Abstract
Intermittent exposure to mildly stressful situations provides opportunities to practice coping in the context of exposure psychotherapies and stress inoculation training. Previously, we showed that stress inoculation modeled in juvenile monkeys enhances subsequent indications of resilience. Here we examine stress inoculation effects in adult female monkeys. We found that stress inoculation prevents social separation stress induced anhedonia measured using sucrose preference tests and reduces the hypothalamic pituitary adrenal (HPA) axis stress hormone response to a novel environment. Stress inoculation also increases glucocorticoid receptor (NR3C1) gene expression in anterior cingulate cortex but not hippocampus. Increased anterior cingulate cortex NR3C1 expression induced by stress inoculation is not associated with significant changes in GR1F promoter DNA methylation. On average, low levels of promoter DNA methylation and limited GR1F expression were evident in monkey anterior cingulate cortex as observed in corticolimbic brain regions of adult humans. Taken together these findings suggest that stress inoculation in adulthood enhances behavioral and hormonal aspects of coping without significantly influencing GR1F promoter DNA methylation as a mechanism for NR3C1 transcription regulation.
Keywords: stress, coping, glucocorticoid receptor, HPA axis, anhedonia, DNA methylation, epigenetic regulation, anterior cingulate cortex, hippocampus
INTRODUCTION
Stress inoculation entails intermittent exposure to mildly stressful situations as a feature of resiliency training for people who work in conditions where performance in the face of adversity is required, e.g., medical and military personnel, police, firefighters, and rescue workers (Meichenbaum and Novaco, 1985; Saunders et al., 1996; Stetz et al., 2007). Exposure psychotherapies likewise teach patients with mood and anxiety disorders to imagine a graded series of stress inducing situations and then encourage direct interaction with these stressors in vivo (McNally, 2007). These procedures promote learning (Craske et al., 2008; Tryon and Misurell, 2008) and provide opportunities to practice coping skills.
Previously, we found that juvenile squirrel monkeys exposed to stress inoculation training sessions comprised of intermittent social separations subsequently show diminished behavioral indications of anxiety and lower stress-levels of cortisol compared to juvenile monkeys not exposed to prior separations (Lyons et al., 2009; Lyons et al., 2010b). Stress inoculation also enhances prefrontal-dependent cognitive control of behavior in juvenile monkeys (Katz et al., 2009; Parker et al., 2005). Here we determine whether an exposure-based form of stress inoculation modeled in adult female monkeys enhances subsequent coping and increases glucocorticoid receptor (NR3C1) gene expression in corticolimbic brain regions that control cognitive, motivational, and emotional aspects of physiology and behavior.
Corticolimbic brain deficits in NR3C1 expression impair glucocorticoid feedback regulation of the hypothalamic pituitary adrenal (HPA) axis resulting in high levels of the stress hormone cortisol in humans with major depression (Alt et al., 2010; Holsboer, 2000; Pandey et al., 2013; Webster et al., 2002). Induced deficits in forebrain NR3C1 expression also diminish sucrose preferences in mice (Boyle et al., 2005) as a measure of anhedonia that reflects a core symptom of major depression in humans (Chourbaji and Gass, 2008). Antidepressant medications that alleviate depressive symptoms increase NR3C1 expression and signaling functions (Anacker et al., 2011a; Okugawa et al., 1999; Peiffer et al., 1991; Seckl and Fink, 1992). Consequently, increased NR3C1 expression and signaling are considered potential antidepressant actions in novel therapies and preventive interventions (Anacker et al., 2011b; McQuade and Young, 2000).
This study tests for stress inoculation effects in adult female monkeys because depressive disorders are more prevalent in women than men (Kessler, 2003; Shively et al., 2005). Specifically, we examine whether stress inoculation enhances subsequent behavioral and hormonal aspects of coping along with increased NR3C1 expression and decreased DNA methylation of a promoter region homologous to GR1F in humans. Low levels of promoter DNA methylation facilitate transcription factor binding and can thereby increase gene expression (Klose and Bird, 2006). Experience dependent epigenetic regulation of NR3C1 expression has been linked to differential DNA methylation of GR1F in humans (McGowan et al., 2009) and a homologous promoter in rats (Weaver et al., 2004).
Materials and Methods
Experimental design
Forty eight adult female squirrel monkeys (Saimiri sciureus) served as subjects. Sixteen monkeys purchased as adults were initially acclimated to the research animal facility and the other 32 monkeys were born and raised at the facility. Prior to the study, non-invasive magnetic resonance brain images were acquired from all monkeys as part of another project. Monkeys were then randomly assigned to stress inoculation or non-inoculated control treatment conditions using block randomization to generate equal sample sizes (n=24) matched for age (6-17 yr) and prior background.
Stress inoculation training sessions were conducted as follows. Monkeys were temporarily separated and housed for 3 wk alone, and then housed for 12 wk in groups each comprised of 2 familiar and 2 unfamiliar female monkeys. Social separations and new group formations are known to reliably increase plasma levels of the stress hormone cortisol in squirrel monkeys (Gonzalez et al., 1981; Lyons et al., 1999). A total of 7 intermittent separations and new group formations were conducted at 15-wk intervals to allow ample time for recovery and provide repeated opportunities for coping in the stress inoculation condition. Non-inoculated control monkeys were continuously housed in groups each comprised of 4 familiar female monkeys.
All monkeys were maintained in climate-controlled rooms with an ambient temperature of 26°C with lights on from 07:00-19:00 hr. To control for ovarian hormone effects, all measures were collected during nonbreeding seasons when ovarian hormones remain stable at non-detectable levels in these seasonally breeding primates (Schiml et al., 1999). Monkeys were housed in species-appropriate environments with various manipulable objects, swinging perches, and foraging activities provided for enrichment. All procedures were conducted at Stanford University in accordance with the Guidelines for Use and Care of Laboratory Animals of the National Research Council. Animal research at Stanford is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Experimental protocols were approved prior to implementation by the Stanford University IACUC.
Sucrose preference tests
Sucrose preference tests were administered 16 days after completion of the treatment conditions. Monkeys were initially separated and housed for 12 hr alone. Standard two-bottle choice tests (Boyle et al., 2005) were subsequently administered from 07:00-11:00 hr to determine preferences for 1% sucrose solution versus plain water during the social separations. Sucrose and water locations were reversed mid-way through the 4 hr test to control for positional effects. Sucrose preference percentage scores were calculated as the amount of sucrose solution consumed relative to total amounts of sucrose solution plus plain water. Absolute amounts were also analyzed to confirm percentage results.
Novel environment stress tests and glucocorticoid feedback
Novel environment stress tests were administered 13 days after completion of the treatment conditions. Monkeys were initially administered a saline injection and then placed for 30 min in a novel test environment. Saline injections and exposure to novelty are known to stimulate stress hormone responses in squirrel monkeys (Coe et al., 1982). Seven days later, glucocorticoid feedback sensitivity was assessed during an identical 30 min novel environment stress test preceded by an injection of 2.5 mg/kg hydrocortisone sodium succinate. Blood samples for hormone determinations were collected immediately after each 30 min test using established procedures (Lyons et al., 1999) between 13:30-15:30 hr to control for circadian effects. At the same time of day, additional samples were collected 1 week before and 1 week after the study in home cage conditions and hormone levels were averaged to provide baseline assessments. Plasma adrenocorticotropic hormone (ACTH) levels determined in duplicate using a validated assay (Lyons et al., 1999) without knowledge of the treatment conditions were used to assess stress hormone responses because cortisol assays cannot distinguish between endogenous cortisol and hydrocortisone. Hydrocortisone was administered because of concerns that dexamethasone may not penetrate the brain (Meijer et al., 1998).
Brain tissue collection
Brain tissue from a randomly selected subset of stress inoculated monkeys (n=6) and non-inoculated controls (n=12) was collected 33 days after completion of the treatment conditions using random block sampling to match for age and prior background. Fewer stress inoculated monkeys than non-inoculated controls were examined to preserve opportunities for future long-term studies of stress inoculation brain effects. All brains were collected using established procedures (Patel et al., 2008) between 08:00-10:00 hr to control for circadian effects. Fresh frozen blocks of anterior cingulate cortex and hippocampus were cut coronally into 20 μm sections, mounted on glass slides, and stored at −80°C for the following studies of NR3C1 expression and GR1F promoter DNA methylation.
Measures of NR3C1 expression
A 1-in-40 series of anterior cingulate cortex sections at the genu of the corpus callosum was examined with in situ hybridization histochemistry for cell layer specific expression of total NR3C1 measured over exon 2 with a squirrel monkey-specific riboprobe (GenBank #AF041834) using methods described elsewhere in detail (Patel et al., 2008). Briefly, for each section of anterior cingulate cortex we placed line transects perpendicular to the pial surface that traversed the full width of cortex. Continuous optical densities were collected along the length of each line transect using NIH image software. These data were used to construct profile plots linearly interpolated to reflect percentage of cortical width as described for human brain tissue (Webster et al., 2002). Cortical cell layer specific measures were derived from the percentages of cortical width for each layer based on criteria for squirrel monkeys (Rosabal, 1967); layer I (1–9%), layer II (10–21%), layer III (22–56%), layer IV is not present, layer V (57–75%), and layer VI (76–100%). The validity of these laminar specifications was confirmed by measuring the depth of each boundary on Nissl stained sections adjacent to those that were used to measure NR3C1 hybridization. Optical density data were background corrected and averaged across two serial sections per monkey to serve as units for statistical analysis.
Measurements of total NR3C1 expression measured over exon 2 were additionally collected from a different 1-in-40 series of sections randomly selected to encompass the entire rostro-caudal extent of hippocampus. On 8–12 hippocampal sections per monkey, we manually traced the borders of dentate gyrus, CA1, and CA2–3 regions determined from adjacent Nissl stained sections and a squirrel monkey brain atlas (Gergen and MacLean, 1962) as described elsewhere (Patel et al., 2008). Optical density data were background corrected and averaged across all serial sections for each monkey to serve as units for statistical analysis. Previously we reported that NR3C1 expression primarily reflects NR3C1 α protein in squirrel monkey brain tissue (Patel et al., 2000a).
In addition to in situ hybridization histochemistry, bulk tissue dissected from anterior cingulate cortex sections was used to simultaneously extract RNA for cDNA libraries and genomic DNA for methylation (see below) using the AllPrep DNA/RNA Micro Kit (Qiagen). Quantitative real time polymerase chain reactions (PCR) were used to measure both total NR3C1 and GR1F expression with GAPDH as the reference gene. Standard SYBR Green methods were used with the following primers derived from
GenBank #AF294761, #AF294761, and #KF042852.
GAPDH forward: 5′ TGCCCCCATGTTCGTGATG 3′
GAPDH reverse: 5′ TAAGCAGTTGGTGGTGCAG 3′
total NR3C1 over exons 2 and 3: forward: 5′ CTCTGAACTTCCCTGGTCGA 3′
total NR3C1 over exons 2 and 3: reverse: 5′ TTGTTGCTGTTGAGGAGCTAGA 3′
GR1F forward: 5′ CGCAGCCCCAGAGAGAC 3′
GR1F reverse: 5′ CTACTCGGATTTTCTTCTTTACC 3′
Measures of methylation
Bisulfite conversion of genomic DNA extracted from anterior cingulate cortex as described above was performed using an EpiTect Kit (Qiagen). The GR1F promoter was amplified and cloned from a single 549 bp fragment generated from 2 rounds of PCR (53°C and 55°C annealing temperatures) and a semi-nested PCR (58°C annealing temperature).
forward 1: 5′ TAATTTTTTAAAAGTGTAAGAAATTTAG 3′
forward 2: 5′ TTTAAAAGTGTAAGAAATTTAGTTTG 3′
forward 3: 5′ AGGGGTGGGGGTTGAATT 3′
reverse 1: 5′ TCCCCAAAAAATAAAACAAAAAAAC 3′
reverse 2: 5′ CTTTCTCCAATTTCTCTTCTC 3′
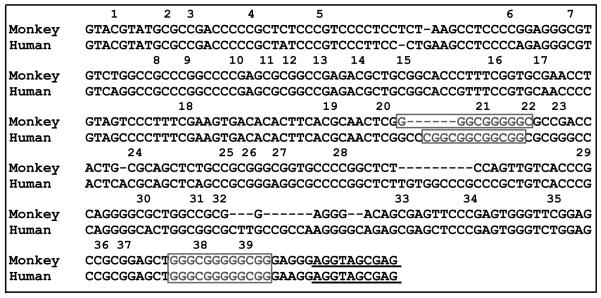
All PCRs were performed using PyroMark PCR (Qiagen) with the following conditions: activation (95°C, 15 min), followed by 40 PCR cycles of 94°C denaturation (30 sec), annealing (see above, 30 sec), 72°C extension (60 sec), and a final extension of 10 min at 72°C. Resulting PCR products were purified, TA cloned into a pDrive cloning vector (Qiagen), and transformed into high efficiency DH5α cells (NEB). A total of 15-20 colonies were picked for each sample and processed for sequencing to determine percent methylation at each of the 39 CpG sites depicted in Fig. 1.
Figure 1.
Genomic sequence for GR1F promoter in humans and squirrel monkeys. Numbers represents CpG dinucleotides analyzed by bisulfite sequencing. Boxes depict NGFI-A (or EGR-1) transcription factor binding sites identified in humans based on experimental evidence (McGowan et al., 2009) and conserved in monkeys based on BIOBASE (Transfac matrix table, 2013.1). Sequence alignment was performed using Geneious 4.8.3. Underline indicates transcription start site.
Data analysis
Sucrose preferences were analyzed by one-sample t-tests and analysis of variance (ANOVA) with body weights controlled as a statistical covariate for 22 stress inoculated monkeys and 23 non-inoculated controls. Hormone data from the same monkeys were analyzed by ANOVA with stress inoculation as a between-subjects factor and sample collection condition as a within-subjects repeated measure. Relationships between hormones and behavior were assessed by linear regression and Spearman rank order correlations. Hormone and behavior data were not collected from 2 stress inoculated monkeys and 1 non-inoculated control monkey that did not complete the 105-wk treatment conditions for reasons unrelated to the study.
Measurements of GR1F promoter DNA methylation in anterior cingulate cortex were analyzed by ANOVA for 6 stress inoculated monkeys and 12 non-inoculated controls. Corresponding measurements of NR3C1 and GR1F expression were not analyzed for 1 of the 6 stress inoculated monkeys because of poor RNA quality. Stress inoculation was considered a between-subjects factor and cortical cell layer and individual CpG sites were treated as within-subjects repeated measures. Relationships between methylation and expression were assessed by linear regression. All descriptive statistics are presented as mean±SEM and test statistics are evaluated with two-tailed probabilities (P<0.05).
RESULTS
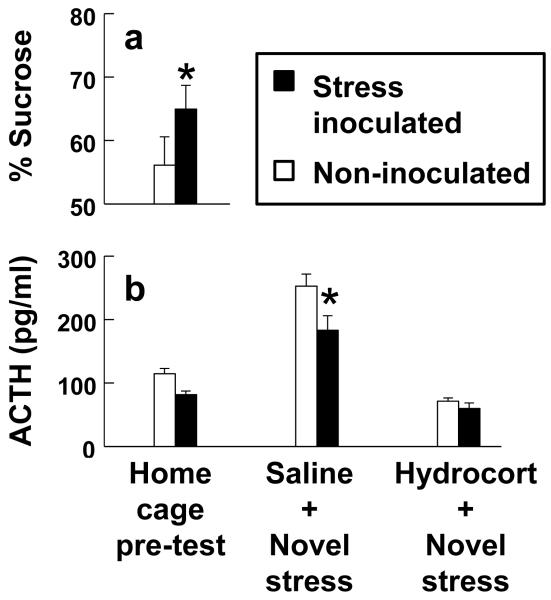
Sucrose preference percentage scores obtained during social separation stress tests (Fig. 2A) were greater in monkeys exposed to stress inoculation compared to non-inoculated controls (F(1,42)=6.91, P=0.012 with body weight controlled as a covariate). Sucrose preference percentage scores without controlling for body weight were also greater than the no-preference 50% threshold for stress inoculated monkeys (t(21)=3.99, P<0.001) but not non-inoculated controls (t(22)=1.36, P=0.187). Absolute amounts of consumption confirmed that stress inoculated monkeys preferred sucrose solution to plain water (F(1,21)=13.04, P=0.002).
Figure 2.
Stress inoculation prevents social separation stress induced anhedonia and reduces stress hormone responses. (a) Sucrose preferences assessed during social separations and (b) ACTH levels measured before and after novel environment stress tests preceded by injection of saline or hydrocortisone. Asterisks indicate significant group differences (P<0.05) with details provided in the text.
Next, we determined whether treatment effects generalize to stress hormone responses and glucocorticoid feedback regulation of the HPA axis assessed by administration of hydrocortisone. Plasma levels of ACTH were measured because cortisol assays cannot distinguish between endogenous cortisol and exogenous hydrocortisone. A stress inoculation main effect (F(1,43)=7.23, P=0.01) and stress inoculation-by-hydrocortisone interaction (F(2,86)=3.41, P=0.038) were discerned for ACTH as depicted in Fig. 2B. Novel environment stress tests preceded by saline increased ACTH above baseline levels measured in undisturbed home cage conditions (F(1,43)=89.94, P<0.001). Post-stress ACTH levels after saline were smaller in stress inoculated monkeys compared to non-inoculated controls (F(1,43)=5.42, P=0.025). Hydrocortisone suppressed post-stress ACTH levels relative to saline (F(1,43)=122.08, P<0.001) but this measure of sensitivity to glucocorticoid feedback did not differ in stress inoculated monkeys compared to non-inoculated controls (F(1,43)=1.26, P=0.268).
In keeping with the possibility that stress inoculation does not modify sensitivity to glucocorticoid feedback and instead reduces responses to stressful qualities of diverse events, we found that diminished post-stress ACTH levels after saline predicted greater protection against social separation stress induced anhedonia (β=−0.41, t(42)=2.79, P=0.008 for the entire sample with body weight controlled as a covariate). A significant relationship was likewise discerned for the entire sample using a non-parametric Spearman rank order correlation not controlling for body weight (rs=−0.37, df 43, P=0.013). Inverse Spearman rank order correlations were also detected for stress inoculated monkeys (rs=−0.26) and non-inoculated controls (rs=−0.31) analyzed separately instead of combined but these correlations were not significant due partly to smaller sample sizes in each of the separate treatment conditions.
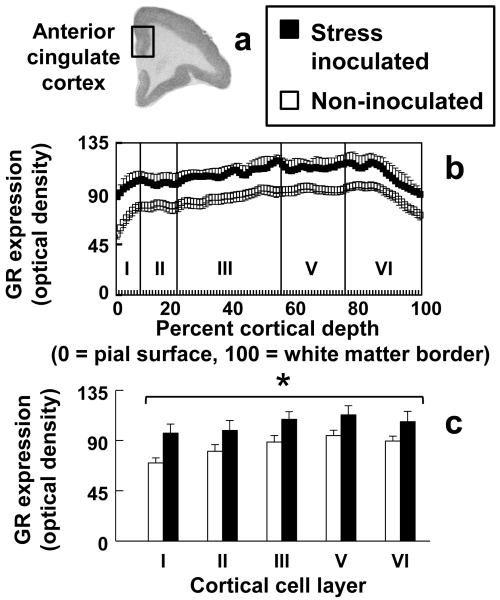
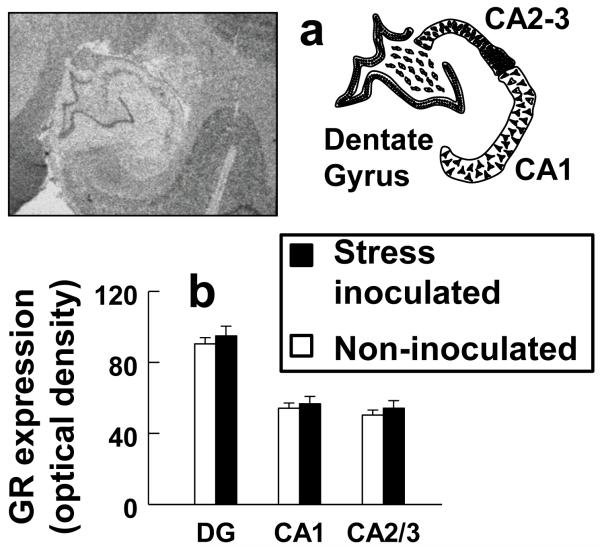
In addition to protecting against separation stress induced anhedonia and reducing the HPA axis stress hormone response, stress inoculation increased NR3C1 expression in all cell layers of anterior cingulate cortex (Fig. 3). Increased NR3C1 expression was confirmed by a stress inoculation main effect (F(1,15)=4.69, P=0.047) and the absence of a stress inoculation-by-cell layer interaction (P=0.235). A stress inoculation main effect was also discerned when NR3C1 expression for anterior cingulate cortex and hippocampus was examined in the same ANOVA (F(1,15)=6.70, P=0.021) but analysis of hippocampus alone did not show stress inoculation effects in any hippocampal sub-region (P=0.502; Fig. 4).
Figure 3.
Anterior cingulate cortex NR3C1 expression measured by in situ hybridization histochemistry is increased in all cell layers by stress inoculation. (a) Representative image of NR3C1 expression, (b) profile plots, and (c) cell layer-specific expression of NR3C1 in anterior cingulate cortex. Asterisk indicates a significant group difference across all cell layers (P=0.047) with details provided in the text.
Figure 4.
Hippocampal NR3C1 expression measured by in situ hybridization histochemistry does not differ significantly by stress inoculation. a) Representative images delineating sub-regions of hippocampus and (b) sub-region measures of NR3C1 expression.
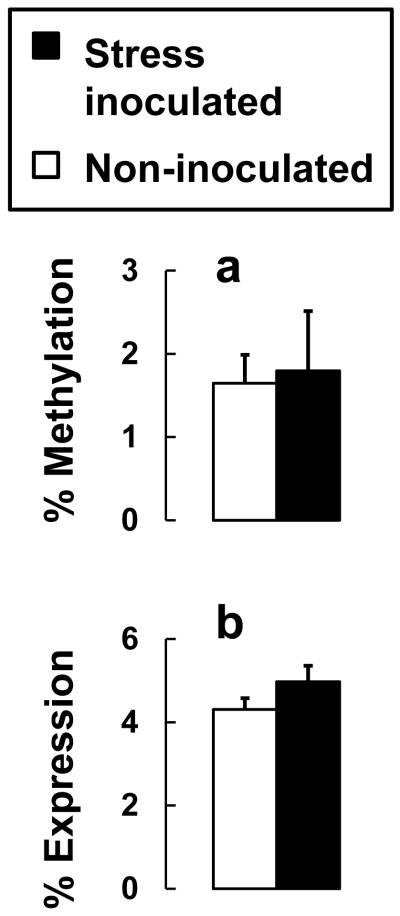
Transcriptional control of NR3C1 is mediated by promoters within a conserved CpG island that renders expression susceptible to epigenetic regulation by DNA methylation. For the promoter region homologous to GR1F in humans (Fig. 1), we focused on monkey anterior cingulate cortex and found no DNA methylation at 6 CpG sites with low levels of DNA methylation at the remaining 33 of 39 CpG sites (Fig. 5A). Methylation differences between CpG sites (P=0.620), stress inoculation main effects (P=0.833), and the stress inoculation-by-CpG site interaction (P=0.186) were not significant for anterior cingulate cortex. DNA methylation analyzed separately at each CpG site, or averaged across all 39 sites, did not predict NR3C1 expression. Relative measures of expression further suggest that GR1F does not robustly regulate NR3C1 expression in monkey anterior cingulate cortex. On average, amounts of GR1F expression are only 4.5% of total NR3C1 expression and do not differ significantly with stress inoculation (Fig. 5B).
Figure 5.
Anterior cingulate cortex GR1F methylation and expression do not differ significantly by stress inoculation. (a) Percentage of methylated clones averaged across 39 CpG sites for GR1F and (b) GR1F expression as a percentage of total NR3C1 expression.
DISCUSSION
Stress inoculation in adult female monkeys protects against subsequent stress induced anhedonia measured using sucrose preference tests. Protection against stress induced anhedonia represents a stressor specific inoculation because sucrose preference tests were administered during social separations that resembled those used for the prior stress inoculation training sessions. Therefore we conducted additional tests in a novel environment that increased HPA axis stress hormone responses and found lower hormone levels in stress inoculated monkeys compared to non-inoculated controls. Follow-up studies with hydrocortisone suggest that stress inoculation does not modify physiologic sensitivity to glucocorticoid feedback regulation and instead reduces responses to stressful qualities of diverse events. Stress inoculation also increases NR3C1 expression in anterior cingulate cortex but not hippocampus and without significantly influencing GR1F promoter DNA methylation.
These findings in adult female monkeys indicate that stress inoculation effects are not restricted to sensitive or critical periods during development as initially suggested by previous research (Lyons et al., 2009; Lyons et al., 2010b). Stress inoculation-like training sessions also increase adult male monkey hippocampal neurogenesis and alter the expression of genes involved in cell proliferation and survival (Lyons et al., 2010a). Antidepressant medications in humans increase hippocampal neurogenesis as a form of adult neuroplasticity through NR3C1 mediated mechanisms (Anacker et al., 2011b). In monkeys we found that stress inoculation increases NR3C1 expression in anterior cingulate cortex but not neurogenic regions of adult hippocampus.
Regional differences in NR3C1 plasticity may be relevant for understanding adaptations involved in physiological versus cognitive aspects of emotion regulation (Caudal et al., 2014; de Kloet et al., 2005). Hippocampus plays important roles in learning, memory, and glucocorticoid feedback control of the HPA axis, whereas frontal brain regions that include anterior cingulate cortex are involved in glucocorticoid feedback, appraisal, and emotion regulation (Etkin et al., 2011; Herman, 2013). Differences in appraisal and habituation as forms of emotion regulation have been found in mice engineered to overexpress forebrain NR3C1 (Hebda-Bauer et al., 2010). Conversely, induced deficits in forebrain NR3C1 expression diminish sucrose preferences as a measure of anhedonia in mice (Boyle et al., 2005; Chourbaji and Gass, 2008). Induced changes in anterior cingulate cortex NR3C1 expression may therefore modify cognitive appraisal without necessarily changing physiologic sensitivity to glucocorticoid feedback control of the HPA axis.
As found for most promoter regions and particularly GR1F in corticolimbic brain regions of adult humans (Alt et al., 2010), we discovered low levels of DNA methylation across 39 CpG sites for GR1F in anterior cingulate cortex of monkeys. These findings diverge from the increases seen in DNA methylation of GR1F along with decreased total NR3C1 expression in adult human hippocampus associated with childhood abuse (McGowan et al., 2009). Decreased total NR3C1 expression in humans with major depression also occurs in amygdala and cingulate gyrus but DNA methylation of GR1F in these brain regions maintains the same low levels observed in healthy human controls (Alt et al., 2010).
Amounts of GR1F expressed as a percentage of total NR3C1 expression are surprisingly limited in monkey anterior cingulate cortex. Low GR1F expression differs from a report that GR1F represents 40-60% of total NR3C1 in human hippocampus (McGowan et al., 2009). Our results agree with evidence that GR1F is not a major promoter as is GR1B or GR1C in hippocampus, amygdala, nucleus accumbens, inferior prefrontal gyrus, and cingulate gyrus (Alt et al., 2010). Site-specific methylation of GR1B and GR1C has been associated with childhood abuse and negatively correlates with total NR3C1 expression in human hippocampus (Labonte et al., 2012). Studies of GR1B and GR1C are needed to determine whether increased NR3C1 expression in monkey anterior cingulate cortex is mediated by differential DNA methylation of these alternative promoters. Epigenetic mechanisms that involve acetylation should also be considered based on recent research results (Charmandari et al., 2011).
Our findings should be interpreted with respect to potential limitations. Results from adult females may or may not hold true for males. Sex differences in sucrose preference tests and HPA axis stress hormone responses have been reported for rodents (Solomon et al., 2012; Young, 1996) and sex differences may warrant attention in monkeys. Stress inoculation training sessions and subsequent test procedures required transfer of animals into new environments. To determine whether habituation or extinction of fear from repeated transfers is crucial, more studies in different contexts are needed. Generally, contextual changes like those used in our test procedures reinstate or increase stress responses instead of producing inoculation-like effects (Bouton et al., 2006; Herman, 2013). Sample sizes for our brain research are small but confirm low levels of DNA methylation and limited GR1F expression in monkey corticolimbic regions as reported for humans (Alt et al., 2010). Although similar patterns of NR3C1 expression are found in human and squirrel monkey brain (Patel et al., 2000a), NR3C1 transactivation differs in humans and monkeys (Patel et al., 2000b). We did not examine NR3C1 protein in this study but other investigations have found that NR3C1 expression corresponds with protein in rhesus monkey and marmoset brain research (Pryce et al., 2005; Sanchez et al., 2000).
In summary, we found that stress inoculation in adult female monkeys prevents social separation stress induced anhedonia and reduces the HPA axis stress hormone response to a novel environment. Stress inoculation also increases NR3C1 expression in anterior cingulate cortex but not hippocampus and does not significantly influence DNA methylation of the GR1F promoter. On average, low levels of DNA methylation and limited GR1F expression are evident in monkey anterior cingulate cortex as reported in humans for corticolimbic brain regions. These findings suggest that stress inoculation in adulthood enhances behavioral and hormonal aspects of coping without significantly influencing GR1F promoter DNA methylation as a mechanism for NR3C1 transcription regulation.
Highlights.
This research suggests that stress inoculation in adulthood enhances behavioral and hormonal aspects of coping without significantly influencing GR1F promoter DNA methylation as a mechanism for NR3C1 transcription regulation.
Acknowledgments
We gratefully acknowledge Mr. Elias Godoy and Mr. Cesar Veloz for excellent care of the animals.
Funding. This research was supported by the National Institutes of Health MH47573 and DA35503, and the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. Dr. Schatzberg reports equity in Merck, Pfizer, Neurocrine, XHale, and Corcept Therapeutics (co-founder). Dr. Schatzberg has received lecture fees from Merck and consulted to Takeda/Lundbeck, Pfizer, and Neuronetics. Dr. Lee, Ms. Buckmaster, Ms. Yi, and Dr. Lyons report no biomedical financial interests or potential conflicts of interest. All authors are employed by or are students at Stanford University.
Contributions. AFS, AGL, and DML designed the research; AGL, CLB, EY, and DML conducted the experiments; AFS, AGL, and DML analyzed data and wrote the paper.
REFERENCES
- Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, Muller CP. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35:544–556. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011a;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry. 2011b;16:738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol. Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc. Natl. Acad. Sci. U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudal D, Jay TM, Godsil BP. Behavioral stress induces regionally-distinct shifts of brain mineralocorticoid and glucocorticoid receptor levels. Front. Behav. Neurosci. 2014;8:19. doi: 10.3389/fnbeh.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Chrousos GP, Lambrou GI, Pavlaki A, Koide H, Ng SS, Kino T. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PloS ONE. 2011;6:e25612. doi: 10.1371/journal.pone.0025612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Gass P. Glucocorticoid receptor transgenic mice as models for depression. Brain Res. Rev. 2008;57:554–560. doi: 10.1016/j.brainresrev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiol. Behav. 1982;29:1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cog. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen JA, MacLean PD. A Stereotaxic Atlas of the Squirrel Monkey’s Brain (Saimiri sciureus) Public Health Service; Bethesda: 1962. [Google Scholar]
- Gonzalez CA, Hennessy MB, Levine S. Subspecies differences in hormonal and behavorial responses after group formation in squirrel monkeys. Am. J. Primatol. 1981;1:439–452. doi: 10.1002/ajp.1350010409. [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Pletsch A, Darwish H, Fentress H, Simmons TA, Wei Q, Watson SJ, Akil H. Forebrain glucocorticoid receptor overexpression increases environmental reactivity and produces a stress-induced spatial discrimination deficit. Neuroscience. 2010;169:645–653. doi: 10.1016/j.neuroscience.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Front. Behav. Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Katz M, Liu C, Schaer M, Parker KJ, Ottet MC, Epps A, Buckmaster CL, Bammer R, Moseley ME, Schatzberg AF, Eliez S, Lyons DM. Prefrontal plasticity and stress inoculation-induced resilience. Dev. Neurosci. 2009;31:293–299. doi: 10.1159/000216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol. Psychiatry. 2012;72:41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Buckmaster PS, Lee AG, Wu C, Mitra R, Duffey LM, Buckmaster CL, Her S, Patel PD, Schatzberg AF. Stress coping stimulates hippocampal neurogenesis in adult monkeys. Proc. Natl. Acad. Sci. U S A. 2010a;107:14823–14827. doi: 10.1073/pnas.0914568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front. Behav. Neurosci. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: implications for understanding resilience. Dev. Psychobiol. 2010b;52:616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Wang OJ, Lindley SE, Levine S, Kalin NH, Schatzberg AF. Separation induced changes in squirrel monkey hypothalamic-pituitary-adrenal physiology resemble aspects of hypercortisolism in humans. Psychoneuroendocrinology. 1999;24:131–142. doi: 10.1016/s0306-4530(98)00065-1. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin. Psychol. Rev. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- McQuade R, Young AH. Future therapeutic targets in mood disorders: the glucocorticoid receptor. Brit. J. Psychiatry. 2000;177:390–395. doi: 10.1192/bjp.177.5.390. [DOI] [PubMed] [Google Scholar]
- Meichenbaum D, Novaco R. Stress inoculation: a preventative approach. Issues Ment. Health Nurs. 1985;7:419–435. doi: 10.3109/01612848509009464. [DOI] [PubMed] [Google Scholar]
- Meijer OC, de Lange EC, Breimer DD, de Boer AG, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139:1789–1793. doi: 10.1210/endo.139.4.5917. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Omori K, Suzukawa J, Fujiseki Y, Kinoshita T, Inagaki C. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. J. Neuroendocrinol. 1999;11:887–895. doi: 10.1046/j.1365-2826.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Dwivedi Y, Palkovits M. Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology. 2013;38:2628–2639. doi: 10.1016/j.psyneuen.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol. Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J. Psychiatric Res. 2000a;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Patel PD, Lyons DM, Zhang Z, Ngo H, Schatzberg AF. Impaired transactivation of the glucocorticoid receptor cloned from the Guyanese squirrel monkey. J. Steroid Biochem. Mol. Biol. 2000b;72:115–123. doi: 10.1016/s0960-0760(00)00023-6. [DOI] [PubMed] [Google Scholar]
- Peiffer A, Veilleux S, Barden N. Antidepressant and other centrally acting drugs regulate glucocorticoid receptor messenger RNA levels in rat brain. Psychoneuroendocrinology. 1991;16:505–515. doi: 10.1016/0306-4530(91)90034-q. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen-Relo A. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur. J. Neurosci. 2005;21:1521–1535. doi: 10.1111/j.1460-9568.2005.04003.x. [DOI] [PubMed] [Google Scholar]
- Rosabal F. Cytoarchitecture of the frontal lobe of the squirrel monkey. J. Comp. Neurol. 1967;130:87–108. doi: 10.1002/cne.901300202. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J. Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders T, Driskell JE, Johnston JH, Salas E. The effect of stress inoculation training on anxiety and performance. J. Occup. Health Psychol. 1996;1:170–186. doi: 10.1037//1076-8998.1.2.170. [DOI] [PubMed] [Google Scholar]
- Schiml PA, Mendoza SP, Saltzman W, Lyons DM, Mason WA. Annual physiological changes in individually housed squirrel monkeys (Saimiri sciureus) Am. J. Primatol. 1999;47:93–103. doi: 10.1002/(SICI)1098-2345(1999)47:2<93::AID-AJP1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Fink G. Antidepressants increase glucocorticoid and mineralocorticoid receptor mRNA expression in rat hippocampus in vivo. Neuroendocrinology. 1992;55:621–626. doi: 10.1159/000126180. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol. Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetz MC, Thomas ML, Russo MB, Stetz TA, Wildzunas RM, McDonald JJ, Wiederhold BK, Romano JA., Jr. Stress, mental health, and cognition: a brief review of relationships and countermeasures. Aviat. Space Environ. Med. 2007;78:B252–260. [PubMed] [Google Scholar]
- Tryon WW, Misurell JR. Dissonance induction and reduction: a possible principle and connectionist mechanism for why therapies are effective. Clin. Psychol. Rev. 2008;28:1297–1309. doi: 10.1016/j.cpr.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry. 2002;7:985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences in response to exogenous corticosterone: a rat model of hypercortisolemia. Mol. Psychiatry. 1996;1:313–319. [PubMed] [Google Scholar]