Abstract
Background
Using blood utilization data acquired from our anesthesia information management system, an updated institution-specific maximum surgical blood order schedule (MSBOS) was introduced. We evaluate whether the MSBOS, along with a remote electronic blood release system (EBRS), reduced unnecessary preoperative blood orders and costs.
Methods
At a large academic medical center, data for preoperative blood orders were analyzed for 63,916 surgical patients over a 34-month period. The new MSBOS and the EBRS (Hemosafe®, Haemonetics Corp., Braintree, MA) were introduced mid-way through this time period. We assessed whether these interventions led to reductions in unnecessary preoperative orders and associated costs.
Results
Among patients having surgical procedures deemed not to require a type and screen or crossmatch (n = 33,216), the percentage of procedures with preoperative blood orders decreased by 38% [from 40.4% (7,167 of 17,740 patients) to 25.0% (3,869 of 15,476 patients), P < 0.001]. Among all hospitalized inpatients, the crossmatch-to-transfusion ratio decreased by 27% (from 2.11 to 1.54; P < 0.001) over the same time period. The proportion of patients who required emergency release uncrossmatched blood increased from 2.2 to 3.1 per 1,000 patients (P = 0.03); however, most of these patients were having emergency surgery. Based on the realized reductions in blood orders, annual costs were reduced by $137,223 ($6.08/patient) for surgical patients, and by $298,966 ($6.20/patient) for all hospitalized patients.
Conclusions
Implementing institution-specific, updated MSBOS-directed preoperative blood ordering guidelines along with an EBRS results in a substantial reduction in unnecessary orders and costs, with a clinically insignificant increase in requirement for emergency release blood transfusions.
Introduction
Optimizing the process of preoperative blood ordering can potentially improve operating room efficiency, increase patient safety, and decrease costs. With medical costs increasingly scrutinized and healthcare stakeholders looking for quality metrics, it is important to standardize care and reduce unnecessary laboratory testing, especially as new patient care models such as “Perioperative Surgical Home”Ω and “Choosing Wisely”Δ are introduced. Over the past decade, a number of medical societies have emphasized the need to reduce unnecessary transfusion by following evidence-based guidelines.1-4 However, reducing the unnecessary ordering and preparation of blood components remains an area of opportunity to improve care and reduce costs.
The maximum surgical blood order schedule (MSBOS), first described in the 1970s, is a list of recommended preoperative blood orders for various types of surgical procedures.5-7 Some primary concerns regarding the MSBOS are that the recommendations are often outdated, based on opinion, do not include recently developed surgical procedures, and are not based on institution-specific blood utilization data. At our institution, we recently created an updated MSBOS based on institution-specific blood utilization data from more than 53,000 patients undergoing 135 categories of surgical procedures.8 In the 2013 publication describing our methods for creating the MSBOS,8 we hypothesized that the MSBOS would reduce the number of unnecessary blood orders and the associated costs for patients having procedures with extremely low rates of transfusion, but until now this hypothesis remained untested.
Preoperative blood ordering refers to obtaining either a type and screen (T/S) or a type and crossmatch (T/C) in anticipation of transfusion for surgical patients. With T/S, a patient specimen is sent to the blood bank, where it is typed for ABO and Rh and screened for the presence of any erythrocyte antibodies. If patients do not have antibodies and their ABO blood group has been assessed at least two times, and the transfusion service has a validated computer system that contains logic to determine discrepancies, an electronic crossmatch may be performed.9,10 Electronic crossmatch relies on this computer system to confirm that ABO-group specific compatible blood will be provided to the patient. Since the electronic crossmatch is substantially faster than the serologic crossmatch and can be performed immediately prior to transfusion, it is likely that the improved blood ordering efficiency as assessed by the crossmatch-to-transfusion ratio (C/T ratio) would be achieved. Today a C/T ratio of 2.0 or lower is considered ideal, and can be used to benchmark clinical practice.11,12
The electronic crossmatch has led to another major advance in transfusion medicine, called the remote electronic blood release system (EBRS). First described over a decade ago,13,14 the EBRS has now evolved to become a “vending machine” for blood, located in the operating room suite, that is electronically linked by a software interface to the blood bank. Early reports of the EBRS describe multiple benefits including a faster delivery of blood products to the patient, improved inventory control, and reduced time and effort for the blood bank staff.13,14 Since we introduced the EBRS simultaneously with the updated MSBOS, we attributed the changes in preoperative blood ordering practices to the combination of these two interventions.
In the current study, we analyzed blood utilization data acquired from our anesthesia information management system (AIMS) to assess changes in preoperative blood ordering practices after the introduction of both the updated MSBOS guidelines and the EBRS. We tested the hypothesis that implementing these measures would result in reductions in blood orders deemed to be unnecessary, the C/T ratio, and overall costs.
Materials and Methods
After receiving approval from the institutional review board at the Johns Hopkins Medical Institutions (Baltimore, Maryland), we analyzed data for patients who had surgery between January 2011 and October 2013. All data for surgical patients were obtained from the AIMS database (Metavision, iMDsoft, Needham, MA). We have previously described the data acquisition and data validation processes for this system.15 During the 34-month time period, 100,789 patients underwent a procedure that required anesthesia. To avoid the confounding influence of special patient populations for whom blood ordering and blood utilization differ substantially, we excluded 22,679 pediatric patients (age <18 yr), 808 obstetrical patients, 6,707 gastrointestinal endoscopy patients, and 16,347 ophthalmologic patients. We also excluded 333 patients because of missing preoperative blood order data. We analyzed the remaining 63,916 patients to assess changes in practice over time as we implemented the new institution-specific MSBOS preoperative blood ordering guidelines and educated providers about these new guidelines.
An updated MSBOS was officially introduced July 1, 2012, approximately halfway through the time period that was studied. The MSBOS, a one-page list of surgical procedure categories and the corresponding recommended preoperative blood orders, was developed from data collected from our AIMS database using algorithms described previously.8 Criteria included in the algorithm were: 1) 5% of more of patients receiving erythrocyte transfusions, 2) Average number of erythrocyte units per patient ≥ 0.3, and 3) median intraoperative estimated blood loss > 50 mL. The MSBOS document is included here (see fig. 1, Supplemental Digital Content 1). The three categories of possible blood orders were: (1) no sample (no blood orders needed), (2) T/S, and (3) T/C. For the third category (T/C), the recommended number of crossmatched units is also indicated on the MSBOS. This MSBOS was the first complete blood order schedule to be published since the original schedule was published in 1976 by Friedman et al.6 To implement the new guidelines, we appended the new MSBOS to the computerized provider order entry system. Additionally, information about the new blood ordering guidelines was disseminated at Grand Rounds presentations and through email notifications to the Departments of Anesthesiology and Surgery. Also included in the educational process was an explanation of the electronic crossmatch procedure that has been in place at our institution for over 10 yr. Many of our medical providers, however, were unaware of this procedure, and the fact that electronic crossmatching takes less than a minute to complete for patients who are eligible. After implementation of the MSBOS, from August 2012 to February 2013, preoperative blood ordering practice audits were conducted, and email feedback was sent to providers when blood orders were placed for patients having surgery deemed not to require blood orders according to the MSBOS. This message is included in the appendix 1.
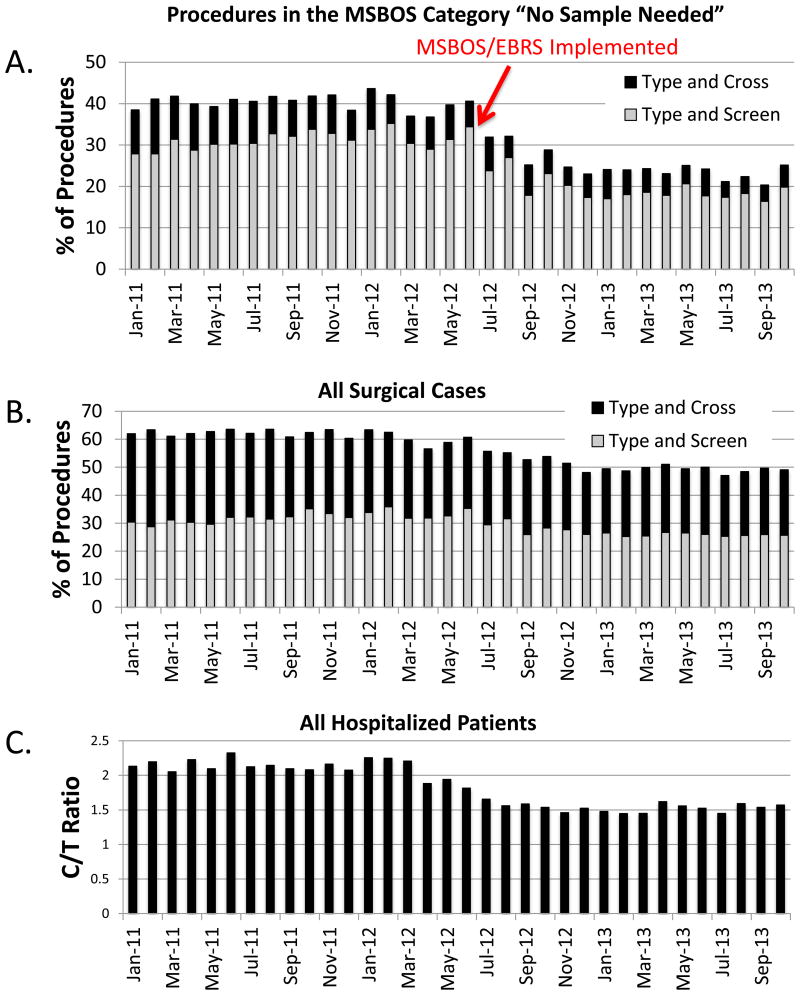
Fig. 1.
Blood ordering practices from the periods before and after release of the updated, institution-specific MSBOS and EBRS were implemented in July 2012. (A) Among patients undergoing surgical procedures in the “no sample needed” category of the MSBOS, blood orders considered to be unnecessary (type and screen and type and cross combined) decreased by 38.1%. (B) For all surgical cases, blood orders decreased by 17.8%. (C) For all hospitalized patients, there was a concomitant decrease in the crossmatch-to-transfusion ratio (C/T ratio). EBRS = remote electronic blood release system, MSBOS = maximum surgical blood order schedule.
The EBRS (Hemosafe®, Haemonetics Corp., Braintree, MA) was introduced in May, 2012, two months prior to release of the updated MSBOS. These remote machines are linked to the blood bank by a software interface (Bloodtrack®, Haemonetics) to report on use and availability of erythrocyte unit inventory. Three of these machines were activated; one for each operating room suite. Because of the 2-month training period, and sequential activation of each machine, both the MSBOS and the EBRS were implemented simultaneously in July, 2012. The EBRS machines were located no further than 30 meters from any given operating room in the surgical suite, which allowed easy and rapid access to blood on demand.
Because the AIMS data are applicable only to those patients having procedures that require surgery and anesthesia, we used a second database (IMPACT Online®, Haemonetics Corp.) to assess the C/T ratio for all hospitalized inpatients (n = 136,640) over the same time period (January, 2011 – October, 2013). The details regarding the extraction and validation for these data have also been described previously.16 We divided the total number of crossmatches by the total number of erythrocyte units transfused for each 1-month period, and determined the percent change in the C/T ratio from the pre- to post-MSBOS/EBRS time periods by comparing the average C/T ratio for all months before release of the MSBOS/EBRS to the monthly average for the C/T ratio after release of the MSBOS/EBRS.
The analysis was designed to compare blood ordering practice for two time periods (pre- vs. post-MSBOS/EBRS), for percentages of patients who had no preoperative blood ordered, T/S ordered, and T/C ordered. The incidence of transfusion with emergency release erythrocytes was also compared for the two time periods. Based on the recommended blood orders from the MSBOS, we performed a subgroup analysis for patients having surgical procedures determined by the MSBOS not to require blood orders, to assess changes in blood orders (both T/S and T/C) pre- and post-MSBOS/EBRS.
An ongoing challenge in our hospital is the substantial number of blood orders placed on the morning of the surgical procedure, which we refer to here as “same-day sample” blood orders. Of concern are the patients who have antibodies present on their T/S and thus are potentially difficult to crossmatch. Such patients are at risk for having a surgical start before blood is available. The implications for patient safety are obvious, and given the urgency of completing such orders, we aimed to reduce the number of same-day orders that were considered to be unnecessary. Data were available for the period of January 2012 to January 2014 for surgical patients whose blood sample for T/S or T/C was not received until the day of the surgical procedure. We analyzed these data to determine whether the total number of same-day samples received per month changed after release of the MSBOS/EBRS. In addition, we assessed whether there was a decrease in the number of same-day blood orders considered to be unnecessary according to the MSBOS.
We calculated the total annualized cost and per patient cost of preoperative blood orders before and after release of the MSBOS/EBRS. Since we defined costs for the purposes of this study as the cost to the payer, not to the hospital, the 2013 Medicare reimbursement rates were used for calculating these costs. This cost for an ABO type, Rh type and antibody screen is $37.30, plus $17.96 for each erythrocyte unit that was crossmatched.Φ These costs were then multiplied by the percentage of patients for which these tests were ordered, and by the annualized number of surgical patients in the pre- and post-MSBOS time periods. To adjust for the 2.4% increase in surgical patient volume in the post-MSBOS period, we used the following formula:
We conducted a second cost analysis based on the change in C/T ratio that included all hospitalized inpatients over the same time period. Although, the MSBOS/EBRS implementation were specifically targeted for surgical patients, awareness and education regarding the electronic crossmatch procedure applied to the care of all inpatients, and therefore we sought to determine if the C/T ratio for all hospitalized patients changed over time. The annualized costs (to the payer) for crossmatches for the two time periods (pre- vs. post-MSBOS/EBRS) were compared by using the 2013 Medicare reimbursement rate. To adjust for the 6.1% increase in inpatient volume in the post-MSBOS period, we used the following formula:
Statistical Analysis
All continuous data were analyzed by Student's t tests and are presented as mean ± SD. Dichotomous data were analyzed by Chi squared tests. Data not normally distributed and ordinal data are presented as median (interquartile range) and were analyzed by the Wilcoxon rank-sum test. Changes over time were analyzed by comparing the mean monthly values prior to and after the interventions took place, in July, 2012. Such time-series analyses are susceptible to misattributing underlying trends that existed before the introduction of the intervention as intervention effects, but no such trends were apparent. All statistical analyses were performed using JMP® (ver 9.0.0, SAS Instiututes Inc., Cary NC) and all hypothesis testing was two-tailed in design. Significance was defined as P < 0.05.
Results
The surgical patient populations studied during the two time periods are compared in table 1. Some parameters had small but statistically significant differences between the two time periods. Notably, after the MSBOS/EBRS was implemented, there was a greater percentage of male patients, but mean age and American Society of Anesthesiologists classification were unchanged. The MSBOS classification was determined for all patients based on the surgical procedures. Mean hemoglobin concentrations upon admission, at the nadir during hospitalization, and upon discharge were similar in the two time periods. Unexpectedly, in the post-MSBOS/EBRS time period, the percentage of patients who were classified to require a T/S increased, and the percentage of patients who required no sample decreased. In addition, the percent of patients who received an erythrocyte transfusion, and the average intraoperative number of erythrocyte units per patient both increased in the post-MSBOS/EBRS time period. This increase in both the number of patients requiring blood orders and in erythrocyte transfusion for the post-MSBOS/EBRS time period suggests that the complexity of our surgical case mix increased over time during the study period.
Table 1. Comparison of Surgical Patients in the Pre-MSBOS/EBRS and Post-MSBOS/EBRS Time Periods.
| Surgical Service | Pre-MSBOS/EBRS (n = 33,457) |
Post-MSBOS/EBRS (n = 30,459) |
P Value |
|---|---|---|---|
| Age (yrs, mean ± SD) | 53 ± 17 | 53 ± 17 | 0.59 |
| Males (n, %) | 15,920 (47.6%) | 14,897 (48.9%) | 0.008 |
| ASA Classification (Median, IQR) | 2 (2, 3) | 2 (2, 3) | 0.74 |
| Blood orders indicated by the MSBOS | 0.004 | ||
| No sample (n, %) | 17,740 (53.0%) | 15,476 (50.8%) | |
| T/S (n, %) | 5,781 (17.3%) | 5,936 (19.5%) | |
| T/C (n, %) | 9,936 (29.7%) | 9,047 (29.7%) | |
| Hb upon admission (g/dL, mean ± SD) | 12.5 ± 2.0 | 12.6 ± 2.0 | 0.09 |
| Hb, hospital nadir (g/dL, mean ± SD) | 9.8 ± 2.4 | 9.9 ± 2.4 | 0.08 |
| Hb upon discharge (g/dL, mean ± SD) | 10.6 ± 1.9 | 10.6 ± 1.9 | 0.91 |
| Estimated blood loss [(median (IQR)] | 100 (50, 300) | 100 (50, 300) | 0.54 |
| Patients receiving erythrocytes (n, %) | 2,598 (7.8%) | 2,587 (8.5%) | 0.006 |
| Intraoperative erythrocyte units/patient (mean ± SD) | 0.25 ± 1.9 | 0.28 ± 1.9 | 0.03 |
ASA = American Society of Anesthesiologists; Hb = hemoglobin; IQR = interquartile range, EBRS = remote electronic blood release system, MSBOS = maximum surgical blood order schedule; T/C = type and crossmatch; T/S = type and screen
Changes over time in preoperative blood orders (both T/S and T/C) for surgical patients and in the C/T ratio for all hospitalized inpatients are shown in figure 1. Among patients in the MSBOS “no sample needed” category, the monthly average for percentage of patients with any preoperative blood orders (either T/S or T/C) decreased by 38.1%, from 40.4 ± 1.9% (7,167 of 17,740 patients) in the pre-MSBOS/EBRS period to 25.0 ± 3.3% (3,869 of 15,476 patients) in the post-MSBOS/EBRS period (P < 0.001; fig. 1A). This change was accounted for by a decrease in both T/S and T/C orders. Among all surgical patients, the monthly average for percentage of patients with any preoperative blood orders decreased by 17.8%, from 61.6 ± 1.9% (20,610 of 33,457 patients) in the pre-MSBOS/EBRS period to 50.6 ± 2.5% (15,412 of 30,459 patients) in the post-MSBOS/EBRS period (P < 0.001; fig. 1B). This change was also accounted for by a decrease in both T/S and T/C orders. For all hospitalized patients, during the same time periods, the C/T ratio decreased by 27.1%. The average monthly C/T ratio was 2.11 ± 0.13 before the MSBOS/EBRS, and 1.54 ± 0.07 after MSBOS/EBRS implementation (P < 0.001; fig. 1C).
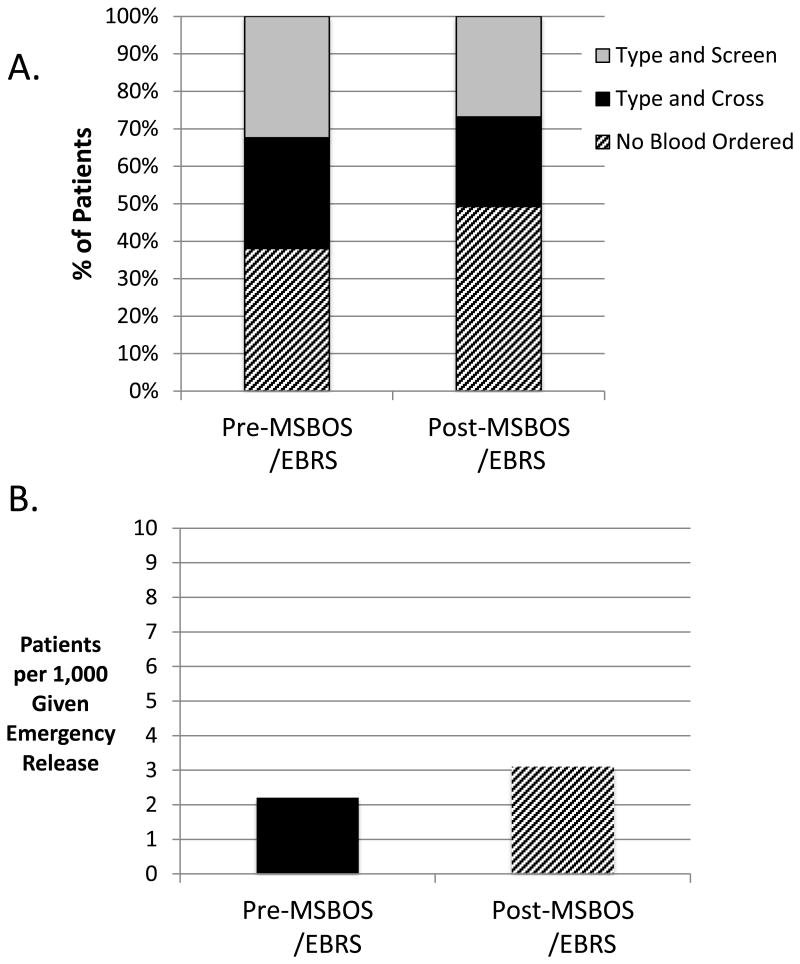
For all surgical patients, we also compared the pre- and post-MSBOS/EBRS time periods for all preoperative blood orders (T/S and T/C) and for those with no preoperative blood orders (fig. 2A). Compared to the pre-MSBOS/EBRS period, the percentage of patients with no blood ordered was significantly greater in the post-MSBOS/EBRS period, and the percentage of patients with T/S and T/C ordered was significantly lower in the post-MSBOS/EBRS period (P < 0.001).
Fig. 2.
A comparison of preoperative blood orders in the pre-MSBOS / EBRS and post-MSBOS / EBRS time periods among all surgical patients. (A) The percentage of patients who received type and screen and type and crossmatch orders decreased while the percentage of patients with no preoperative blood orders increased. (B) This change in practice was associated with a small increase of one additional patient per thousand that required emergency release type-O, uncossmatched erythrocyte transfusion. EBRS = remote electronic blood release system, MSBOS = maximum surgical blood order schedule.
Transfusion of uncrossmatched emergency release (Type O) blood was also evaluated. Pre-MSBOS/EBRS, the rate of emergency release of erythrocyte transfusions was 2.2 patients per 1,000, and post-MSBOS/EBRS it was 3.1 patients per 1,000 (fig. 2B). Despite this exceedingly low incidence of emergency release blood transfusions, this increase was statistically significant (P = 0.03). Over the entire 34-month time period, 162 patients required emergency release blood, and 98 (60%) of those patients were having emergency surgery. Of those patients in the “no sample needed” category of the MSBOS, only 7 of 17,740 (0.4 in 1,000 patients) and 16 of 15,476 (1.0 in 1,000 patients) required transfusion with emergency release blood in the pre- and post-MSBOS time periods, respectively (P = 0.05).
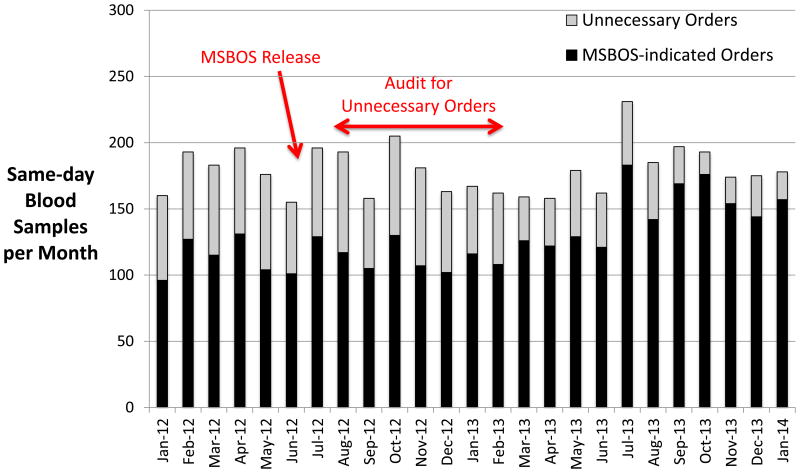
Figure 3 shows a comparison of same-day sample orders. Although the total number of same-day samples per month did not decrease, the number and percentage of these blood orders considered to be unnecessary according to the MSBOS decreased over time (from 36 ± 3% pre-MSBOS/EBRS to 26 ± 2% post-MSBOS/EBRS, P = 0.02). The decrease in unnecessary same-day sample orders decreased even further after the audits with feedback were sent to providers (from 36 ± 3% to 18 ± 6%, P < 0.001).
Fig. 3.
The total number of preoperative blood orders placed on the day of surgery (same-day blood sample orders) is shown for each month in the study period. Although the total number of same-day blood orders did not change over time, the number of same-day preoperative blood orders considered to be unnecessary decreased, especially after audits were conducted to inform providers when preoperative blood orders were considered to be unnecessary. MSBOS = maximum surgical blood order schedule.
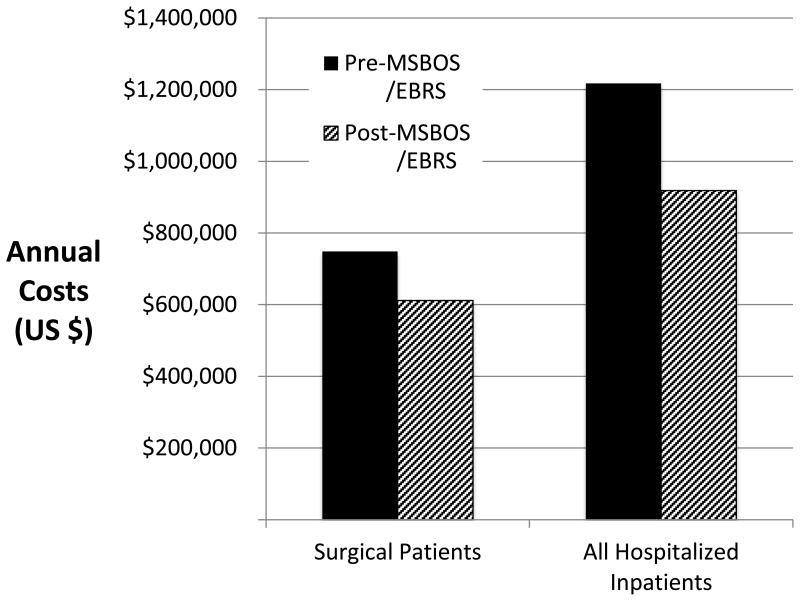
The calculated hospital costs for preoperative blood orders are shown in figure 4. When cost was analyzed by using the number of surgical patients who had a T/S and T/C ordered and the 2013 Medicare reimbursement rates, the annual costs at our institution decreased by $137,223 ($6.08/patient) after implementation of the MSBOS/EBRS. When the annualized cost of crossmatches for all hospitalized inpatients was assessed according to the C/T ratios before (n = 67,782 annualized crossmatches) and after (n = 54,279 annualized crossmatches) the new MSBOS was implemented, the decrease in cost was $298,966 per year ($6.20/patient).
Fig. 4.
Annualized costs for type and screen and type and crossmatch blood orders are shown for all surgical and all hospitalized patients included in the study. After implementation of the MSBOS and EBRS, annual costs decreased by $137,223 in surgical patients ($6.08/patient) and by $298,966 ($6.20/patient) in all hospitalized patients. EBRS = remote electronic blood release system, MSBOS = maximum surgical blood order schedule.
Discussion
At our institution, we recently implemented a new MSBOS based on current institution-specific, AIMS-acquired data for 135 categories of surgical procedures.8 At the same time we initiated an EBRS, which has been previously shown to allow faster and more efficient delivery of blood products to the patient, primarily due to the electronic crossmatch procedure and the closer proximity of blood to the operating rooms.17 Using these two interventions, we achieved a 38% decrease in preoperative blood orders for procedures where orders were deemed to be unnecessary. In addition, the C/T ratio for the entire hospital decreased from 2.11 to 1.54 after the changes were implemented. This decrease in excessive blood ordering has resulted in an annual cost reduction of almost $300,000. These changes in practice align nicely with the goals of the American Society of Anesthesiologists-endorsed Perioperative Surgical Home and Choosing Wisely campaigns, both of which advocate for standardization of care, elimination of duplicate or unnecessary tests, and overall cost reduction.
Updating and implementing an MSBOS has substantial benefits. Since the first MSBOS was published in the 1970s,5-7,18 there have been numerous changes in surgical procedures and blood management, including the introduction of laparoscopic and robotic techniques, new cautery devices, intraoperative autologous blood salvage, hemostatic agents, and a generalized improvement in surgical techniques. For example, of the 1,137 robotic radical prostatectomy procedures included in the current study, only one patient received an erythrocyte transfusion. Emergency release type-O blood, which was administered in this particular case, is associated with a 0.2% incidence of a mild delayed hemolytic reaction.19,20 We propose that such a reaction would occur in only 2 patients out of every 1,000,000 who have this procedure without preoperative blood orders (1 per 1,000 transfused × 2 per 1,000 with reaction). The likelihood of a major ABO incompatibility hemolytic reaction is greater, based on the incidence of clerical errors and the wrong unit being transfused, which is reported to occur in 1.5 per 100,000 transfused patients.21,22 The MSBOS clearly helps anesthesiologists, surgeons, and transfusion medicine physicians and staff to identify which surgical cases might require blood and which do not. Implementation of the MSBOS also decreased the number of unnecessary blood orders on the same day of surgery. However, we were disappointed to note that the total number of same-day surgery samples received in the blood bank did not change over time. Although we did not specifically assess these occurrences, by clearly defining ahead of time the need for blood orders, these new guidelines should decrease the number of surgical cases that do not have blood available before the surgical start time, a practice not in compliance with The Joint Commission guidelines.23 Thus, the MSBOS may also decrease delays in starting surgical cases,24 decrease excess ordering of laboratory tests,18 and promote the goals of patient blood management programs.
Since methods to improve patient blood management are multifactorial, it is common to have more than one intervention occurring at the same time. We recognize that both the updated MSBOS and the EBRS interventions occurred simultaneously, and therefore we were unable to determine which of these contributed to the reduction in unnecessary blood orders. It seems logical to conclude, however, that the MSBOS would be more likely to reduce unnecessary T/S orders, while the EBRS, which relies on the electronic crossmatch, is more likely to reduce T/C orders and thus the C/T ratio. This theory is based on the MSBOS algorithm we used, which is designed to identify surgical procedures with exceedingly low transfusion rates, and thus no need for either a T/S or T/C. The EBRS, however, along with education of providers regarding the electronic crossmatch procedure, should reduce T/C orders, since a T/C can be quickly accomplished and blood units quickly delivered to the operating room or bedside. For these reasons, we decided to assess both surgical patients and all hospitalized patients since implementation and education regarding the electronic crossmatch is relevant not only for surgical patients.
The combination of the updated MSBOS and EBRS was associated with a reduction in preoperative blood orders placed unnecessarily. Healthcare spending has increased at a faster rate than overall gross domestic product,25 and the cost of blood transfusion has also increased dramatically over the past two decades.26-28 The portion of total hospital costs attributable to blood transfusion varies by disease and procedure, with transfusion representing 1% or less of total costs for most conditions.29 However, transfusion plays a more substantial financial role (5-9% of total hospital costs) for other medical treatments associated with higher transfusion rates, such as liver transplantation and complex cardiac and vascular procedures. Thus, it is important to identify cost-saving measures related to patient blood management, besides reducing the number of units transfused.
The C/T ratio has been used traditionally to evaluate the appropriateness of erythrocyte orders, with an optimal ratio described as < 2.0.11,12,30 Our interventions significantly decreased the C/T ratio, and in our case was associated with lowering the ratio to less than 2.0. There are limitations, however in using the C/T ratio. For example, patients having massive transfusion for trauma will artificially lower the ratio. Thus, it is important to keep the C/T ratio in context.
The MSBOS/EBRS intervention we describe does not appear to have compromised patient safety. In the current era of decreased staffing, the MSBOS can help providers to focus on the most critical laboratory activities. Historically, the serologic crossmatch was performed before all transfusions to ensure that the patient received the correct ABO type and that no unexpected erythrocyte antibodies were present.9 However, as technology progressed, the antibody screen has been enhanced to be 99.99% effective in preventing transfusion of incompatible blood.31
The electronic crossmatch has now replaced the serologic crossmatch for the vast majority of patients who have had at least two T/S samples tested and do not have any previously detected alloantibodies.9 In one published study, no acute hemolytic transfusion reactions occurred among 161 patients receiving 581 units of uncrossmatched, type O erythrocytes.19 In addition, delayed hemolytic transfusion reactions are rare at <0.5% for transfusion of uncrossmatched erythrocytes.20 Thus, the increase in patients who received emergency release blood after implementation of the MSBOS/EBRS, does not appear to have had any clinically relevant patient safety implications. Moreover, most individuals in our study who received uncrossmatched erythrocytes were trauma patients or those having emergency surgery, and the MSBOS/EBRS intervention did not change the blood orders for these types of patients. Given the very low frequency of adverse events in patients receiving uncrossmatched type-O blood,19 a study powered to compare event rates before and after our interventions would require an exceedingly large sample size. That being said, we are unaware of any patient in our series that suffered from a clinically evident hemolytic reaction.
This retrospective study of blood ordering has several limitations. First, there were small but statistically significant differences in baseline characteristics of the patients in the two time periods, due to the large number of patients in this study. A greater percentage of patients required blood orders and a greater percentage of patients were transfused in the post-MSBOS/EBRS period than in the pre-MSBOS/EBRS period. This difference is suggestive of increased overall surgical procedure complexity in the post-MSBOS/EBRS time period. Nevertheless, fewer preoperative blood orders were placed in the post-MSBOS/EBRS period, with only a small increase in the use of emergency release transfusions. Although this increase was statistically significant, we believe that the clinical significance of 2 versus 3 in 1,000 patients administered uncrossmatched type-O erythrocytes is negligible. Furthermore, the use of emergency release blood for patients in the “no sample needed” category of the MSBOS was only increased from 0.4 to 1.0 per 1,000 cases, and no clinically significant cases of hemolytic reactions were recognized. Another limitation is that these findings are from a single institution. Thus, further applicability of our findings may or may not be relevant for other institutions. Another limitation of the MSBOS in general, is that blood orders should be modified for patients with preoperative anemia, as we suggested in our original publication.8 Finally, the cost of an EBRS system should also be taken into account in the overall cost analysis. At our institution we calculate that the cost savings achieved in 1 yr amounts to the approximate cost of one EBRS machine. However, it is also possible that implementing the MSBOS-based blood ordering alone, without the EBRS, would also result in substantial cost savings.
The definition of the term “costs” has been debated in the literature. When considering the internal affairs of the hospital, costs often refer to the actual costs of performing a test (e.g., labor, disposables, reagents), whereas charges refer to the charge to the patient, Medicare, or a third-party payer. However, when the payer is considered, the hospitals' charges become the payers' costs, a concept that is important to consider when attempting to introduce “cost-effective” changes in practice. To avoid confusion over these terms, we use the term “costs” to indicate the cost to the payer (the Medicare reimbursement rate), not to the hospital.
In summary, implementing an updated, institution-specific MSBOS along with an EBRS has substantial advantages that include (1) decreasing the number of preoperative blood orders considered to be unnecessary, (2) reducing the C/T ratio, and (3) decreasing overall costs without evidence of impact on patient safety. In an era of cost reduction and pressure to reduce tests and procedures that are not beneficial to patient care, optimizing the practice of preoperative blood ordering is an important advance in perioperative medicine. Furthermore, by standardizing care and reducing unnecessary testing and costs, this change in practice aligns nicely with the goals of two national campaigns, Choosing Wisely and the Perioperative Surgical Home.
Supplementary Material
Acknowledgments
The authors would like to thank Claire Levine, M.S., E.L.S. (Editorial Services, Department of Anesthesiology/Critical Care Medicine, Johns Hopkins Medicine, Baltimore, Maryland) for editorial assistance, Sharon Paul, B.S., M.S. (Department of Anesthesiology/Critical Care Medicine, Johns Hopkins Medicine, Baltimore, Maryland) for assistance with data acquisition, Joan Boyd, M.T.(A.S.C.P.) S.B.B., (Transfusion Medicine Division, Department of Pathology, Johns Hopkins Medicine, Baltimore, Maryland), Allen Valentine, M.B.A., C.(A.S.C.P.), Medical Laboratories, Department of Pathology, Johns Hopkins Medicine, Baltimore, Maryland) and Christi Marshall, M.S. (Department of Pathology, Johns Hopkins Medicine, Baltimore, Maryland) for assistance with accessing the blood bank's database.
A.A.R.T. was supported by the National Institutes of Health (Bethesda, Maryland) 1K23AI093152-01A1, and Doris Duke Charitable Foundation (New York, New York) Clinician Scientist Development Award (#22006.02). All other support was provided from institutional and/or departmental sources.
Footnotes
Competing Interests: S.M.F. has received compensation for speaking and consulting, as well as research funding from Haemonetics Corp., (Braintree, Massachusetts).
TOC Statement: Use of a Maximum Surgical Blood Order Schedule in a tertiary hospital reduced blood over-ordering from 40% to 25% of patients. Emergency release of uncrossmatched blood increased from 0.22% to 0.31%. Combining the Maximum Surgical Blood Order Schedule with electronic crossmatching reduced costs by $6 per patient.
https://www.asahq.org/For-Members/Perioperative-Surgical-Home.aspx. Last accessed May 17, 2014.
http://www.choosingwisely.org/doctor-patient-lists/american-society-of-anesthesiologists/. Last accessed May 17, 2014.
AABB: 2013 Proposed Medicare Payments for Hospital Outpatient Services http://www.aabb.org/programs/reimbursementinitiatives/Pages/13hoppsruleprop.aspx 2013. Last accessed May 17, 2014.
References
- 1.American Society of Anesthesiologists Task Force on Perioperative Blood T, Adjuvant T. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Napolitano LM, Kurek S, Luchette FA, Anderson GL, Bard MR, Bromberg W, Chiu WC, Cipolle MD, Clancy KD, Diebel L, Hoff WS, Hughes KM, Munshi I, Nayduch D, Sandhu R, Yelon JA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Workgroup EPM. American College of Critical Care Medicine Taskforce of the Society of Critical Care Medicine: Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. J Trauma. 2009;67:1439–42. doi: 10.1097/TA.0b013e3181ba7074. [DOI] [PubMed] [Google Scholar]
- 3.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B. Clinical Transfusion Medicine Committee of the AABB: Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 4.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion. Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, International Consortium for Evidence Based Perfusion. Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG. 2011 Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 5.Mintz PD, Nordine RB, Henry JB, Webb WR. Expected hemotherapy in elective surgery. N Y State J Med. 1976;76:532–7. [PubMed] [Google Scholar]
- 6.Friedman BA, Oberman HA, Chadwick AR, Kingdon KI. The maximum surgical blood order schedule and surgical blood use in the United States. Transfusion. 1976;16:380–7. doi: 10.1046/j.1537-2995.1976.16476247063.x. [DOI] [PubMed] [Google Scholar]
- 7.Henry JB, Mintz P, Webb W. Optimal blood ordering for elective surgery. JAMA. 1977;237:451. [PubMed] [Google Scholar]
- 8.Frank SM, Rothschild JA, Masear CG, Rivers RJ, Merritt WT, Savage WJ, Ness PM. Optimizing preoperative blood ordering with data acquired from an anesthesia information management system. Anesthesiology. 2013;118:1286–97. doi: 10.1097/ALN.0b013e3182923da0. [DOI] [PubMed] [Google Scholar]
- 9.Judd WJ. Requirements for the electronic crossmatch. Vox Sang. 1998;74(Suppl 2):409–17. doi: 10.1111/j.1423-0410.1998.tb05450.x. [DOI] [PubMed] [Google Scholar]
- 10.Technical Manual. 16. Bethesda: AABB; 2008. [Google Scholar]
- 11.Krier DB, Richards FE. Transfusion-to-cross-match community comparison data. Am J Med Qual. 1996;11:68–72. doi: 10.1177/0885713X9601100203. [DOI] [PubMed] [Google Scholar]
- 12.Nuttall GA, Santrach PJ, Oliver WC, Jr, Horlocker TT, Shaughnessy WJ, Cabanela ME, Bryant S. The predictors of red cell transfusions in total hip arthroplasties. Transfusion. 1996;36:144–9. doi: 10.1046/j.1537-2995.1996.36296181927.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng G, Chiu DS, Chung AS, Wong HF, Chan MW, Lui YK, Choy FM, Chan JC, Chan AH, Lam ST, Fan TC. A novel system for providing compatible blood to patients during surgery: “Self-service” electronic blood banking by nursing staff. Transfusion. 1996;36:347–50. doi: 10.1046/j.1537-2995.1996.36496226151.x. [DOI] [PubMed] [Google Scholar]
- 14.Cox C, Enno A, Deveridge S, Seldon M, Richards R, Martens V, Woodford P. Remote electronic blood release system. Transfusion. 1997;37:960–4. doi: 10.1046/j.1537-2995.1997.37997454025.x. [DOI] [PubMed] [Google Scholar]
- 15.Frank SM, Savage WJ, Rothschild JA, Rivers RJ, Ness PM, Paul SL, Ulatowski JA. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology. 2012;117:99–106. doi: 10.1097/ALN.0b013e318255e550. [DOI] [PubMed] [Google Scholar]
- 16.Frank SM, Resar LM, Rothschild JA, Dackiw EA, Savage WJ, Ness PM. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052–9. doi: 10.1111/trf.12227. [DOI] [PubMed] [Google Scholar]
- 17.Staves J, Davies A, Kay J, Pearson O, Johnson T, Murphy MF. Electronic remote blood issue: A combination of remote blood issue with a system for end-to-end electronic control of transfusion to provide a “total solution” for a safe and timely hospital blood transfusion service. Transfusion. 2008;48:415–24. doi: 10.1111/j.1537-2995.2007.01545.x. [DOI] [PubMed] [Google Scholar]
- 18.Mintz PD, Lauenstein K, Hume J, Henry JB. Expected hemotherapy in elective surgery. A follow-up. JAMA. 1978;239:623–5. [PubMed] [Google Scholar]
- 19.Dutton RP, Shih D, Edelman BB, Hess J, Scalea TM. Safety of uncrossmatched type-O red cells for resuscitation from hemorrhagic shock. J Trauma. 2005;59:1445–9. doi: 10.1097/01.ta.0000198373.97217.94. [DOI] [PubMed] [Google Scholar]
- 20.Goodell PP, Uhl L, Mohammed M, Powers AA. Risk of hemolytic transfusion reactions following emergency-release RBC transfusion. Am J Clin Pathol. 2010;134:202–6. doi: 10.1309/AJCP9OFJN7FLTXDB. [DOI] [PubMed] [Google Scholar]
- 21.Nuttall GA, Abenstein JP, Stubbs JR, Santrach P, Ereth MH, Johnson PM, Douglas E, Oliver WC., Jr Computerized bar code-based blood identification systems and near-miss transfusion episodes and transfusion errors. Mayo Clin Proc. 2013;88:354–9. doi: 10.1016/j.mayocp.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Linden JV, Wagner K, Voytovich AE, Sheehan J. Transfusion errors in New York State: An analysis of 10 years' experience. Transfusion. 2000;40:1207–13. doi: 10.1046/j.1537-2995.2000.40101207.x. [DOI] [PubMed] [Google Scholar]
- 23.Gammon HM, Waters JH, Watt A, Loeb JM, Donini-Lenhoff A. Developing performance measures for patient blood management. Transfusion. 2011;51:2500–9. doi: 10.1111/j.1537-2995.2011.03406.x. [DOI] [PubMed] [Google Scholar]
- 24.McWilliams B, Yazer MH, Cramer J, Triulzi DJ, Waters JH. Incomplete pretransfusion testing leads to surgical delays. Transfusion. 2012;52:2139–44. doi: 10.1111/j.1537-2995.2012.03568.x. quiz 2145. [DOI] [PubMed] [Google Scholar]
- 25.Keehan SP, Cuckler GA, Sisko AM, Madison AJ, Smith SD, Lizonitz JM, Poisal JA, Wolfe CJ. National health expenditure projections: Modest annual growth until coverage expands and economic growth accelerates. Health Aff (Millwood) 2012;31:1600–12. doi: 10.1377/hlthaff.2012.0404. [DOI] [PubMed] [Google Scholar]
- 26.Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfus Med. 2003;13:205–18. doi: 10.1046/j.1365-3148.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 27.Amin M, Fergusson D, Aziz A, Wilson K, Coyle D, Hebert P. The cost of allogeneic red blood cells: A systematic review. Transfus Med. 2003;13:275–85. doi: 10.1046/j.1365-3148.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 28.Kacker S, Frick KD, Tobian AA. The costs of transfusion: Economic evaluations in transfusion medicine, Part 1. Transfusion. 2013;53:1383–5. doi: 10.1111/trf.12188. [DOI] [PubMed] [Google Scholar]
- 29.Jefferies LC, Sachais BS, Young DS. Blood transfusion costs by diagnosis-related groups in 60 university hospitals in 1995. Transfusion. 2001;41:522–9. doi: 10.1046/j.1537-2995.2001.41040522.x. [DOI] [PubMed] [Google Scholar]
- 30.Vibhute M, Kamath SK, Shetty A. Blood utilisation in elective general surgery cases: Requirements, ordering and transfusion practices. J Postgrad Med. 2000;46:13–7. [PubMed] [Google Scholar]
- 31.Boral LI, Henry JB. The type and screen: A safe alternative and supplement in selected surgical procedures. Transfusion. 1977;17:163–8. doi: 10.1046/j.1537-2995.1977.17277151923.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.