life stress, depressive symptoms, and trait rumination. These findings support our central hypothesis that state rumination would be associated with non-habituation of the HPA axis to repeated stress. Our finding of HPA axis habituation to repeated stress is consistent with the existing literature (Kirschbaum et al., 1995; Wüst et al., 2005). Additionally, our data are in line with previous work that has shown a relationship between rumination and patterns of physiological activity, such as cardiovascular reactivity to novel and repeated stressors (Brosschot et al., 2006; Johnson et al., 2012). Our results further confirm the previously described relationship between post-stress rumination and cortisol responses (Denson et al., 2009; Zoccola & Dickerson, 2012), but we were unable to replicate previously described relationships between trait rumination and HPA axis stress responses (Zoccola, 2008; Zoccola et al., 2010). Our findings build on this research by linking rumination with adaptation of the HPA axis to repeated stress exposure, thus documenting the relationship between rumination and patterns of cortisol reactivity.
Rumination has been described as a response style; specifically, it is theorized that when stressed, some people respond by ruminating and negatively dwelling on the stressor itself (Simonson, Sánchez, Arger, & Mezulis, 2012). The current study found a moderate positive relationship between trait rumination and post-stress rumination, indicating that those who tend to engage in negative memories in general also showed increased post-stress rumination. Participants who reported greater post-stress rumination after an initial stressor had increased cortisol responses. On a cognitive level, participants who were unable to stop re-experiencing the stress test might have prolonged their state of arousal. By focusing on details of the stressor, such as how upset they felt during the stress test, an individual who ruminates may, after the stress test, experience increased stress-related emotions which could serve to amplify the cortisol response. However, this concept does not fully explain why state rumination after a first-time stressor would predict second-time cortisol responses. One possible explanation is that individuals who are more likely to ruminate, and thus have higher trait rumination and higher state rumination measures, are simply more physiologically reactive to psychosocial stress. However, this is unlikely due to the missing relationship between trait rumination measures and cortisol responses on either day of testing. An alternative theory explaining the relationship between state rumination and cortisol reactivity across multiple stressful experiences may be that state rumination is directly inhibiting coping mechanisms that would otherwise allow an individual to adapt to a stressor. Focusing on thoughts about poor performance and feelings of stress and anxiety may preoccupy ruminators, and limit the cognitive resources needed to formulate a plan to handles future stressors (Takano & Tanno, 2009; Teasdale, 1999). Thus, coping mechanisms could be attenuated, leading to perceived poorer performance when the stressor is encountered again, ultimately resulting in increased stress hormone production. On the other end of the spectrum, those who do not engage in post-stress state rumination, might be better able to develop a coping strategy for adapting to future stressors. This could lead to greater feelings of confidence and better perceived performance when non-ruminators encounter a stressor again; therefore, stress hormone reactivity could be attenuated, leading to habituation of the HPA axis to repeated acute stress. If state rumination impairs post-stress coping mechanisms, such as planning for the future, this could explain how rumination inhibits HPA axis habituation. Unfortunately, we did not have measures of coping in the current study so that these considerations remain speculative and will need to be addressed in future studies. It is also possible that the way in which one appraises the stressor could affect post-stress rumination; for example, those who feel more threatened may also ruminate more after a stressful experience. Feeling threatened has been shown to be related to increased cortisol responses (Schlotz, Hammerfald, Ehlert, & Gaab, 2011); therefore, it would be ideal to investigate other variables, such as primary and secondary appraisal mechanisms, that may mediate the relationship between post-stress rumination and cortisol response habituation.
HPA axis habituation to acute stress is considered to be adaptive and thought to promote healthier physiological functioning; moreover, non-habituation may be prospectively related with the long-term development of disease. Specifically, it is thought that non-habituation of the HPA axis could increase allostatic load, thereby leading to poor health outcomes such as neurodegenerative, psychiatric, and metabolic disease (Danese & McEwen, 2012; Juster et al., 2010; Parente, Hale, & Palermo, 2013; Power & Schulkin, 2012). Therefore, non-habituation of the HPA axis is considered to be maladaptive. In the present study, we found that post-stress rumination was related with higher second day HPA axis responses, which are indicative of this maladaptive stress response pattern. In combination with prior research, these findings help to advance our understanding of the role post-event emotional processing, such as perseverative cognition, may have in shaping the biological stress response. This relationship implies that modification of an individual's engagement in rumination after a stressful event might be an avenue for reducing maladaptive response patterns, more specifically to improve HPA axis responses to repeated stress. It should also be noted that we did not find a relationship between state rumination after a second stress exposure and cortisol responses on either day, which might be attributed to the lower cortisol responses on this second day of stress exposure.
These findings must be interpreted in light of some limitations. The measure of trait rumination focused mostly on the tendency to rehearse emotionally distressing events; it does not fully capture all components of rumination, including subscales such as brooding. This may explain the missing relationship between trait rumination and cortisol response patterns. More deeply assessing trait rumination in future studies may be essential in determining which components of rumination are most closely linked to physiological adaptation. Similarly, the measure of state rumination used here was a thought questionnaire that did not distinguish between rumination and reflection (Abbott & Rapee, 2004). Reflection is defined as past-centered thinking that is contemplative in nature (Harrington & Loffredo, 2010). Reflection was not measured, and it may be possible that participants both ruminated and then reflected; for example, someone may have had thoughts about how poorly he performed, but then followed up those thoughts with reflections on how well he performs at other tasks. It may be important to further characterize exactly what kinds of thoughts participants are engaging in after stress, to further elucidate the relationship between state rumination, coping mechanisms, and HPA axis response patterns. An additional limitation to consider is that some participants may have experienced delayed and/or prolonged rumination that was not captured by the post-TSST assessment; for example, in the twenty-four hours between the two stressors, we did not measure how often participants mentally re-lived the first stressor. As rumination is, by definition, a repetitive process, it could be useful to determine exactly how repetitive it is in our participants after stress. More specifically, someone who shows a great deal of post-stress rumination initially but then stops ruminating within a short amount of time may be more likely to adapt to the stressor by the second exposure than someone who stays awake all night ruminating between the two stressors. In other words, longer duration of rumination may have more pronounced effects on physiological adaptation. However, measuring prolonged rumination is difficult to do. Even asking participants to fill out questionnaires or diaries may actually cause them to ruminate, thus disallowing the goal of truly assessing spontaneously occurring rumination. Regardless, future studies will include measurements of post-stress rumination several hours after the stressor, possibly including a measurement in the evening between two days of stress testing.
In summary, the findings of the current study provide support for the hypothesis that rumination may influence HPA axis responses to repeated acute stress. Specifically, results indicate that higher levels of state rumination after encountering an initial stressor are predictive of amplified initial and second-time cortisol responses to a repeated stressor. Overall, these results suggest that post-stress rumination may inhibit coping, decrease habituate of the HPA axis, and could increase allostatic load over time. These data imply that post-stress rumination may have profound impacts on physiological functioning and thus affect future health. In order to further understand these findings and their implications, additional measures, outcomes, and methodologies could be used. For example, future research examining the impact of state rumination on HPA axis response patterns may benefit from more complete measures that distinguish between rumination and reflection. Moreover, assessing the relationship between other physiological variables, such as peripheral inflammatory habituation to stress, and state rumination may allow us comes. Additionally, it is notably difficult to draw causal conclusions about the nature of rumination and HPA axis responses to repeated stressors. Therefore, an experimental design where participants are randomly assigned to rumination or non-rumination conditions could be implemented.
Highlights.
State rumination predicts cortisol responses to initial stress exposure
State rumination after stress predicts cortisol responses to subsequent stress
Associations of state rumination with cortisol are independent of trait rumination
Acknowledgments
This work was supported by NIH training grant T32-GM084907-01, and a research grant from the American Federation for Aging Research (AFAR). MVT acknowledges funding from the Swiss National Science Foundation (SNF). We would like to thank the research assistants in the Laboratory for Biological Health Psychology at Brandeis University who assisted with data collection.
Role of the funding source: The study was partially funded by a research grant from the American Federation for Aging Research (AFAR). AFAR did not have any role in study design, analyses, and interpretation of data.
Footnotes
Conflict of interest: None of the authors has any conflicts to report.
Contributors: DG, MVT, and NR designed the study. DG, LH, XC, and MVT carried out data collection. DG, NR, and JB analyzed the data. DG, NR, JB, and PMZ wrote the manuscript. All authors agreed with the current version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott MJ, Rapee RM. Post-Event Rumination and Negative Self-Appraisal in Social Phobia Before and After Treatment. Journal of Abnormal Psychology. 2004;113(1):136–144. doi: 10.1037/0021-843x.113.1.136. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research; Journal of Psychosomatic Research. 2006 doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Byrd-Craven J, Granger DA, Auer BJ. Stress reactivity to co-rumination in young women's friendships: Cortisol, alpha-amylase, and negative affect focus. Journal of Social and Personal Relationships. 2011;28(4):469–487. doi: 10.1177/0265407510382319. [DOI] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. doi:298/14/1685 [pii]10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. doi:10.1016/j.physbeh.2011.08.019S0031-9384(11)00404-5 [pii] [DOI] [PubMed] [Google Scholar]
- Dannahy L, Stopa L. Post-event processing in social anxiety. Behav Res Ther. 2007;45(6):1207–1219. doi: 10.1016/j.brat.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, Miller N. Cognitive Appraisals and Emotions Predict Cortisol and Immune Responses: A Meta-Analysis of Acute Laboratory Social Stressors and Emotion Inductions. Psychol Bull. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- Edwards S, Rapee R, Franklin J. Postevent Rumination and Recall Bias for a Social Performance Event in High and Low Socially Anxious Individuals. Cognitive Therapy and Research. 2003;27(6):603–617. doi: 10.1023/a:1026395526858. [DOI] [Google Scholar]
- Epel E, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Ickovics JR. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic medicine. 2000;62(5):623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2012;31(2):264–268. doi: 10.1037/a0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse JB, Junker BW. Exploratory statistical methods, with applications to psychiatric research. Psychoneuroendocrinology. 1992;17(5):423–441. doi: 10.1016/0306-4530(92)90001-n. [DOI] [PubMed] [Google Scholar]
- Harrington R, Loffredo DA. Insight, rumination, and self-reflection as predictors of well-being. Journal of Psychology: Interdisciplinary and Applied. 2010;145(1):39–57. doi: 10.1080/00223980.2010.528072. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Lavoie KL, Bacon SL, Carlson LE, Campbell TS. The Effect of Trait Rumination on Adaptation to Repeated Stress. Psychosomatic medicine. 2012;74(3):258–262. doi: 10.1097/PSY.0b013e31824c3ef2. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. doi:10.1016/j.neubiorev.2009.10.002S0149-7634(09)00148-1 [pii] [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner CJ, Stone AA, H D. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Vol. 57. Philadelphia, PA: Lippincott Williams & Wilkins; 1995. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, von Känel R, Preckel D, Zgraggen L, Mischler K, Fischer JE. Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biological Psychology. 2006;72(2):147–153. doi: 10.1016/j.biopsycho.2005.09.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. New York, NY, ETATS-UNIS: Elsevier; 2003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and Damage from Acute and Chronic Stress: Allostasis and Allostatic Overload and Relevance to the Pathophysiology of Psychiatric Disorders. Annals of the New York Academy of Sciences. 2004;1032(1):1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lasley EN. Allostatic load: When protection gives way to damage. Advances in Mind-Body Medicine. 2003;19(1):28–33. [PubMed] [Google Scholar]
- Morris MC, Rao U. Cortisol response to psychosocial stress during a depressive episode and remission. Stress. 2014;17(1):51–58. doi: 10.3109/10253890.2013.857398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. doi: 10.1037/0021-843x.109.3.504. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102(1):20–28. doi: 10.1037/0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Parente V, Hale L, Palermo T. Association between breast cancer and allostatic load by race: National Health and Nutrition Examination Survey 1999–2008. Psychooncology. 2013;22(3):621–628. doi: 10.1002/pon.3044. [DOI] [PubMed] [Google Scholar]
- Power ML, Schulkin J. Maternal obesity, metabolic disease, and allostatic load. Physiol Behav. 2012;106(1):22–28. doi: 10.1016/j.physbeh.2011.09.011. doi:10.1016/j.physbeh.2011.09.011S0031-9384(11)00457-4 [pii] [DOI] [PubMed] [Google Scholar]
- Puterman E, O'Donovan A, Adler NE, Tomiyama AJ, Kemeny M, Wolkowitz OM, Epel E. Physical activity moderates effects of stressor-induced rumination on cortisol reactivity. Psychosomatic medicine. 2011;73(7):604–611. doi: 10.1097/PSY.0b013e318229e1e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Robinson MS, Alloy LB. Negative Cognitive Styles and Stress-Reactive Rumination Interact to Predict Depression: A Prospective Study. Cognitive Therapy and Research. 2003;27(3):275–291. doi: 10.1023/a:1023914416469. [DOI] [Google Scholar]
- Roger D, Najarian B. The construction and validation of a new scale for measuring emotion control. Personality and Individual Differences. 1989;10(8):845–853. http://dx.doi.org/10.1016/0191-8869(89)90020-2. [Google Scholar]
- Schlotz W, Hammerfald K, Ehlert U, Gaab J. Individual differences in the cortisol response to stress in young healthy men: Testing the roles of perceived stress reactivity and threat appraisal using multiphase latent growth curve modeling. Biological Psychology. 2011;87(2):257–264. doi: 10.1016/j.biopsycho.2011.03.005. http://dx.doi.org/10.1016/j.biopsycho.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation Between Reactivity of the Hypothalamus-Pituitary-Adrenal Axis and the Sympathetic-Adrenal-Medullary System to Repeated Psychosocial Stress. Psychosomatic medicine. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Tsao JCI, Alden LE, Craske MG. Worry and rumination: Repetitive thought as a concomitant and predictor of negative mood. Cognitive Therapy and Research. 2000;24(6):671–688. doi: 10.1023/a:1005587311498. [DOI] [Google Scholar]
- Simonson J, Sánchez O, Arger C, Mezulis AH. Integrating affective and cognitive vulnerabilities to depression: Examining individual differences in cognitive responses to induced stress. Cognitive Therapy and Research. 2012;36(5):474–482. doi: 10.1007/s10608-011-9383-x. [DOI] [Google Scholar]
- Strauss J, Muday T, McNall K, Wong M. Response Style Theory Revisited: Gender Differences and Stereotypes in Rumination and Distraction. Sex Roles. 1997;36(11):771–792. doi: 10.1023/a:1025679223514. [DOI] [Google Scholar]
- Takano K, Tanno Y. Self-rumination, self-reflection, and depression: self-rumination counteracts the adaptive effect of self-reflection. Behav Res Ther. 2009;47(3):260–264. doi: 10.1016/j.brat.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behav Res Ther. 1999;37(Suppl 1):S53–77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24(4):479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006;20(1):40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko IS, van Rossum EFC, Koper JW, Hellhammer DH. Habituation of cortisol responses to repeated psychosocial stress—further characterization and impact of genetic factors. Psychoneuroendocrinology. 2005;30(2):199–211. doi: 10.1016/j.psyneuen.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Young EA, Nolen-Hoeksema S. Effect of ruminations on the saliva cortisol response to a social stressor. Psychoneuroendocrinology. 2001;26(3):319–329. doi: 10.1016/s0306-4530(00)00059-7. [DOI] [PubMed] [Google Scholar]
- Zoccola PM. Rumination and cortisol responses to laboratory stressors. Psychosomatic medicine. 2008;70(6):661. doi: 10.1097/PSY.0b013e31817bbc77. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS. Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research. 2012;73(1):1–9. doi: 10.1016/j.jpsychores.2012.03.007. http://dx.doi.org/10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, Zaldivar FP. Rumination and cortisol responses to laboratory stressors. Psychosom Med. 2008;70(6):661–667. doi: 10.1097/PSY.0b013e31817bbc77. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Figueroa WS, Rabideau EM, Woody A, Benencia F. Differential Effects of Poststressor Rumination and Distraction on Cortisol and C-Reactive Protein. Health Psychology, No Pagination Specified. 2014 doi: 10.1037/hea0000019. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Quas JA, Yim IS. Salivary cortisol responses to a psychosocial laboratory stressor and later verbal recall of the stressor: The role of trait and state rumination. Stress. 2010;13(5):435–443. doi: 10.3109/10253891003713765. [DOI] [PubMed] [Google Scholar]