Abstract
Reward dysfunction is thought to play a core role in the pathophysiology of major depressive disorder (MDD). Event-related potential (ERP) and functional magnetic resonance imaging (fMRI) studies have identified reward processing deficits in MDD, but these methods have yet to be applied together in a single MDD sample. We utilized multimodal neuroimaging evidence to examine reward dysfunction in MDD. Further, we explored how neurobiological reward dysfunction would map onto subtypes of MDD. The feedback negativity (FN), an ERP index of reward evaluation, was recorded in 34 unmedicated depressed individuals and 42 never-depressed controls during a laboratory gambling task. Ventral striatal (VS) activation to reward was recorded in a separate fMRI session, using an identical task, among a subgroup of 24 depressed individuals and a comparison group of 18 non-depressed controls. FN amplitude was blunted in MDD. This effect was driven by a MDD subgroup characterized by impaired mood reactivity to positive events, a core feature of melancholic MDD. A similar pattern was observed for VS activation, which was also blunted among the MDD subgroup with impaired mood reactivity. Neither FN amplitude nor VS activation were related to the full, DSM-defined melancholic or atypical MDD subtypes. Across the MDD sample, FN amplitude and VS activation were correlated, indicating convergence across methods. These results indicate that not all MDD is characterized by reward dysfunction, and that there is meaningful heterogeneity in reward processing within MDD. The current study offers neurobiological evidence that impaired mood reactivity is a key phenotypic distinction for subtyping MDD, and further suggests that the existing melancholic phenotype may require further refinement.
Keywords: ERP, fMRI, Reward, Depression, Feedback Negativity, Striatum
1. Introduction
Major depressive disorder (MDD) ranks among the world’s most common (Kessler & Wang, 2009) and economically burdensome illnesses (Berto, D'Ilario, Ruffo, Di Virgilio, & Rizzo, 2000; Luppa, Heinrich, Angermeyer, Konig, & Riedel-Heller, 2007). A cardinal symptom of MDD is anhedonia, a pervasive lack of interest or pleasure in normally enjoyable activities (American Psychiatric Association, 2013), and it has been suggested that anhedonia may be what distinguishes MDD from other disorders, including anxiety disorders (Joiner, Catanzaro, & Laurent, 1996; Watson, Clark, et al., 1995; Watson, Weber, et al., 1995) and schizophrenia (Joiner, Brown, & Metalsky, 2003). Recently, there is growing interest in translating findings from basic neuroscience to characterize anhedonia with regard to quantitative deficits in reward processing (Nestler & Carlezon, 2006; Pizzagalli, Dillon, Bogdan, & Holmes, 2011; Russo & Nestler, 2013). Behavioral studies have linked MDD with insensitivity to reward contingencies (Henriques & Davidson, 2000; Pizzagalli, Iosifescu, Hallett, Ratner, & Fava, 2008), which correlates with self-reported anhedonia severity (Pizzagalli, Jahn, & O'Shea, 2005) and predicts a poor response to treatment (Vrieze et al., 2012).
Building upon this behavioral data, functional magnetic resonance imaging (fMRI) studies have begun to shed light on the pathophysiology of reward dysfunction in MDD. The striatum is a core region involved in reward processing (X. Liu, Hairston, Schrier, & Fan, 2011), and studies on MDD have consistently found blunted reward-related activity within this region, including in the ventral striatum (VS) (Pizzagalli et al., 2009; Steele, Kumar, & Ebmeier, 2007), caudate (Forbes et al., 2006; Forbes et al., 2009; Olino et al., 2011; Smoski et al., 2009), and putamen (Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008). VS hypoactivation in particular has been related to anhedonia severity rather than other symptoms of depression or anxiety (Keedwell, Andrew, Williams, Brammer, & Phillips, 2005; Wacker, Dillon, & Pizzagalli, 2009); deep brain stimulation of the VS, meanwhile, is effective for treating refractory MDD (Schlaepfer et al., 2008).
Converging evidence has also emerged from electrophysiological research, using event-related potentials (ERPs) to index reward dysfunction in MDD. ERP studies have focused on the feedback negativity (FN), a component that is more positive for rewards and more negative for non-rewards (Foti, Weinberg, Dien, & Hajcak, 2011; Gehring & Willoughby, 2002; Holroyd, Pakzad-Vaezi, & Krigolson, 2008). The FN is maximal at frontocentral electrodes 300 ms following reward feedback and reflects the early evaluation of rewards compared to non-rewards (Foti, Weinberg, et al., 2011; Holroyd et al., 2008). In non-depressed individuals, FN amplitude has been shown to correlate with both behavioral and self-reported reward sensitivity (Bress & Hajcak, 2013). While traditionally thought to be generated within the anterior cingulate cortex (Gehring & Willoughby, 2002), it has been proposed that the FN may also reflect reward-related activity within the striatum (Foti, Weinberg, et al., 2011). Two recent multimodal studies have supported this perspective: In an unselected sample in which ERP and fMRI data were recorded in separate sessions, FN amplitude covaried directly with VS BOLD signal to reward and midbrain gray matter volume (Carlson, Foti, Harmon-Jones, & Proudfit, 2014; Carlson, Foti, Mujica-Parodi, Harmon-Jones, & Hajcak, 2011); a subsequent study examining simultaneous ERP-fMRI recordings found trial-by-trial associations between FN amplitude to reward feedback and activation in the VS and cingulate cortex (Becker, Nitsch, Miltner, & Straube, 2014).
Notably, blunted FN amplitude is associated with MDD symptoms in both clinical (W. H. Liu et al., 2014) and nonclinical samples (Bress, Smith, Foti, Klein, & Hajcak, 2012; Foti & Hajcak, 2009), an association which appears to be specific to symptoms of MDD and not anxiety (Bress, Meyer, & Hajcak, 2013). Blunted FN amplitude may represent a neurobiological mechanism of risk for MDD, such that it is more pronounced among individuals with a family history of MDD (Foti, Hajcak, Kotov, & Klein, 2011) and has been shown to prospectively predict first episode onset of MDD over and above other known risk factors (Bress, Foti, Kotov, Klein, & Hajcak, 2013).
Building upon these findings of impaired reward processing in MDD, we sought to shed further light on the specificity of this dysfunction using a multimodal neuroimaging approach. Both diminished VS activation and FN amplitude have been implicated in MDD, yet these neurobiological measures have yet to be considered together. Here, we integrated ERP and fMRI data on reward dysfunction within a single MDD sample, testing for convergence across methods (i.e., association between FN amplitude and VS activation). In a previous report, we demonstrated a link between these hemodynamic and electrophysiological indices of reward functioning (Carlson et al., 2011); here, we sought to extend these findings by using multimodal neuroimaging data to quantify reward dysfunction in MDD. This allowed us to examine the incremental utility in combining fMRI and ERP measures to capture group differences in reward processing.
A further goal was to move beyond diagnostic correlates and leverage neural information of reward dysfunction to identify biologically distinct subgroups within MDD. We tested whether there would be significant between-subjects variation among depressed individuals which could allow us to subtype MDD based on the presence of reward dysfunction. Indeed, evidence of meaningful MDD subtypes has been inconsistent (Hadzi-Pavlovic & Boyce, 2012). Although there has long been a distinction between melancholic and atypical MDD, the validity of these subtypes remains equivocal with regard to putative etiology, treatment response, and illness characteristics. From its initial conception, melancholic MDD was thought to represent an endogenous syndrome (Robertson, 1911). While some specific biological abnormalities have been identified in melancholic MDD, notably hypothalamic-pituitary-adrenal axis dysregulation (Stetler & Miller, 2011), reliable biomarkers with diagnostic utility are lacking. Melancholic and atypical MDD are also thought to respond to different types of treatment, yet a study of 481 patients found that MDD subtype did not predict treatment response (Bobo et al., 2011). Lastly, a recent study of 818 patients indicated that the melancholic and atypical subtypes—as currently defined—do not separate cleanly using latent class analysis (Lamers et al., 2010), casting doubt on whether these represent meaningfully distinct subgroups.
This mixed evidence suggests that the melancholic and atypical MDD phenotypes may be inadequate and require further refinement. Rather than starting with these pre-existing categories and seeking neurobiological indicators, it may instead be beneficial to adopt a different approach: Identify novel subgroups based on dysfunction in basic, well-established processes, irrespective of existing diagnostic boundaries, and then build outward toward a revised clinical phenotype. This approach is articulated within the Research Domain Criteria Project (RDoC) (Insel et al., 2010), as part of a broader effort to improve the classification of psychopathology by more fully integrating clinical and basic science.
Here, we examined whether neural evidence of reward dysfunction could be used to validate and potentially refine the existing melancholic phenotype. As described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013), the primary criteria for melancholic MDD are pervasive anhedonia and impaired mood reactivity to positive events; atypical MDD is characterized by intact mood reactivity. Based on these definitions, we expected individuals with melancholic MDD to exhibit profound reward dysfunction, as evidenced by diminished FN amplitude and VS activation. Beyond these primary criteria, however, both subtypes are defined by a number of other, secondary symptoms: melancholic MDD with a poorer morning mood, early wakening, loss of appetite, agitation, and inappropriate guilt; atypical MDD with hypersomnia, increased appetite, limb heaviness, and interpersonal sensitivity. Considering this symptom heterogeneity, we examined whether reward dysfunction would map more closely onto the full melancholic subtype or instead to the primary criteria of pervasive anhedonia and impaired mood reactivity. It is noteworthy that, within the DSM-5, mood reactivity to positive events is the sole illness characteristic that uniquely distinguishes between the melancholic and atypical subtypes; impaired mood reactivity precludes a diagnosis of atypical MDD, and intact mood reactivity precludes a diagnosis of melancholic MDD. One possibility, therefore, is that anhedonia and impaired mood reactivity—the two criteria that are critical for differentiating the melancholic and atypical subtypes within the DSM-5—may represent more powerful ‘joints’ for parsing MDD into biologically distinct subgroups and elucidating a more specific profile of reward dysfunction in MDD. In this way, it may be possible to refine the relevant phenotypes in a manner that is guided by empirical, multimodal evidence of neurobiological reward dysfunction.
2. Methods
2.1 Participants
The MDD group consisted of 34 female adults recruited from the community; only female participants were recruited for the current study given that prevalence rates of MDD are significantly higher in women than in men (Kessler et al., 2003). The inclusion criterion was a clinical diagnosis of unipolar depression (i.e., current MDD and/or dysthymic disorder); exclusion criteria were diagnoses of current generalized anxiety disorder, lifetime obsessive compulsive disorder, lifetime substance abuse/dependence, more than one other current comorbid Axis I disorder, or current prescription of psychiatric medication (past two months). Current comorbid disorders included specific phobia (n=6), panic disorder (2), social phobia (1), and body dysmorphic disorder (1). The healthy control group consisted of 42 female adults with no diagnosable lifetime Axis I disorder and no history of neurological illness. Diagnoses were determined using the Structured Interview for DSM Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 2001).
ERP data was collected from all 34 depressed and 42 control participants. Of these, 24 depressed participants and 6 controls also completed the fMRI gambling task. To yield an adequate comparison group for the fMRI task, we included 12 non-depressed participants drawn from a separate, larger sample who completed the identical fMRI task (Carlson et al., 2011). These 12 were all female, were comparable in age to controls and depressed participants (p’s>.20), and were free of any current depressive symptoms (depression score of 0–4 on the 21-item version of the Depression Anxiety Stress Scale) (Lovibond & Lovibond, 1995). This yielded final fMRI samples sizes of 24 depressed patients and 18 non-depressed controls. Participants received monetary compensation for completing the study. This research protocol was approved by the institutional review board at Stony Brook University, and written informed consent was obtained from all participants.
2.2 Symptom Measures
Past-week anhedonia and symptom severity was assessed using the Mood and Anxiety Symptom Questionnaire (MASQ), a scale designed in accordance with the tripartite model of depression and anxiety (Watson, Clark, et al., 1995; Watson, Weber, et al., 1995). Four subscales were considered: the Anhedonic Depression and Anxious Arousal subscales capture symptoms specific to depression and anxiety, respectively; the General Distress—Depression and General Distress—Anxiety subscales capture symptoms more common to both disorders. The MASQ has good internal consistency in clinical and non-clinical samples (α >.80), and the disorder-specific subscales exhibit convergent and discriminant validity (Watson, Weber, et al., 1995). Of interest here was the Anhedonic Depression subscale, which has been linked to blunted VS activation in a non-clinical sample (Wacker et al., 2009).
Mood reactivity was coded from the item on the melancholic MDD module of the SCID for the current depressive episode: “During the times when you’re feeling depressed, if something good happens to you or if someone tries to cheer you up, do you feel better, at least for a while?” Responses were coded in a binary manner (intact vs. partially/fully impaired).
2.3 Task
A laboratory gambling task was used to elicit the FN and VS activation (Carlson et al., 2011; Foti, Weinberg, et al., 2011). The ERP version was administered using Presentation software (Neurobehavioral Systems, Inc., Albany, CA). On each trial, participants were shown a graphic displaying two doors (occupying 6° of the visual field vertically and 8° horizontally) and chose one door to open using either the left or right mouse button. Participants were told that one door contained a prize on each trial. Following each choice, a feedback stimulus indicated whether they won or lost money on that trial. A green ‘↑’ indicated a gain of $0.40, and a red ‘↓’ a loss of $0.20 (each occupying 3° of the visual field vertically and 1° horizontally). The task consisted of 50 trials (25 wins, 25 losses), presented pseudorandomly. Stimuli were presented as follows: (i) the two doors until a response was made, (ii) a fixation mark for 1000 ms, (iii) a feedback arrow for 2000 ms, (iv) a fixation mark for 1500 ms, and (v) ‘Click for the next round’ until a response was made. Prior to the main task, participants completed five practice trials. Halfway through the task, participants received a break and the amount of money won at that point was displayed.
The fMRI version of the task was administered using E-Prime (Psychology Software Tools, Pittsburgh, PA), with identical stimuli and a similar design. Stimuli were presented as follows: (i) the two doors for 4000 ms, during which individuals made a response, (ii) a fixation mark for 500 ms, (iii) a feedback arrow for 1000 ms, (iv) a blank black screen for a jittered intertrial interval of 1500–14000 ms (M = 4000 ms). The spacing between events was determined using the genetic algorithm to optimally sample across the entire hemodynamic response (Wager & Nichols, 2003). The task consisted of 60 trials (30 wins, 30 losses) presented pseudorandomly. Participants first completed two practice trials. Participants were instructed that if they did not make a response while the doors were presented, the computer would randomly pick a door for them.
2.4 Procedure
The experiment was conducted in a single laboratory session lasting three hours: The SCID was administered. The ERP and fMRI sessions were conducted in a random order by an experimenter blind to group membership. Participants then completed the MASQ. All participants were paid their task winnings ($5.00 per task) and were compensated for their time.
2.5 ERP Data Acquisition
The electroencephalogram was recorded using a custom cap (Cortech Solutions, Wilmington, NC, USA) and the ActiveTwo Biosemi system (BioSemi, Amsterdam, Netherlands). The signal was preamplified at the electrode with a gain of one and was digitized at 24-bit resolution with a least significant bit value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth-order sinc filter with a -3 dB cutoff of 204.8 Hz. Recordings were taken from 34 scalp electrodes based on the 10/20 system (including FCz and Iz), and two mastoid electrodes. The electrooculogram was recorded from electrodes 1 cm above and below the left eye and 1 cm adjacent to each eye. Electrodes were measured online relative to a common mode sense electrode forming a monopolar channel. Brain Vision Analyzer (Brain Products, Munich, Germany) was used for offline analysis. Data were re-referenced to the mastoid average and band-pass filtered from 0.01–30 Hz. The signal was segmented from −500 to 1000 ms relative to feedback onset and was corrected for blinks and eye movements using a regression method (Gratton, Coles, & Donchin, 1983). Channels were rejected trial-wise using a semi-automated procedure, with artifacts identified as: a step of 50 μV between samples, a 300 μV difference within a trial, or a difference of less than 0.5 μV within 100-ms intervals. Additional artifacts were identified visually. ERPs were averaged separately for wins and losses, and the FN was scored as the mean activity at Fz/FCz from 250–350 ms, with a baseline of −200 to 0 ms.
2.6 fMRI Data Acquisition
A 3 Tesla Siemens Trio whole body scanner was used to acquire 242 T2-weighted whole-brain volumes with an EPI sequence sensitive to BOLD signal, using the following parameters: TR=2500 ms, TE=22 ms, flip angle=83°, matrix dimensions=96x96, FOV=224x224mm, slices=40, slice thickness=3.5mm, and gap=0. Standard preprocessing procedures were performed in SPM8 utilizing default parameters, including image realignment corrections for head movements, slice timing corrections for acquisition order, normalization to standard 2x2x2 mm Montreal Neurological Institute space, and spatial smoothing with a Gaussian full-width-at-half-maximum 8 mm filter. An event-related fixed-effects general linear model (GLM) was created for each participant. The data was analyzed in an event-related design (i.e., stick function), which used the onsets of the win and loss feedback cues to define our conditions. Win and loss cues were modeled separately, and t-contrasts were created for each participant to examine activation to wins in comparison to loss (Win>Loss contrast); the lack of an explicit fixation period precluded the analysis of either win or loss feedback alone (e.g., Win>Fixation, Loss>Fixation). Between-subjects effects were examined by creating a second-level mixed-effects GLM with win minus loss as a fixed effect and subject as a random effect. A one-way t-test was calculated to examine reward related activity across the entire group. Resultant whole-brain t-maps were thresholded at p<.001, uncorrected with a minimum cluster size of 10 voxels. Data were extracted for each individual, using SPM’s principle eigenvariate extraction, from a 6mm sphere centered on the group-wise maximal activation within the anatomical VS.
3. Results
3.1 Sample Characteristics
Demographic and symptom characteristics are presented in Table 1. The MDD and control groups were well-matched on age, ethnicity, education level, and marital status. There was a trend toward a group effect on race, with a somewhat higher proportion of Caucasian participants within the depressed group.1 As expected, self-reported symptoms of depression and anxiety were more severe among the MDD group.
Table 1.
Demographic and Symptom Characteristics of Sample
| Depressed (n = 34) | Control (n = 42) | Group Comparison | |||
|---|---|---|---|---|---|
| N | % | n | % | Fisher’s Exact Test (p) | |
| Ethnicity | |||||
| Hispanic/Latino | 2 | 5.9 | 3 | 8.6 | 1.00 |
| Other | 32 | 94.1 | 32 | 91.4 | |
| Race | |||||
| Caucasian | 24 | 72.7 | 17 | 50.0 | .08† |
| Other | 9 | 27.3 | 17 | 50.0 | |
| Education | |||||
| Part College or Less | 21 | 61.8 | 30 | 71.4 | .46 |
| College Degree | 13 | 38.2 | 12 | 28.6 | |
| Marital Status | |||||
| Ever married | 8 | 23.5 | 7 | 17.1 | .57 |
| Never married | 26 | 76.5 | 34 | 82.9 | |
|
| |||||
| M | SD | M | SD | t(df) | |
|
| |||||
| Age | 26.00 | 8.89 | 23.79 | 7.12 | 1.21(74) |
| Symptoms | |||||
| Anhedonic Depression | 64.36 | 12.74 | 40.00 | 11.10 | 8.35(65)*** |
| General Distress, Depression | 38.09 | 11.60 | 19.09 | 5.72 | 8.51(65)*** |
| Anxious Arousal | 30.36 | 10.90 | 21.00 | 4.59 | 4.61(65)*** |
| General Distress, Anxiety | 25.21 | 8.65 | 16.50 | 4.67 | 5.15(65)*** |
Note: Symptoms are subscales from the Mood and Anxiety Symptom Questionnaire.
p<.10,
p<.001
MDD subtype profiles are presented in Table 2. Eleven individuals (32.4%) met full DSM criteria for the melancholic subtype, 6 (17.6%) for atypical, and 17 (50.0%) did not meet full criteria for either. No significant group differences in symptoms were observed across DSM subtypes. Separate from these pre-defined categories, 11 individuals (32.4%) reported impaired mood reactivity to positive events and 23 (67.6%) reported intact mood reactivity. Impaired mood reactivity was associated with more severe Anhedonic Depression symptoms.
Table 2.
Symptom Severity and Reward Sensitivity by Depression Subtype
| Mood Reactivity to Positive Events | Impaired (n = 11) | Intact (n = 23) | Group Comparison | ||
|---|---|---|---|---|---|
|
| |||||
| M | SD | M | SD | F(df) | |
| Neural Response to Reward | |||||
| Feedback Negativity | −.90 | 3.56 | 4.41 | 3.70 | 15.68(1,32)*** |
| Ventral Striatum | .24 | .60 | .80 | .63 | 4.66(1,22)* |
| Symptoms | |||||
| Anhedonic Depression | 71.70 | 10.40 | 61.17 | 12.52 | 5.41(1,31)* |
| General Distress, Depression | 42.40 | 8.14 | 36.22 | 12.59 | 2.02(1,31) |
| Anxious Arousal | 32.40 | 8.87 | 29.48 | 11.74 | .49(1,31) |
| General Distress, Anxiety | 28.90 | 8.65 | 23.61 | 8.33 | 2.75(1,31) |
| Age of Depression Onset | 14.56 | 3.32 | 17.50 | 6.00 | 1.90(1,29) |
| DSM Subtype | Melancholic (n = 11) | Atypical (n = 6) | Neither (n = 17) | Group Comparison | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | SD | M | SD | M | SD | F(df) | |
| Neural Response to Reward | |||||||
| Feedback Negativity | 3.72 | 5.14 | 3.59 | 3.28 | 1.71 | 4.23 | .84(2,31) |
| Ventral Striatum | .59 | .69 | .68 | .12 | .52 | .79 | .08(2,21) |
| Symptoms | |||||||
| Anhedonic Depression | 61.40 | 11.70 | 66.17 | 10.50 | 65.47 | 14.33 | .38(2,30) |
| General Distress, Depression | 36.50 | 10.72 | 34.33 | 16.37 | 40.35 | 10.55 | .71(2,30) |
| Anxious Arousal | 31.90 | 8.36 | 26.67 | 11.22 | 30.76 | 12.34 | .44(2,30) |
| General Distress, Anxiety | 26.10 | 8.29 | 19.00 | 5.37 | 26.88 | 9.17 | 2.04(2,30) |
| Age of Depression Onset | 17.40 | 5.60 | 15.80 | 5.68 | 16.44 | 5.65 | .16(2,28) |
Note: Symptoms are subscales from the Mood and Anxiety Symptom Questionnaire. Amplitude of the Feedback Negativity (Win vs. Loss) was converted to a positive number, such that larger numbers indicate greater reward-related neural activity.
p<.05,
p<.001
3.2 Reward Sensitivity: ERP Data
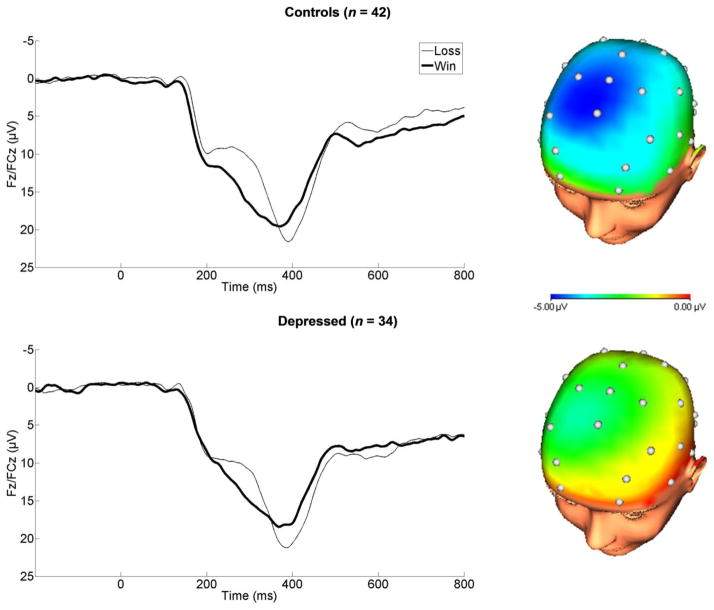
Consistent with previous studies, FN amplitude (Win vs. Loss) was maximal at 300 ms following feedback onset and at frontocentral electrodes (Figure 1). The timing and scalp distribution of the FN was comparable across the MDD and control groups. We analyzed FN amplitude using a mixed-model ANOVA including Feedback as the within-subjects factor (Win vs. Loss) and Group as the between-subjects factor (MDD vs. Control). The ANOVA yielded a main effect of Feedback (F(1,74) = 71.73, p < .001, ηp 2= .49) and an interaction with Group (F(1,74) = 6.08, p < .05, ηp2 = .08). This interaction indicates that the modulation of FN amplitude by reward versus non-reward (i.e., Loss minus Win) was blunted in the MDD group (M = −2.69, SD = 4.39 μV) compared to controls (M = −4.90, SD = 3.43 μV; group comparison: t(74) = 2.47, p<.05, d = .56); when predicting the FN to win and loss trials separately (as opposed to the difference score), the effects of Group was non-significant (p’s > .30, d’s < .23). Importantly, within each group the Win vs. Loss contrast was significant (Control: t(41) = 9.28, p < .001, d = 1.44; MDD: t(33) = 3.57, p = .001, d = .67). While the FN was blunted in MDD, significant reward-related neural activity was still present in this group.
Figure 1.
Feedback negativity (FN) elicited by monetary reward among control (top) and depressed groups (bottom). Headmaps represent the difference between loss and win outcomes from 250–350 ms, where the FN was scored.
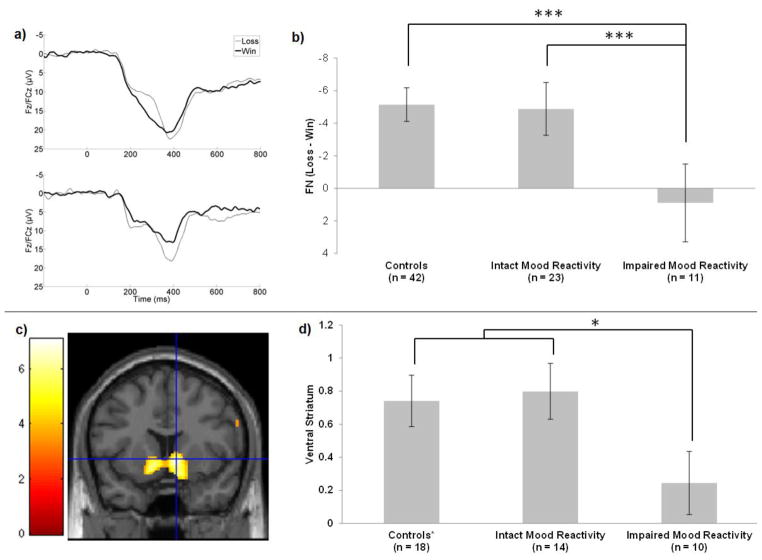
Next, the possibility of neurobiologically distinct MDD subgroups was explored. FN amplitude (Win vs. Loss) was not predicted by DSM subtype (Table 2). FN amplitude was strongly predicted, however, by mood reactivity to positive events (Figure 2a–b). The FN was substantially blunted among those depressed individuals reporting impaired mood reactivity (Impaired vs. Intact Mood Reactivity MDD subgroups: t(32) = 3.96, p < .001, d = 1.46; Impaired Mood Reactivity vs. Controls: t(51) = 4.96, p < .001, d = 1.66); depressed individuals with intact mood reactivity and never-depressed controls were indistinguishable in their FN amplitude (t(63) = .54, p = .59, d = .14). This group difference was driven primarily by a reduced response to reward feedback on win trials (Impaired vs. Intact Mood Reactivity MDD subgroups: t(32) = 2.74, p < .05, d = .99; Impaired Mood Reactivity vs. Controls: t(51) = 2.77, p < .01, d = .91), rather than to monetary loss (p’s > .30, d’s < .38). The within-subjects contrast (Win vs. Loss) revealed robust reward-related neural activity within the intact mood reactivity MDD subgroup (t(22) = 5.71, p < .001, d = 1.21), but not within the impaired mood reactivity MDD subgroup (t(10) = .84, p = .42, d = .30).
Figure 2.
Reduced reward-related neural activity among depressed individuals reporting impaired mood reactivity. (a) Feedback negativity (FN) among depressed individuals with intact (top) and impaired (bottom) mood reactivity. (b) Group means and standard errors for FN amplitude. (c) Ventral striatal (VS) activation across the full sample (y = 10). (d) Group means and standard errors for VS activation; controls include 12 non-depressed individuals not represented in part b. *p<.05, ***p<.001
Across the full MDD group, no significant associations were observed between blunted FN amplitude and anhedonia, symptom severity, or age of onset (r’s from .17 to −.32, all p’s > .05). Next, we examined the unique impact of illness characteristics on FN amplitude using multiple linear regression; mood reactivity status and scores on the two depression subscales of the MASQ (Anhedonic Depression and General Distress—Depression) were entered as simultaneous predictors. Controlling for anhedonia and symptom severity in this manner, the link between impaired mood reactivity and blunted FN amplitude remained significant (rpartial = .53, p< .01); the unique effects of the two symptom subscales were non-significant (both p’s > .15).
3.3 Reward Sensitivity: fMRI Data
Reward-related activity was isolated using a whole brain analysis for the Win>Loss contrast. This revealed robust bilateral activation in the VS across the full sample (Figure 2c), with a global maximum in the right VS (k = 1160, t(41) = 7.07, peak at MNI: 10, 10, -2).2 To capture between-subjects variation in VS activity, the modulating effects of MDD and mood reactivity were examined. Unlike the FN, there was no main effect of Group (MDD vs. Non-Depressed) on VS activity (t(40) = .84, p = .41, d = .26). As with FN amplitude, however, VS activity was blunted among those depressed individuals with impaired mood reactivity compared to the rest of the sample (Figure 2d; t(40) = 2.29, p < .05, d = .84); depressed individuals with intact mood reactivity were indistinguishable from the non-depressed comparison group in terms of VS activation (t(30) = .25, p = .80, d = .09). The within-subjects contrast revealed robust reward-related VS activation in the non-depressed comparison group (t(17) = 4.76, p < .001) and in the MDD subgroup with intact mood reactivity (t(13) = 4.73, p < .001), but not within the MDD subgroup with impaired mood reactivity (t(9) = 1.28, p = .23). This effect of mood reactivity was not captured by DSM subtype, which was unrelated to VS activation within the full depressed group (Table 2).3
Across the full MDD group, no significant associations were observed between reduced VS activation and anhedonia, symptom severity, or age of onset (r’s from .02 to −.40, p’s > .05). Next, we examined the unique impact of illness characteristics on VS activation using multiple linear regression; mood reactivity and scores on the two depression subscales of the MASQ (Anhedonic Depression and General Distress—Depression) were entered as simultaneous predictors. Controlling for anhedonia and symptom severity, the link between impaired mood reactivity and reduced VS activation was no longer significant (rpartial = .28, p = .21); the effects of the two symptom subscales were also non-significant (both p’s > .30).
3.4 Convergence Across ERP and fMRI Data
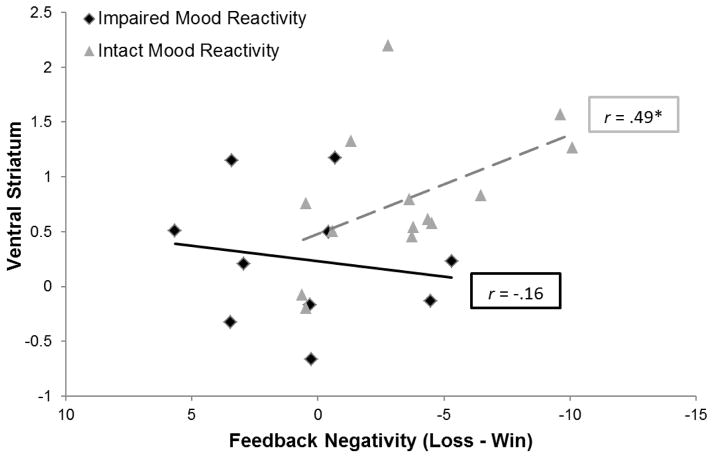
Among depressed individuals, there was convergence across electrophysiological and hemodynamic measures of reward sensitivity (Figure 3), with a significant correlation between FN amplitude and VS activation (r = .39, pone-tailed < .05). This association was driven by the MDD subgroup with intact mood reactivity (r = .49, pone-tailed < .05), and was not apparent within the MDD subgroup with impaired mood reactivity (r = −.16, pone-tailed = .33). The comparison of these correlation coefficients approached significance (z = 1.44, pone-tailed = .07).
Figure 3.
Scatterplot depicting the convergence across electrophysiological and hemodynamic measures of reward-related neural activity within depressed group, as a function of mood reactivity to positive events. *pone-tailed<.05
To examine for unique effects of FN amplitude and VS activation, we entered each as simultaneous predictors using binary logistic regression. When predicting Group (MDD vs. Non-Depressed), an effect was observed for FN amplitude (OR = .68, p < .01, 95% CI = .52–.88) but not VS activation (OR = .95, p = .93, 95% CI = .29–3.1). When predicting Mood Reactivity, an effect was again observed for FN amplitude (OR = .64, p < .01, 95% CI = .47–.87) but not VS activation (OR = .31, p = .18, 95% CI = .06–1.7).
4. Discussion
The current study sheds new light on the nature of reward dysfunction in MDD in three ways: First, building upon past ERP research in non-clinical samples (Bress, Meyer, et al., 2013; Bress et al., 2012; Foti & Hajcak, 2009) and replicating a recent report from a clinical sample (W. H. Liu et al., 2014), MDD was associated with reduced reward-related neural activity, as indicated by FN amplitude. Second, we found converging evidence across ERP and fMRI measures, such that FN amplitude and VS activation to reward were correlated within the MDD group. This replicates and extends the findings of a previous study conducted in an unselected sample (Carlson et al., 2011) and is the first application of a multimodal neuroimaging approach for characterizing reward insensitivity in MDD. Third, moving beyond standard diagnostic comparisons, we found that blunted FN amplitude was driven specifically by an MDD subgroup characterized by impaired mood reactivity to positive events; among the MDD subgroup with intact mood reactivity, FN amplitude was unaffected and indistinguishable from never-depressed controls. This effect of mood reactivity was independent of self-reported anhedonia, which did not significantly predict FN amplitude. Similarly, and demonstrating further convergence across ERP/fMRI measures, VS activation to rewards was blunted specifically among depressed individuals with impaired mood reactivity. These novel findings go beyond diagnostic differences in reward functioning related to MDD, linking hyposensitivity to reward to a more specific phenotype. In contrast, no effects were observed for the full, DSM-defined melancholic and atypical MDD subtypes, which did not adequately map onto neural data of reward dysfunction.
Mood reactivity was an effective ‘joint’ for dividing the MDD sample into two distinct subgroups: one characterized by reward dysfunction, and one by normal reward processing. This was not the case for the existing DSM subtypes of melancholic and atypical MDD, which did not predict impairment in either FN amplitude or VS activation. Within the DSM-5, impaired versus intact mood reactivity is recognized as a key illness characteristic for subtyping MDD (American Psychiatric Association, 2013). The current results lend biological support for this fundamental distinction, across both ERP and fMRI measures. Mood reactivity alone, however, is insufficient to subtype MDD within the current diagnostic framework, as a number of other, secondary symptoms must also be present. Considered in light of the current results, it is possible that the melancholic phenotype—as currently defined is—inadequate, which may explain the inconsistent support for melancholia as a distinct MDD subtype (Hadzi-Pavlovic & Boyce, 2012). The existing phenotype consists of a heterogeneous cluster of symptoms, but it is specifically the primary criterion of impaired mood reactivity which appears to be linked to abnormal reward processing. Building outward from these findings, it may be possible to continue to iteratively refine more specific MDD phenotypes in a manner that is directly grounded in basic neuroscience (Insel et al., 2010).
Depressed individuals with impaired mood reactivity also reported more severe anhedonia, but only mood reactivity captured variation in FN amplitude and VS activation. Anhedonia was measured using the Anhedonic Depression subscale of the MASQ, which combines numerous facets of hedonic capacity, including anticipatory and consummatory pleasure (Watson, Clark, et al., 1995; Watson, Weber, et al., 1995). As such, this general measure of anhedonia may be conflating subjective experiences that map onto the distinct reward components, such as ‘liking’ and ‘wanting’ (Berridge, Robinson, & Aldridge, 2009). Mood reactivity to positive events, by contrast, is a more narrowly defined construct: A persistently low, nonreactive mood even in the face of positive events. This construct relates more directly to ‘liking’, particularly the RDoC construct of Initial Responsiveness to Reward Attainment. The current pattern of results indicates a specific link between a facet of reward dysfunction (i.e., ‘liking’) and the key illness characteristic of mood reactivity, rather than a more global deficit in positive affect.
Other relevant reward facets, as outlined by RDoC, include Approach Motivation (i.e., ‘wanting’) and Reward Learning—both of which have been related to MDD. For example, there is evidence that while viewing pleasurable stimuli, MDD patients exhibit a deficit in subjective anticipatory but not consummatory pleasure (Sherdell, Waugh, & Gotlib, 2012). Other work has linked MDD to a behavioral deficit in the acquisition of reward contingencies (Pizzagalli et al., 2008; Vrieze et al., 2012). Considered alongside the current results, this line of evidence suggests that MDD is associated with disruptions in multiple facets of reward processing—but not necessarily within the same individuals. Indeed, basic research indicates that the facets of reward processing are mediated by dissociable neural circuitry (Smith, Berridge, & Aldridge, 2011), suggesting that some cases of MDD may be characterized primarily by a deficit in ‘liking’, others by deficits in ‘wanting’ or learning, and others by a combination of deficits in multiple reward facets. The RDoC initiative provides an essential framework for integrating the existing reward literature in MDD and further exploring this possibility.
Aside from anhedonia, individuals with impaired mood reactivity reported comparable symptoms of depression and anxiety. Thus, the impaired mood reactivity subgroup was not more ill overall, and classifying the current MDD sample based on mood reactivity provided unique clinical information not apparent from the other symptom measures. Whereas the DSM-defined subtypes of melancholic and atypical MDD have been criticized for being largely unrelated to treatment response (Bobo et al., 2011; Uher et al., 2011), a refined melancholic phenotype that is informed by neurobiological dysfunction may help to inform treatment approaches by explaining clinical heterogeneity that, at present, is not well understood. If the core role of mood reactivity status is validated in future research, this could be tested directly by assessing the moderating role of mood reactivity status on treatment outcome, separate from the DSM-defined subtypes. Conversely, it would also be informative to test the extent to which mood reactivity improves over the course of successful treatment—and whether this maps onto normalization of reward-related neural activity.
Given comparable severity of the current depressive episode across mood reactivity subgroups, it may be of interest to test for differences in course of illness (Klein, 2008). There is some evidence that melancholic MDD is more chronic than atypical MDD (Gili et al., 2012), a distinction which may become more pronounced as the phenotype is further refined. By isolating the subgroup of MDD indicated here—characterized by reward dysfunction and non-reactive mood—it may be possible to more accurately understand and predict illness trajectory. Indeed, preliminary evidence that reduced FN amplitude uniquely and prospectively predicts first-onset MDD in adolescence, over and above the influence of family history, trait neuroticism, and sub-threshold depressive symptoms (Bress, Foti, et al., 2013). Considered in light of the current findings, reduced FN amplitude and VS hypoactivation may be relevant biomarkers for the course of illness specifically within the subgroup of MDD with impaired mood reactivity. Furthermore, while age of onset was unrelated to reward dysfunction here, other research has linked an early age of onset to reduced left frontal neural activity during reward anticipation (Shankman, Klein, Tenke, & Bruder, 2007). It will be valuable for future work to evaluate how these different types of reward-related neural activity change over time in MDD and whether such changes can be used to better understand the course of illness, leading to more accurate predictions of symptom onset, remission, and recurrence.
In contrast to the subgroup with non-reactive mood, those depressed individuals with intact mood reactivity exhibited robust reward-related neural activity across ERP and fMRI indices. This implies that not all MDD is characterized by reward-related abnormalities; in some individuals with MDD, reward processing may be unaffected. In addition, FN amplitude and VS activation were directly correlated with one another, a novel result in the MDD literature. This is consistent with two previous studies in unselected samples (Becker et al., 2014; Carlson et al., 2011), indicating convergence across electrophysiological and hemodynamic measures of reward sensitivity. FN elicited appears to be a highly effective tool for quantifying reward dysfunction in MDD and clarifying the pathophysiology of melancholia, one which covaries directly with VS hypoactivation.
One limitation of the current study is the relatively small sizes of the MDD subgroups. Given that impaired mood reactivity was a fairly common phenomenon within the MDD sample (32.4%), it will be feasible to extend these findings to larger clinical samples. Impairment in mood reactivity was also not assessed among controls due to the assessment hierarchy of the SCID; future work may clarify whether mood reactivity similarly modulates reward-related neural activity within populations that do not meet full diagnostic criteria for MDD, including healthy controls as well as patients with subthreshold depressive symptoms. A second limitation is that mood reactivity was assessed via a single item from the SCID, which limits the reliability of this characteristic. We view the current results as a necessary first step for refining the melancholic phenotype and, if the link between impaired mood reactivity and reward dysfunction is further substantiated by future research, it will be important to develop a more thorough assessment tool for evaluating this illness characteristic.
The current study focused on an unmedicated, female sample with limited comorbidity, and it will be of interest to replicate these results in more heterogeneous patient populations. By considering a wider range of diagnostic and demographic categories, it will be possible to test how neurobiological evidence of reward dysfunction may be utilized to further improve our understanding of the boundaries between MDD and other co-occurring disorders. In the same way that the present study leveraged neural information of reward dysfunction to better understand individuals differences within MDD, it will be possible to apply this same approach to more clinically complex populations with numerous diagnoses, potentially utilizing evidence of reward dysfunction to identify novel, distinct subgroups that are not captured within the current diagnostic framework. For example, it would be of interest to consider the interplay between depressive symptoms and other characteristics known to impact reward processing, such as trait impulsivity and symptoms of attention-deficit hyperactivity disorder (Plichta & Scheres, 2014).
Reward dysfunction is thought to play a central role in the pathophysiology of MDD, and the current study builds upon the existing literature by utilizing a multimodal neuroimaging approach. We found converging evidence across methods, such that FN amplitude covaried with VS activation. Further, both methods indicated the possibility of a neurobiologically distinct subgroup of MDD characterized by impaired mood reactivity to positive events, a core feature of melancholic MDD. This subgroup exhibited significant reward dysfunction, whereas in the subgroup with intact mood reactivity reward processing was unaffected. The full, DSM-defined subtypes of melancholic and atypical MDD, however, did not map onto reward dysfunction. The existing subtypes may be strengthened by integrating clinical definitions more closely with evidence of biological dysfunction. Mood reactivity is a key illness feature that may represent a fundamental divide between distinct subgroups of MDD—indicated by reward dysfunction—and offers a route for further refinement of the melancholic phenotype.
Highlights.
We empirically link fMRI/ERP measures of abnormal reward processing in depression
Reward dysfunction was specific to depressed subjects with impaired mood reactivity
The feedback negativity (FN) ERP and ventral striatal (VS) activation were blunted
Reward dysfunction was not explained by the full, DSM-defined depression subtypes
VS activation and FN amplitude were positively correlated in the depressed group
Acknowledgments
This research was funded in part by Dr. Foti’s grant (NIMH, F31-MH090658).
Footnotes
Race did not moderate the current results: Across the full sample, FN amplitude and VS activation (Win vs. Loss) were comparable across Race category (both p’s > .25). Within the MDD group, the effect of impaired mood reactivity on both measures of reward-related neural activity remained significant after adjusting for Race (FN: F(1,29) = 9.10, p < .01; VS: F(1,20) = 5.56, p < .05).
Although data were not corrected for multiple comparisons during whole-brain analyses, an examination of cluster-wise and peak-wise corrections revealed that both the cluster (p < .001 FWE-corrected) and peak-voxel (p < .01 FWE-corrected) passed correction for multiple comparisons.
Due to the different healthy comparison groups used for the ERP and fMRI analyses, we also replicated these between-groups comparisons when considering only those participants with full data (MDD: n = 24; Controls: n = 6). An identical pattern of results emerged: The FN (Win vs. Loss) was blunted among the MDD group compared to Controls (F(1,28) = 4.68, p<.05), and this effect was driven specifically by the MDD subgroup with impaired mood reactivity (Impaired Mood Reactivity vs. Control: t(14) = 3.97, p < .01; Impaired vs. Intact Mood Reactivity: t(22) = 2.83, p < .05; Intact Mood Reactivity vs. Control: t(18) = 1.29, p = .21). Similarly, the effect of mood reactivity on VS activation remained significant, with the MDD subgroup with impaired mood reactivity exhibiting blunted VS activation compared to the rest of the sample (t(28) = 2.10, p < .05); the Intact Mood Reactivity subgroup and Controls did not differ from one another (t(18) = .50, p = .62).
Financial Disclosures
The authors of this report have no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Becker MP, Nitsch AM, Miltner WH, Straube T. A Single-Trial Estimation of the Feedback-Related Negativity and Its Relation to BOLD Responses in a Time-Estimation Task. J Neurosci. 2014;34(8):3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berto P, D'Ilario D, Ruffo P, Di Virgilio R, Rizzo F. Depression: Cost-of-illness studies in the international literature, a review. Journal of Mental Healthy Policy and Economics. 2000;3(1):3–10. doi: 10.1002/1099-176x(200003)3:1<3::aid-mhp68>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Chen H, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Shelton RC. Randomized comparison of selective serotonin reuptake inhibitor (escitalopram) monotherapy and antidepressant combination pharmacotherapy for major depressive disorder with melancholic features: a CO-MED report. J Affect Disord. 2011;133(3):467–476. doi: 10.1016/j.jad.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bress JN, Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50(7):610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Hajcak G. Differentiating Anxiety and Depression in Children and Adolescents: Evidence From Event-Related Brain Potentials. J Clin Child Adolesc Psychol. 2013 doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symtpoms from late childhood to early adolescence. Biol Psychol. 2012;89:156–162. doi: 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Harmon-Jones E, Proudfit GH. Midbrain volume predicts fMRI and ERP measures of reward reactivity. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0725-9. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders--Patient Edition (SCID-I/P 2/2001 Revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biol Psychol. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Kotov R, Klein DN. Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. J Abnorm Child Psychol. 2011;39(7):913–924. doi: 10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from non-rewards: Temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gili M, Roca M, Armengol S, Asensio D, Garcia-Campayo J, Parker G. Clinical patterns and treatment outcome in patients with melancholic, atypical and non-melancholic depressions. PLoS One. 2012;7(10):e48200. doi: 10.1371/journal.pone.0048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hadzi-Pavlovic D, Boyce P. Melancholia. Curr Opin Psychiatry. 2012;25(1):14–18. doi: 10.1097/YCO.0b013e32834dc147. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14(5):711–724. [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model's prediction of anhedonia's specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Research. 2003;119(3):243–250. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Catanzaro SJ, Laurent J. Tripartite structure of positive and negative affect, depression, and anxiety in child and adolescent psychiatric inpatients. J Abnorm Psychol. 1996;105(3):401–409. doi: 10.1037//0021-843x.105.3.401. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. Epidemiology of depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. 2. New York: Guilford Press; 2009. pp. 5–22. [Google Scholar]
- Klein DN. Classification of depressive disorders in the DSM-V: proposal for a two-dimension system. J Abnorm Psychol. 2008;117(3):552–560. doi: 10.1037/0021-843X.117.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, de Jonge P, Nolen WA, Smit JH, Zitman FG, Beekman AT, Penninx BW. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA) J Clin Psychiatry. 2010;71(12):1582–1589. doi: 10.4088/JCP.09m05398blu. [DOI] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, Chan RC. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. Sydney: Psychology Foundation; 1995. [Google Scholar]
- Luppa M, Heinrich S, Angermeyer MC, Konig HH, Riedel-Heller SG. Cost-of-illness studies of depression: A systematic review. J Affect Disord. 2007;98(1–2):29–43. doi: 10.1016/j.jad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Dahl RE, Ryan ND, Silk JS, Birmaher B, Forbes EE. “I won, but I'm not getting my hopes up”: depression moderates the relationship of outcomes and reward anticipation. Psychiatry Res. 2011;194(3):393–395. doi: 10.1016/j.pscychresns.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Dillon DG, Bogdan R, Holmes AJ. Reward and punishment processing in the human brain: Clues from affective neuroscience and implications for depression research. New York, NY: Psychology Press; 2011. [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GM. The differentiation of melancholia, the depressive phase of manic-depressive insanity. Journal of Mental Science. 1911;57(238):415–457. [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: a biobehavioral study. J Abnorm Psychol. 2007;116(1):95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121(1):51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108(27):E255–264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130(Pt 9):2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73(2):114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Uher R, Dernovsek MZ, Mors O, Hauser J, Souery D, Zobel A, Farmer A. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J Affect Disord. 2011;132(1–2):112–120. doi: 10.1016/j.jad.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Claes S. Reduced Reward Learning Predicts Outcome in Major Depressive Disorder. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]