Abstract
Transplantation of major histocompatibility complex (MHC)-mismatched mouse neural precursor cells (NPCs) into mice persistently infected with the neurotropic JHM strain of mouse hepatitis virus (JHMV) results in rapid rejection that is mediated, in part, by T cells. However, the contribution of the innate immune response to allograft rejection in a model of viral-induced neurological disease has not been well defined. Herein, we demonstrate that the natural killer (NK) cell-expressing activating receptor NKG2D participates in transplanted allogeneic NPC rejection in mice persistently infected with JHMV. Cultured NPCs derived from C57BL/6 (H-2b) mice express the NKG2D ligand retinoic acid early precursor transcript (RAE)-1 but expression was dramatically reduced upon differentiation into either glia or neurons. RAE-1+ NPCs were susceptible to NK cell-mediated killing whereas RAE-1- cells were resistant to lysis. Transplantation of C57BL/6-derived NPCs into JHMV-infected BALB/c (H-2d) mice resulted in infiltration of NKG2D+CD49b+ NK cells and treatment with blocking antibody specific for NKG2D increased survival of allogeneic NPCs. Further, transplantation of differentiated RAE-1- allogeneic NPCs into JHMV-infected BALB/c mice resulted in enhanced survival, highlighting a role for the NKG2D:RAE-1 signaling axis in allograft rejection. We also demonstrate that transplantation of allogeneic NPCs into JHMV-infected mice resulted in infection of the transplanted cells suggesting that these cells may be targets for infection. Viral infection of cultured cells increased RAE-1 expression, resulting in enhanced NK cell-mediated killing through NKG2D recognition. Collectively, these results show that in a viral-induced demyelination model, NK cells contribute to rejection of allogeneic NPCs through an NKG2D signaling pathway.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) involving immune responses directed against self-antigens within the CNS resulting in neuroinflammation and demyelination1, 2. Ultimately, myelin and axonal loss culminates in extensive disability through defects in neurological function3-6. Although myelin repair can occur during the course of the disease, it is often transient and not sustained7, 8. Therefore, an important unmet clinical need for MS patients is an effective method to induce sustained remyelination while limiting disease progression and ongoing demyelination 9, 10. In recent years, considerable effort has focused on cell replacement therapies through use of neural precursor cells (NPCs) to promote remyelination. Indeed, in animal models of autoimmune neuroinflammatory demyelination there is evidence that transplantation of NPCs results in improved clinical outcome accompanied by reduced neuroinflammation and myelin repair11-15.
Using a viral model of demyelination, we have demonstrated that intraspinal transplantation of mouse NPCs into animals with established demyelination results in improved motor skills along with limited spread of demyelination accompanied by axonal sparing and remyelination16. Intracranial infection with the neuroadapted JHM strain of mouse hepatitis virus (JHMV) results in an acute encephalomyelitis followed by chronic immune-mediated demyelinating disease similar clinically and histologically to the human demyelinating disease multiple sclerosis (MS)17-19. While the etiology of MS is unknown, both genetic factors as well as environmental influences (e.g. viral infection) have long been considered important in triggering disease20-23. Therefore, defining mechanisms contributing to demyelination as well as remyelination in animals in which disease is initiated by a persistent infection with a neurotropic virus is clinically relevant. With this in mind, we have shown that following intraspinal injection of syngeneic NPCs into JHMV-infected mice, transplanted cells are well-tolerated, preferentially differentiate into cells of an oligodendrocyte lineage, and selectively colonize areas of white matter damage within the spinal cord16, 24.
While the findings from our transplantation studies emphasize the therapeutic potential of NPCs in ameliorating disease in JHMV-infected mice, the majority of transplantation studies have utilized syngeneic NPCs for CNS engraftment and do not address the important issue of whether MHC-mismatched NPCs are recognized as foreign by the host immune system and subsequently rejected. Evidence argues that unmatched grafts are well-tolerated within the CNS due to muted immunogenicity of NPCs and clinical studies support that transplantation of allogeneic NPCs results in prolonged survival25-27. However, the immunoprivileged status of NPCs has recently been questioned28 and more recent studies argue that allogeneic NPCs exhibit diminished survival upon transplantation29-31. Our recent studies demonstrate that transplantation of MHC-mismatched NPCs into the CNS of mice infected with the JHMV results in rapid rejection in which T cells participate in recognition and rejection of allogeneic cells32. These findings, along with others33-35, indicate an important role for T cells in contributing to rejection of foreign cells following CNS injection and argue for sustained immunosuppression using drugs targeting T cells. More recently, Palmer and colleagues36 have provided compelling evidence indicating an important role for the innate immune system (e.g. natural killer (NK) cells) in recognizing and killing allogeneic NPCs via NKG2D signaling upon injection into the CNS. These studies build upon a growing literature illustrating the importance of the innate immune system in contributing to allograft rejection34, 37, 38.
Retinoic acid early precursor transcript (RAE)-1 is the ligand for the NK cell activating receptor NKG2D. Numerous studies have highlighted a functional role for RAE-1 as a target for NKG2D recognition and killing of virally-infected cells and tumor cells as well as contributing to allograft rejection39-44. RAE-1 is expressed on NPCs and is thought to be important in regulating proliferation suggesting a non-immune functional role during development45. In this study, we demonstrate that transplantation of allogeneic NPCs into the spinal cords of mice persistently infected with JHMV results in rapid rejection that is mediated, in part, through an NKG2D-dependent pathway. Further, JHMV infection of cultured NPCs increases expression of RAE-1 and these cells are susceptible to NK cell-mediated lysis that is enhanced upon NKG2D recognition. These findings support a role for NKG2D signaling in allograft rejection as well as killing virally infected NPCs in a model of viral-induced demyelination.
Material and Methods
Animals and Virus
Age-matched (5-7wk) C57BL/6 (H-2b for syngeneic transplants, National Cancer Institute (NCI, Frederick, MD), BALB/c (H-2d for allogeneic transplants, NCI), and SCID/NCr (H-2d for allogeneic transplant, NCI) mice were infected intracranially (i.c.) with 150 (C57BL/6), 15,000 (BALB/c), or 2,000 (SCID/NCr) plaque forming units (PFU) of mouse hepatitis virus (MHV) strain J2.2v-1 (JHMV) in 30 μl sterile HBSS24. SCID/NCr are immunodeficient for B and T lymphocytes but have normal numbers of NK cells, macrophages, and granulocytes. Mice were sacrificed at defined times post-infection (p.i.) by either perfusion with 1× PBS or 4% paraformaldehyde in PBS and spinal cords were removed and processed for analysis. All animal experiments were approved by the University of California, Irvine Institutional Animal Care and Use Committee.
Cell culture, transplantation, and reagents
Enhanced Green Fluorescent Protein expressing NPCs (eGFP-NPCs) were cultured in the absence of growth matrix in NPC media consisting of DMEM/F12+glutamax (1×, Gibco, cat# 10565-018), 100 μg/ml ciproflox (Cellgro), 50μg/ml gentamicin (Sigma), 2.5 μg/ml fungizone (Invitrogen), 1000U/ml Penicillin/streptomycin (Gibco), 1× N2 (Gibco), and 20ng/ml human EGF (Sigma). Cells were passaged with 0.05% trypsin for 30 sec, followed by quenching with cold NPC media. The addition of human epidermal growth factor (hEGF) is necessary to maintain undifferentiated NPCs. eGFP-NPCs were differentiated by culturing on matrigel (1:25, BD Biosciences)-coated plates for 5 days in eGFP-NPC media (as previously described16) in the absence of hEGF. Media were changed every other day for both the undifferentiated and differentiated cultures. Undifferentiated or differentiated eGFP-NPCs were transplanted (2.5 × 105 in 2.5 μl Hank's balanced salt solution (HBSS)/mouse) at spinal cord T9 at day 14 p.i. into C57BL/6 (syngeneic) and BALB/c (allogeneic) mice or at day 7 p.i. into SCID/NCr mice. As a sham control, virally-infected mice were transplanted with HBSS alone (vehicle only)16. Recombinant mouse IFN-γ was purchased from Cell Sciences (Canton, MA). eGFP-NPCs were infected with JHMV (0.1 moi) overnight at which point media were replaced with fresh media without virus for 24 hr. YAC-1 cells, used as a positive control for NK cell-mediated lysis, were grown in RPMI-1640 medium with 10% fetal bovine serum, glutamax (1×), and penicillin/streptomycin (1000 U/ml).
Flow cytometry
Lymphocytes were isolated from the spinal cord (9 mm rostral and 9 mm caudal to the transplant site) of C57BL/6 and BALB/c mice on day 8 following transplantation with eGFP-NPCs or vehicle only using a discontinuous Percoll gradient as previously described 32, 46-49. Following block of FC receptors with anti-CD16 + CD32 mAb (clone 2.4G2; BD Biosciences) for 20 min at 4°C, cells were stained using the following mAbs: PerCp or PE/Cy5-conjugated anti-CD3e (BD Biosciences), PE or APC-conjugated anti-CD49b (BD Biosciences), and PE or APC-conjugated anti-NKG2D (eBioscience). Cultured eGFP-NPCs were trypsinized with 0.05% trypsin (Invitrogen), blocked with anti-CD16 + CD32 mAb as described above, and stained with either PE-conjugated anti-MHC class I (eBioscience), anti-MHC class II (BD Biosciences), anti-pan RAE-1 (R&D Systems), or APC-conjugated anti-CD133 (Biolegend). Cells were analyzed using a FACStar flow cytometer (BD Biosciences) or LSRII flow cytometer (BD Biosciences) with FlowJo software (Tree Star, OR). All data are shown as percentage of gated single (forward scatter height versus forward scatter area) live (forward scatter versus side scatter) eGFP+ cells. Appropriate isotype-matched control Ig's were used for each antibody. eGFP-NPCs were stained for RAE-1 as described above and eGFP+RAE-1+ or eGFP+RAE-1- cells were sorted using a FACS Aria III (BD Biosciences). Sorted RAE-1+ and RAE-1- eGFP-NPCs were plated in eGFP-NPC media.
NK cell isolation
NK cells were isolated from the blood of BALB/c mice using an EasySep mouse NK cell enrichment kit (Stem Cell Technologies, Vancouver, BC). Briefly, red blood cells were lysed by treatment (twice) with 2ml ACK buffer (0.15M NH4Cl, 10mM KHCO3, 0.1mM EDTA in double distilled H20) for 90 sec at room temperature (RT). Following the final wash, cells were resuspended at 1×108 cells/ml in EasySep buffer (1× PBS + 2% fetal bovine serum + 1mM EDTA) in a 14 ml polystyrene tube. Fifty μl/ml EasySep negative selection mouse NK cell enrichment cocktail was added for 15 min, followed by 200 μl/ml EasySep biotin selection cocktail for 15 min, followed by 200 μl/ml EasySep D magnetic particles for 10 min. All incubations were done at RT. Cell suspension was brought to a volume of 5 ml with EasySep buffer and placed in “the big easy” EasySep magnet for 5 min at RT. Following incubation, non-labeled cells were transferred to a new tube and counted. NK cell (CD3- CD49b+) purity was determined to be >90% as determined by flow cytometry.
Non-Radioactive Cytotoxicity Assay
Cytotoxicity was determined by readout of lactase dehydrogenase (LDH) released from dying cells using a CytoTox 96 non-radioactive cytotoxicity kit (Promega, Madison, WI). eGFP-NPCs (target, T) were plated in 100 μl at 2×104 cells per well in 96-well flat-bottom plates and allowed to adhere prior to the addition of 100 μl of NK cells (effector, E) at 20:1, 10:1, and 5:1 E:T ratio. Cells were incubated for 4.5 hr at 37° C, plates were centrifuged at 250 × g for 3 min and 50 μl from each well was transferred to a corresponding well of another 96-well flat-bottom plate. Fifty μl of substrate mix was added to each well, the plate was incubated for 30 min at RT in the dark, and then 50 μl of stop solution was added to each well. Absorbance was recorded for each well at 490 nm using a Synergy HT plate reader (BioTek; Winooski, VT). Control wells included NK cells only to determine spontaneous effector LDH release, eGFP-NPCs only to determine spontaneous target LDH release, eGFP-NPCs plus lysis solution (Promega) to determine maximum lysis, eGFP-NPC media only for background control, and eGFP-NPC media plus lysis solution for volume correction control. Forty-five minutes prior to harvesting supernatant, 20μl of lysis solution was added to maximum lysis and volume correction control wells. Percent cytotoxicity was calculated as:
Histopathology
Animals were euthanized by inhalation of halothane (Sigma) and fixed by cardiac perfusion. Spinal cords were extracted and processed for OCT sections as previously described16. The number of eGFP-positive cells was counted and data are presented as average±SEM. For immunofluorescent staining, we used mouse-anti-JHMV (specific for the nucleocapsid (N) protein, kindly provided by Dr. Stanley Perlman, University of Iowa), rat-anti-CD49b (DX5), and rat-anti-NKG2D (CX5). Alexa 594-conjugated secondary antibodies used were: goat anti-mouse (detection of anti-N; Invitrogen), goat anti-rat IgM (for anti-CD49b; Invitrogen), and goat anti-rat IgG (for anti-NKG2D; Invitrogen). DAPI Fluoromount-G (Southern Biotech, Birmingham, AL) was used to visualize nuclei. Images were taken on an Eclipse Ti inverted microscope (Nikon, Melville, NY).
Antibody Treatment
JHMV-infected mice were intraperitoneally (i.p.) treated with 100μg/mouse of anti-NKG2D (CX5), or control Rat IgG (Sigma) in 300μl sterile HBSS at days -1, 1, 3, 5, 12, and 19 post-transplantation (p.t.) and sacrificed at day 21 p.t.
Statistical Analysis
Statistical analysis was performed using an unpaired or paired Student's t test and p≤0.05 was considered significant.
Results
NK cells target RAE-1+ NPCs
Expression of the NKG2D ligand RAE-1 on cultured NPCs expressing eGFP (eGFP-NPCs, derived from mice on the C57BL/6 background) was determined by flow cytometric analysis. We first determined that 85.1±1.6% of eGFP-NPCs expressed CD133 (Figure 1A). Subsequently, gating on the eGFP+CD133+ cells revealed 64.3±0.7% of dual-positive cells expressed RAE-1 while 48.3±2.0% of eGFP+CD133- cells expressed RAE-1 (Figure 1A). Although we did not phenotype the CD133+RAE-1- cells, we believe this population most likely represents NPCs in varying states of either proliferation and/or differentiation. Similarly, we believe the eGFP+CD133- population represents cells undergoing differentiation. We next demonstrated that in vitro differentiation of eGFP-NPCs into glial-enriched culture16 resulted in dramatically diminished expression of RAE-1 (<1% when compared to undifferentiated cultures), suggesting that RAE-1 is restricted to undifferentiated NPCs (Figures 1B and C). We have previously shown that treatment of cultured NPCs with the proinflammatory cytokine IFN-γ increases expression of MHC class I and II32; however, exposure to IFN-γ (100U/ml) resulted in an average 5.4±1.5% reduction in RAE-1 expression compared to untreated cells (p<0.05) (Figures 1B and D). We did not detect expression of the NK cell-activating minor histocompatibility antigen H60 on either undifferentiated or differentiated NPCs (data not shown).
Figure 1. RAE-1 expression on cultured NPCs.
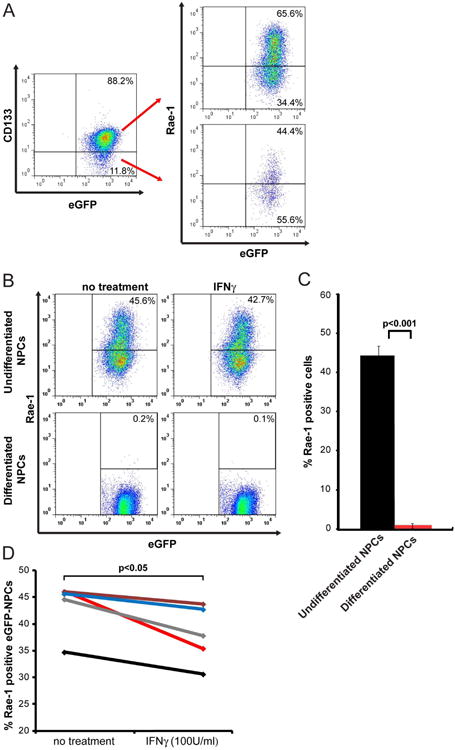
(A) Representative dot blot showing staining for the NPC marker CD133 and eGFP; 85.1±1.6 % of cultured eGFP-NPCs expressed CD133. Subsequent staining for RAE-1 on eGFP+CD133+ and eGFP+CD133- revealed 64.3±0.7% of dual-positive cells expressed RAE-1 while 48.3±2.0% of eGFP+CD133- cells expressed RAE-1. (B,C) Differentiated and undifferentiated cultured eGFP-NPCs were treated with IFN-γ (100 U/ml) for 24 hr and RAE-1 expression was determined by flow cytometry. (B) Representative flow analysis for RAE-1 expression on IFN-γ-treated or non-treated differentiated and undifferentiated eGFP-NPCs is shown. (C) Quantification of RAE-1 expression on differentiated and undifferentiated non-treated eGFP-NPCs. Data represents five independent experiments and data is shown as average±SEM; p<0.05. (D) Quantification of RAE-1 expression on non-treated and IFN-γ-treated undifferentiated eGFP-NPCs. Paired data from five independent experiments showing decreased RAE-1 expression following IFN-γ treatment. The average decrease from all experiments is 12.3±3.3% SEM; each line represents an individual experiment; p<0.05.
We next tested whether NK cells could lyse cultured eGFP-NPCs using an in vitro cytolytic killing assay. Primary NK cells (CD3-CD49b+) were isolated from the blood of BALB/c mice to greater than 90% purity (data not shown). Cultured NPCs were sorted into RAE-1+ and RAE-1- populations (Figure 2A) and cultured with NK cells. Enriched NK cells killed allogeneic RAE-1+ NPCs over a range of effector-to-target ratios similar to NK cell-mediated killing of YAC-1 cells that were used as a positive control (Figure 2B). Enriched NK cells from C57BL6 mice recognized and killed syngeneic NPCs at low levels (data not shown). These findings indicate that RAE-1 expression by NPCs is regulated, in part, by the differentiation fate of cells and that RAE-1 expression on allogeneic NPCs allows for recognition by NK cells that participate in allograft rejection.
Figure 2. NK cell lysis of RAE-1+ NPCs.
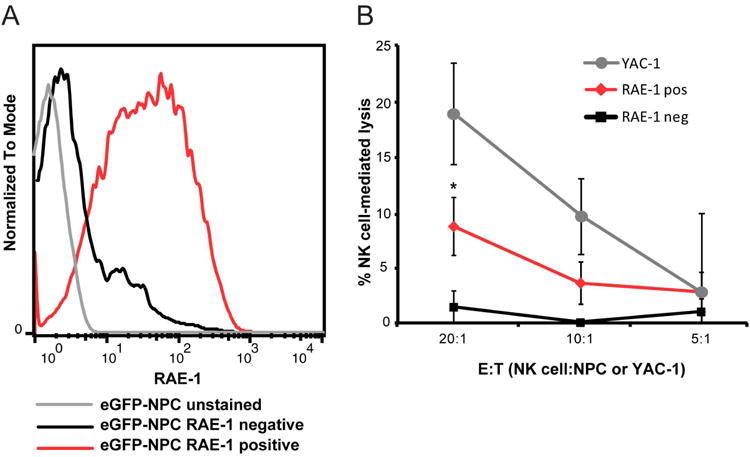
(A) Representative histogram depicting RAE-1+ (red line) and RAE-1- (black line) eGFP-NPCs sorted by FACS. eGFP-NPCs stained with isotype control antibody are indicated by grey line. (B) RAE-1+ (red line) eGFP-NPCs, RAE-1- (black line) eGFP-NPCs, or YAC-1 (grey line) cells (control for NK cell lysis) were cultured with allogeneic NK cells in an LDH assay and the percentage of NK cell-mediated lysis at three different E:T ratios is shown. Data represent three independent experiments; *p<0.05, unpaired student's T test between RAE-1+ and RAE-1- eGFP-NPCs.
Antibody blockade of NKG2D increases survival of allogeneic NPCs
To examine if NK cells contribute to allograft rejection, JHMV-infected BALB/c mice were intraspinally transplanted with either C57BL/6-derived eGFP-NPCs or HBSS (vehicle control) at day 14 p.i. that represents a time in which persistent virus is present within the CNS and demyelination is established24, 32. In addition, JHMV-infected C57BL/6 mice were transplanted with syngeneic eGFP-NPCs via intraspinal injection at day 14 p.i. Experimental mice were sacrificed at day 8 post-transplantation (p.t.), and infiltrating lymphocytes were isolated from a defined area of the spinal cord, 9 mm rostral and caudal to the transplantation site, and were immunophenotyped by flow cytometry. Transplantation of allogeneic eGFP-NPCs into infected BALB/c mice resulted in a significant (p<0.01) increase in the number of CD3-CD49b+NKG2D+ NK cells migrating into the spinal cord of mice compared to infected C57BL/6 mice receiving syngeneic eGFP-NPCs (Figures 3A and B). Importantly, allogeneic and syngeneic transplants were normalized to vehicle only transplant controls to account for NK cell infiltration into the spinal cord as a result of JHMV infection or due to trauma from needle injection.
Figure 3. NK cell infiltration into spinal cords following allogeneic NPC transplantation.
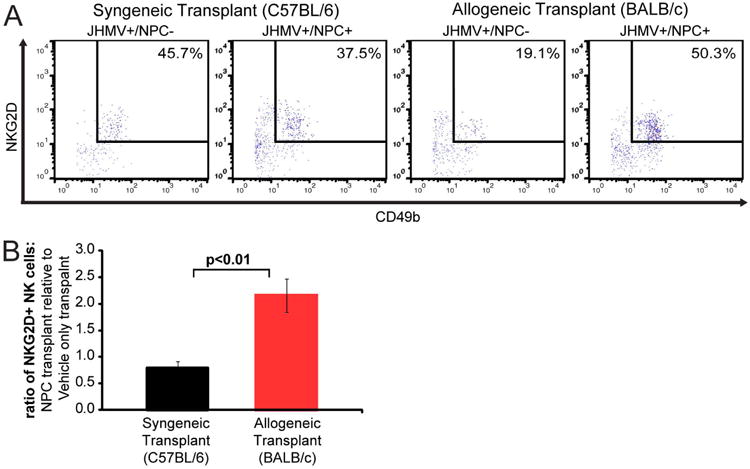
Vehicle only or eGFP-NPCs were transplanted into JHMV-infected C57BL/6 (syngeneic transplant) and JHMV-infected BALB/c (allogeneic transplant) mice on day 14 post-JHMV-infection. (A) Mice were sacrificed at day 8 p.t. and the frequency of NKG2D+ NK cells among total lymphocytes in the spinal cord (9mm rostral and caudal to transplant site) of recipient mice was determined by flow cytometry. Representative flow analysis of CD3−CD49b+NKG2D+ NK cells in syngeneic and allogeneic eGFP-NPC transplanted, and vehicle only transplanted mice is shown. (B) Quantification of NK cells in allogeneic and syngeneic transplanted mice normalized to vehicle only transplant. Data is presented as average±SEM and is 1 of 2 representative experiments with a minimum of 3 mice per group; p<0.01.
To determine if NKG2D+ NK cells were recruited to the site of allogeneic transplant in the absence of viral infection, non-infected mice were transplanted with allogeneic eGFP-NPCs or vehicle alone. Following allogeneic eGFP-NPCs or vehicle-only transplantation, spinal cords were removed at day 8 p.t. and NK cell infiltration was determined. Transplantation of allogeneic eGFP-NPCs into non-infected Balb/c mice resulted in an increase in the percentage of CD3-CD49b+ NK cells (Supp. Figure 1A) as well as the percentage of NKG2D+ NK cells within the spinal cord compared to vehicle only-transplanted mice (Supp. Figures 1A and B).
To test the role of NKG2D signaling in allograft rejection, allogeneic recipients were treated with either the non-depleting, neutralizing anti-NKG2D mAb or an isotype-matched control antibody. As an additional control, syngeneic recipients received isotype control antibody. Administration of isotype control antibody to syngeneic recipients did not affect either transplanted eGFP-NPC migration rostral or caudal to implantation site or colonization of white matter tracts (Figures 4A and D) whereas eGFP-NPCs were not detected within the spinal cords of allogeneic recipients treated with control antibody (Figures 4B and D). Treatment of infected mice receiving allogeneic eGFP-NPCs with a blocking antibody specific to NKG2D resulted in graft survival at day 21 p.t. in 4 of 5 mice (Figures 4C and D). There was a significant (p<0.001) increase in the frequency of surviving cells (17.7% at transplant site) when compared to allogeneic transplants receiving injections with an isotype control antibody, in which 0 of 4 mice had a surviving graft (0%) (Figure 4D). Survival of allogeneic NPCs in mice treated with anti-NKG2D antibody remained lower when compared to mice receiving syngeneic NPCs (Figures 4C and D).
Figure 4. Blocking NKG2D increases survival of transplanted allogeneic NPCs.
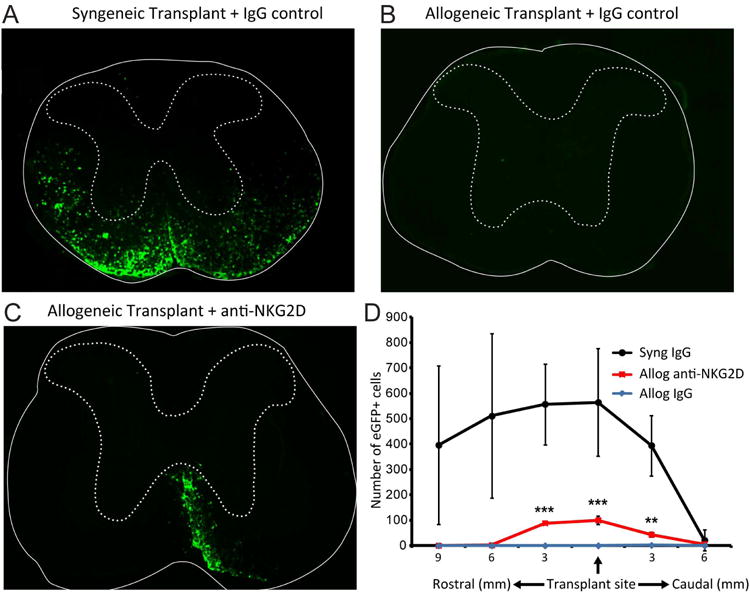
Representative coronal spinal cord sections of the transplant site from JHMV-infected mice receiving either syngeneic eGFP-NPCs treated with IgG control antibody (A), allogeneic eGFP-NPCs plus anti-NKG2D (B) or IgG control antibody (C). Experimental mice were sacrificed at day 21 p.t. and migration/survival of transplanted cells was evaluated by visualization of eGFP-expression from transplanted cells. (D) Dual-positive DAPI and eGFP-NPCs were counted in coronal sections (9 mm rostral and 6 mm caudal to transplant site at 3 mm intervals) from mice syngeneically transplanted treated with an IgG control antibody (n=5), allogeneically transplanted treated with anti-NKG2D (n=5), and allogeneically transplanted treated with an IgG control antibody (n=4). Increased numbers of eGFP-NPCs (**p<0.01, ***p<0.001) were present within the spinal cords of allogeneically transplanted mice treated with anti-NKG2D antibody compared to allogeneically transplanted mice treated with an IgG control antibody. 100% (5/5) syngeneically transplanted mice treated with an IgG control antibody, 80% (4/5) allogeneically transplanted mice treated with anti-NKG2D, and 0% (0/4) allogeneically transplanted treated with an IgG control antibody had a surviving graft at day 21 p.t.
Glial differentiation of allogeneic NPCs increases survival following transplantation
Our findings argue that expression of RAE-1 by NPCs renders these cells susceptible to NKG2D recognition and subsequent lysis of allografts by infiltrating NK cells. Additionally, upon differentiation of cultured NPCs, RAE-1 expression was dramatically reduced resulting in limited NK cell killing of allogeneic cells by NK cells and this further supports the notion that NK cells recognize and kill allogeneic NPCs through NKG2D recognition of RAE-1. As an additional test, we transplanted either eGFP-NPCs that constitutively express RAE-1 or differentiated eGFP-NPCs in which RAE-1 expression is greatly reduced into JHMV-infected mice. Consistent with our earlier findings16, differentiation of eGFP-NPCs resulted in ∼80% of cells expressing oligendroglia markers NG2 and PDGFRα (data not shown). Initially, we transplanted undifferentiated and differentiated eGFP-NPCs into JHMV-infected C57BL/6 mice, representing a syngeneic transplant, in order to compare migration of the two cell populations. Examination of coronal sections of spinal cords at day 21 p.t. indicated similar numbers of eGFP-NPCs present within the white matter tracts of animals receiving undifferentiated NPCs compared to differentiated cells (Figures 5A and B). Surviving grafts were found in 100% of C57BL/6 mice that received either undifferentiated (n=13) or differentiated (n=12) syngeneic eGFP-NPCs. Quantification of cell numbers within transplanted mice revealed similar numbers of differentiated cells compared to undifferentiated cells and cell migration rostral and caudal to the implantation site was almost identical (Figure 5C). Consistent with our earlier studies32, allogeneic undifferentiated eGFP-NPCs were rejected below the level of detection by day 21 p.t. (Figures 6A and C), whereas eGFP-NPCs that were differentiated prior to transplant were found in 50% (6 of 12) of allogeneically transplanted mice (Figures 6B and C). Quantification of transplanted cells demonstrated increased numbers (p<0.01) of differentiated allogeneic eGFP-NPCs within spinal cords as compared to undifferentiated allogeneic eGFP-NPCs and surviving cells migrated rostral and caudal to the implantation site (Figure 6C). Immunohistochemical staining at day 8 p.t. for the NK cell marker CD49b50 demonstrated accumulation of these cells in areas in which undifferentiated cells are present while CD49b staining was not detected around differentiated cells (Figure 6D). These findings provide further support for RAE-1 expression on undifferentiated NPCs as a target for NK cell recognition and killing of allografts.
Figure 5. Differentiated NPCs migrate following transplantation.
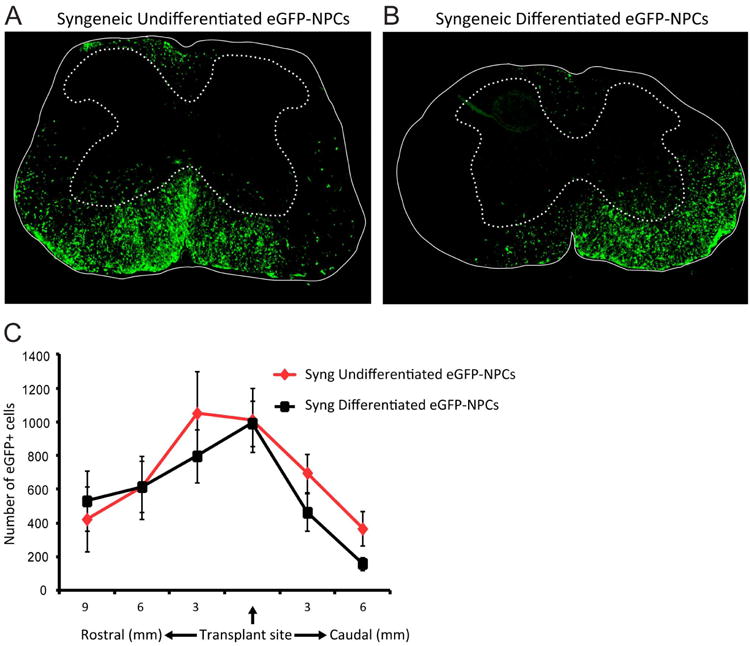
Undifferentiated and differentiated eGFP-NPCs were transplanted into C57BL/6 mice (syngeneic transplant) on day 14 post-JHMV-infection. Representative coronal spinal cord sections of the transplant site from JHMV-infected mice receiving syngeneic undifferentiated eGFP-NPCs (n=13; A) or syngeneic differentiated eGFP-NPCs (n=12; B). Experimental mice were sacrificed at day 21 p.t. and migration and/or survival of transplanted cells evaluated by visualization of eGFP-expression from transplanted cells. (C) eGFP-NPCs were counted in coronal sections (9 mm rostral and 6 mm caudal to transplant site at 3 mm intervals) from mice syngeneically transplanted with undifferentiated (n=11) or differentiated (n=11) eGFP-NPCs. There was no significant difference between the numbers of undifferentiated or differentiated eGFP-NPCs.
Figure 6. Transplanted allogeneic differentiated NPCs.
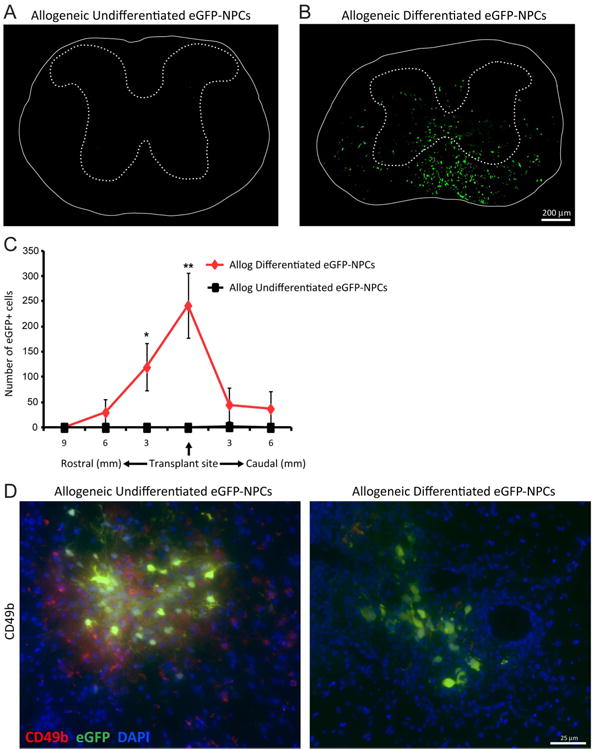
display increased survival following transplantation. Undifferentiated and differentiated eGFP-NPCs were transplanted into BALB/c mice (allogeneic transplant) on day 14 post-JHMV-infection. Representative coronal spinal cord sections of the transplant site from JHMV-infected mice receiving allogeneic undifferentiated eGFP-NPCs (A) or allogeneic differentiated eGFP-NPCs (B). Experimental mice were sacrificed at day 21 p.t. and migration and/or survival of transplanted cells evaluated by visualization of eGFP-expression from transplanted cells. (C) eGFP-NPCs were counted in coronal sections 9mm rostral and 6mm caudal to transplant site at 3mm intervals from mice transplanted with undifferentiated (n=4) or differentiated (n=6) eGFP-NPCs. Increased numbers of eGFP-NPCs (*p<0.05, **p=0.01) were present within the spinal cords of mice transplanted with differentiated eGFP-NPCs compared to undifferentiated allogeneic NPCs. (D) Representative immunofluorescence images showing CD49b+ NK cells (red) and eGFP-NPCs (green) with DAPI-stained nuclei (blue) at day 8 p.t. in coronal sections of spinal cords from mice transplanted with undifferentiated and differentiated allogeneic eGFP-NPCs.
Elevated RAE-1 expression by NPCs in response to JHMV infection
NK cells exhibit a rapid response following viral infection and participate in initiating an effective anti-viral immune response, as well as directly eliminating infected cells51-53. As we are implanting NPCs into the CNS of mice infected with a neurotropic virus, it is possible that transplanted cells may become infected with virus and serve as targets for immune recognition and destruction. In support of this possibility, NPCs are susceptible to infection with neurotropic coxsackie virus54-56 and we have previously shown that differentiated NPCs support replication of JHMV57. To determine if JHMV is capable of infecting NPCs, we first infected SCID mice, which lack T and B lymphocytes but retain functional NK cells, with JHMV and subsequently transplanted the mice with eGFP-NPCs at day 7 p.i. Our rationale for using SCID mice for these studies is that virus-specific T lymphocytes recruited to the CNS following JHMV infection control viral spread and we wished to avoid an adaptive immune response specific for virus 47, 58, 59. As shown in Figure 7A, JHMV antigen is readily detectable within transplanted eGFP-NPCs as determined by immunohistochemical staining. Approximately 50% of surviving NPCs at day 7 p.t. were infected with JHMV (data not shown). We next tested whether JHMV infection of eGFP-NPCs increases susceptibility to NK cell-mediated death through a RAE-1 signaling pathway. Following 24 hr infection of cultured eGFP-NPCs with JHMV, expression of RAE-1 significantly (p<0.01) increased from 44.4±3.2% of non-infected NPCs to 75.4±3.4% of JHMV-infected NPCs, representing an ∼2-fold increase in expression of RAE-1 (p<0.01) compared to non-infected cells (Figures 7B and C). To test whether increased RAE-1 expression following JHMV infection increased susceptibility to NK cell-mediated lysis, a cytotoxicity assay was performed. Infected (24 hr) and non-infected C57BL/6-derived NPCs were co-cultured with allogeneic BALB/c NK cells and target cell lysis was determined. NK cell-mediated killing of eGFP-NPCs was significantly (p<0.05) increased in infected cells compared to non-infected cells at an effector to target (E:T) ratio of 20:1 and killing diminished with decreased E:T ratios (Figure 7D). Inclusion of anti-NKG2D blocking antibody (20 μg/ml) resulted in diminished NK cell-mediated killing of allogeneic JHMV-infected eGFP-NPCs that trended down with decreasing E:T ratios (Figure 7E). A similar increase in susceptibility was observed when JHMV-infected NPCs were co-cultured with syngeneic C57BL/6 NK cells; approximately 40% of JHMV-infected NPCs were lysed by syngeneic NK cells at an E:T ratio of 20:1, compared to <10% of non-infected NPCs (data not shown).
Figure 7. JHMV-infection increases RAE-1 expression on NPCs and elevates susceptibility to NK cell mediated lysis.
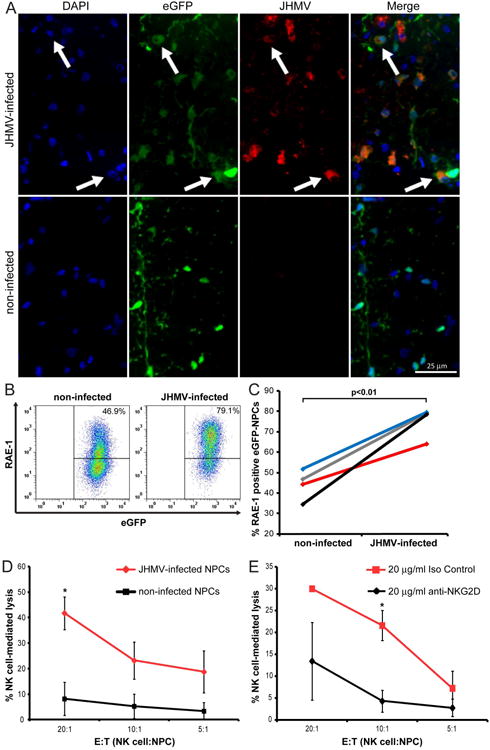
(A) Representative immunofluorescence images revealing co-localization (white arrows) of JHMV (spike protein; red) with eGFP (green) and DAPI-stained nuclei (blue) at day 7 p.t. in coronal sections of spinal cords from JHMV-infected (top panels) and non-infected (bottom panels) SCID mice transplanted with allogeneic eGFP-NPCs. (B) Cultured eGFP-NPCs were infected with JHMV (0.1 moi) for 24 hr and RAE-1 expression determined by flow cytometry. Representative flow analysis for RAE-1 expression on non-infected or JHMV-infected eGFP-NPCs is shown. (C) Paired data from four independent experiments showing increased (p<0.01) RAE-1 expression following JHMV infection. Each line represents and individual experiment. (D) Non-infected (black line) and JHMV-infected (red line) eGFP-NPCs were cultured with allogeneic NK cells in an LDH assay and the percentage of NK cell-mediated lysis at three different E:T ratios is shown. Data represent five independent experiments; *p<0.05. (E) JHMV-infected eGFP-NPCs were cultured with allogeneic NK cells plus 20 μg/ml anti-NKG2D (black line) or 20 μg/ml isotype-matched control Ig (red line) in an LDH assay and the percentage of NK cell-mediated lysis at three different E:T ratios is shown. Data represents three independent experiments; *p<0.05.
Discussion
NPCs have emerged as a viable replacement therapy for demyelinating disease, such as MS9, 10. Preclinical animal models of MS have convincingly shown the ability of transplanted NPCs to improve clinical outcome that is associated with enhanced remyelination of axons. The ability of NPCs to preferentially differentiate into either glial lineage cells or neurons is considered important within the context of regaining motor function through investment of new myelin as well as potential immunomodulatory activity9, 16, 24. While originally believed to be immunologically inert, we have recently demonstrated that transplantation of allogeneic NPCs into the spinal cords of JHMV-infected mice results in rejection that is mediated, in part, by T lymphocytes.32 Although cultured NPCs do not constitutively express high levels of MHC class I or II, upon exposure to the proinflammatory cytokine IFN-γ expression of both of these molecules increases dramatically29, 32, 60 suggesting these molecules aid in immune recognition and destruction. In addition, Palmer and colleagues36 have recently demonstrated an important role for NK cells in recognizing and killing allogeneic NPCs through NKG2D recognition of RAE-1 that is expressed on the surface of NPCs. Further evidence for the involvement of the innate immune response in rejection of NPCs comes from a recent study of human NPCs, which showed a robust innate immune response directed to human NPCs transplanted into the CNS61. Therefore, a better understanding of how the innate immune response contributes to rejection of allografts within the context of the JHMV model of demyelination is merited and is the focus of this report. Our findings support and extend recent studies examining the role of the innate immune response in recognizing and killing transplanted allogeneic NPCs36. Phillips et al. elegantly demonstrated improved survival of allogeneic NPCs following transplantation into the CNS of mice lacking NKG2D (Klrk1-/- mice)36. Our findings are consistent with these results by showing that cultured RAE-1-expressing NPCs facilitate recognition and killing by NK cells through an NKG2D-dependent pathway and blocking NGK2D dampened NK cell-mediated lysis of cultured NPCs. Importantly, using the JHMV model of neuroinflammatory demyelination, we show that administration of anti-NKG2D antibody increased survival of transplanted allogeneic NPCs. Blocking NKG2D did not result in 100% survival of allografts highlighting the importance of infiltrating T lymphocytes or other cells in killing32. It is possible that NKG2D signaling on T cells may also contribute to recognition and killing of allogeneic NPCs. NKG2D functioning as a co-stimulatory molecule on T cells has been implicated in allograft rejection41 although the majority of studies emphasize either a direct role for NKG2D killing via NK cells39, 40, 44 or indirectly through T cell activation62. Furthermore, we have previously shown a role for NKG2D in enhancing CD8+ T cell-mediated lysis in response to JHMV infection of the CNS63. Also, it is possible some cell loss following transplantation can be a result of failed engraftment due to the injection, or rejection from other cells besides T cells or NK cells. Nonetheless, our findings demonstrating that killing of undifferentiated allogeneic NPCs is muted in the absence of RAE-1 as well as blocking NKG2D signaling diminishes NK-mediated lysis support a role for NKG2D-mediated lysis of transplanted allogeneic cells by infiltrating NK cells. Furthermore, these results build upon previous studies showing that NKG2D is involved in non-CNS transplant allograft transplant rejection44.
Our results support the hypothesis that regulation of RAE-1 expression by allogeneic NPCs is important in increasing survival. Treatment of cultured NPCs with the proinflammatory cytokine IFN-γ resulted in a marginal reduction in RAE-1 expression which is in contrast to the dramatic increase in both MHC class I and II following IFN-γ exposure29, 32, 60. Ultimately, IFN-γ-treatment of NPCs did not diminish NK-mediated lysis, indicating that sufficient surface levels of RAE-1 remain thus allowing recognition by NK cells. Whether this is the result of compensation by other NKG2D ligands such as MULT-1, which is weakly expressed on NPCs36, is not know at this time.
Our data indicate that upon differentiation of cultured NPCs, RAE-1 expression dramatically declines to almost undetectable levels as determined by flow cytometry. Following differentiation of cultured NPCs, the majority (∼80%) of cells are GalC+ and NG2+ oligodendroglia with remaining populations comprised of GFAP-positive astrocytes and Tuj1+ neurons16. Our results demonstrate that transplantation of allogeneic differentiated NPCs lacking RAE-1 results in enhanced survival when compared to transplantation of RAE-1+ undifferentiated NPCs. In addition, differentiated cells are able to successfully migrate and colonize areas of white matter damage in a manner similar to undifferentiated cells16, 24. Within the context of our model of viral-induced demyelination, we propose that following allogeneic transplantation of NPCs into the spinal cord JHMV-infected mice, these cells are likely first targeted by NK cells as we observed increased numbers of infiltrating NK cells within the spinal cords of allograft recipients that was associated with a rapid reduction in numbers of transplanted cells. Over time, surviving cells differentiate and this is accompanied by diminished RAE-1 expression that limits NK cell-mediated rejection. However, inflammatory T cells presumably recognize allogeneic transplanted NPCs through an MHC-mediated pathway following exposure to IFNγ culminating in complete rejection. Collectively, our in vitro and in vivo data argue that reducing RAE-1 expression on allogeneic stem cell populations may increase survival and improve both motor skills and histology outcomes in preclinical animal models of MS.
In addition to viral pathogens being an environmental factor associated with MS, there are numerous viruses capable of persisting within the CNS, including JC virus and Epstein-Barr virus64-66. In the absence of immune surveillance, e.g. as a result of immunosuppressive therapy necessary for transplantation, viral recrudescence is a valid concern67. A clinically relevant example is the development of progressive multifocal leukoencephalopathy (PML) due to JC virus in MS patients resulting from administration of natalizumab which impairs T cell infiltration into the CNS. This leads to the question of whether NPCs transplanted to treat demyelinating diseases would be susceptible to viral infection. In vitro, RAE-1 expression was elevated in response to JHMV infection, and this enhanced NKG2D-dependent NK cell-mediated death of allogeneic NPCs, demonstrating that RAE-1 and NKG2D are mediators of NK cell lysis activity following JHMV infection. NPCs transplanted into immune-deficient mice succumb to infection; although, it is not clear whether virally infected transplanted NPCs die as a result of viral-mediated or NK cell-mediated lysis; in vitro, ∼20% of NPCs initially die following JHMV infection68. While some studies have examined the susceptibility of NPCs to various neurotropic viruses, reports are limited with regards to how viral infection of allogeneic NPCs following transplant affects immune recognition and survival. Recently, Basu and colleagues69 determined that Japanese Encephalitis Viral infection of NPCs alters their immunogenicity, resulting in recognition by allogeneic T cells, and subsequent T cell proliferation. We believe our findings, in conjunction with others, highlights the need for additional information regarding the immunogeneic affect of viral infection of NPCs within the context of allogeneic transplant.
Conclusion
Our findings demonstrate that in addition to T cell suppression, modulation of NKG2D-RAE-1 signaling may be necessary for long-term survival of allografts transplanted into the CNS. Possible mechanisms include elimination of NK cells, blocking NK cell activation, or limiting RAE-1 expression on transplanted allogeneic NPCs. However, the consequences of these potential therapeutic interventions must be considered within the context of possible viral infection of transplanted NPCs and the consequences on optimal host defense.
Supplementary Material
Non-infected (N.I.) mice were transplanted with eGFP-NPCs or vehicle only at day 14 p.i. (A,B) Mice were sacrificed at days 0 and 8 p.t. and the frequency of NKG2D+ NK cells among total lymphocytes in the spinal cord (9mm rostral and caudal to transplant site) of recipient mice was determined by flow cytometry. Representative flow analysis of CD3-CD49b+ NK cells (% of total live cells, A), and NKG2D+ NK cells (% of CD3-CD49b+ cells, B) in allogeneic eGFP-NPC- and vehicle only-transplanted mice are shown. (C) Quantification of percentage of NKG2D+ NK cells in spinal cords of allogeneic eGFP-NPC- and vehicle only-transplanted mice. Data is presented as average+SEM with 4 mice per group; p<0.05.
Acknowledgments
This work was funded by National Institutes of Health (NIH) Grant R01 NS074987 and a National Multiple Sclerosis Society (NMSS) Collaborative Center Research Award (CA1058-A-8) to T.E.L. L.L.L. is and American Cancer Society Professor and supported by NIH AI066897 and CA095137. J.G.W. is supported by NMSS postdoctoral fellowship FG 1960-A-1.
Footnotes
Author Contributions: Jason G. Weinger designed experiments, collected data for analysis and interpretation, and wrote the manuscript; Warren C. Plaisted and Sonia M. Maciejewski collected data for analysis and interpretation; Craig M. Walsh provided financial support; Lewis L. Lanier provided study material and assisted in manuscript writing; Thomas E. Lane designed experiments, analyzed and interpreted data, provided financial support, and wrote the manuscript.
Potential Conflict of Interest: L.L.L. and University of California (San Francisco, CA) has licensed intellectual property rights relating to NKG2D for potential therapeutic development.
Literature Cited
- 1.Markovic-Plese S, Pinilla C, Martin R. The initiation of the autoimmune response in multiple sclerosis. Clin Neurol Neurosurg. 2004;106:218–222. doi: 10.1016/j.clineuro.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGavern DB, Murray PD, Rivera-Quinones C, et al. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123(Pt 3):519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Stefano N, Matthews PM, Fu L, et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121(Pt 8):1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- 5.Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(Suppl 5):v3–9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- 6.Lucchinetti CF, Parisi J, Bruck W. The pathology of multiple sclerosis. Neurol Clin. 2005;23:77–105. vi. doi: 10.1016/j.ncl.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Kipp M, Victor M, Martino G, et al. Endogeneous remyelination: findings in human studies. CNS Neurol Disord Drug Targets. 2012;11:598–609. doi: 10.2174/187152712801661257. [DOI] [PubMed] [Google Scholar]
- 8.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 9.Pluchino S, Zanotti L, Brini E, et al. Regeneration and repair in multiple sclerosis: the role of cell transplantation. Neurosci Lett. 2009;456:101–106. doi: 10.1016/j.neulet.2008.03.097. [DOI] [PubMed] [Google Scholar]
- 10.Sher F, Balasubramaniyan V, Boddeke E, et al. Oligodendrocyte differentiation and implantation: new insights for remyelinating cell therapy. Curr Opin Neurol. 2008;21:607–614. doi: 10.1097/WCO.0b013e32830f1e50. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Hur T, Goldman SA. Prospects of cell therapy for disorders of myelin. Ann N Y Acad Sci. 2008;1142:218–249. doi: 10.1196/annals.1444.014. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Rostami A, Zhang GX. Cellular remyelinating therapy in multiple sclerosis. J Neurol Sci. 2009;276:1–5. doi: 10.1016/j.jns.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Martino G, Franklin RJ, Baron Van Evercooren A, et al. Stem cell transplantation in multiple sclerosis: current status and future prospects. Nat Rev Neurol. 2010;6:247–255. doi: 10.1038/nrneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 14.Groves AK, Barnett SC, Franklin RJ, et al. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- 15.Pluchino S, Quattrini A, Brambilla E, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 16.Totoiu MO, Nistor GI, Lane TE, et al. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol. 2004;187:254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane TE, Buchmeier MJ. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bender SJ, Weiss SR. Pathogenesis of murine coronavirus in the central nervous system. J Neuroimmune Pharmacol. 2010;5:336–354. doi: 10.1007/s11481-010-9202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virtanen JO, Jacobson S. Viruses and multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11:528–544. doi: 10.2174/187152712801661220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvetti M, Giovannoni G, Aloisi F. Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol. 2009;22:201–206. doi: 10.1097/WCO.0b013e32832b4c8d. [DOI] [PubMed] [Google Scholar]
- 22.Christensen T. Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes viruses. Rev Med Virol. 2005;15:179–211. doi: 10.1002/rmv.465. [DOI] [PubMed] [Google Scholar]
- 23.Fotheringham J, Jacobson S. Human herpesvirus 6 and multiple sclerosis: potential mechanisms for virus-induced disease. Herpes. 2005;12:4–9. [PubMed] [Google Scholar]
- 24.Carbajal KS, Schaumburg C, Strieter R, et al. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hori J, Ng TF, Shatos M, et al. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Baroja ML, Majumdar A, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–456. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 27.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 28.Anderson AJ, Haus DL, Hooshmand MJ, et al. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient? Regen Med. 2011;6:367–406. doi: 10.2217/rme.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Phillips LK, Gould E, et al. MHC mismatch inhibits neurogenesis and neuron maturation in stem cell allografts. PLoS One. 2011;6:e14787. doi: 10.1371/journal.pone.0014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearl JI, Lee AS, Leveson-Gower DB, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearl JI, Kean LS, Davis MM, et al. Pluripotent stem cells: immune to the immune system? Sci Transl Med. 2012;4:164ps125. doi: 10.1126/scitranslmed.3005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinger JG, Weist BM, Plaisted WC, et al. MHC mismatch results in neural progenitor cell rejection following spinal cord transplantation in a model of viral-induced demyelination. Stem Cells. 2012;30:2584–2595. doi: 10.1002/stem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Xu CJ, Lu HZ, et al. Long-term fate of allogeneic neural stem cells following transplantation into injured spinal cord. Stem Cell Rev. 2010;6:121–136. doi: 10.1007/s12015-009-9104-y. [DOI] [PubMed] [Google Scholar]
- 34.Preynat-Seauve O, de Rham C, Tirefort D, et al. Neural progenitors derived from human embryonic stem cells are targeted by allogeneic T and natural killer cells. J Cell Mol Med. 2009;13:3556–3569. doi: 10.1111/j.1582-4934.2009.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laguna Goya R, Busch R, Mathur R, et al. Human fetal neural precursor cells can up-regulate MHC class I and class II expression and elicit CD4 and CD8 T cell proliferation. Neurobiol Dis. 2011;41:407–414. doi: 10.1016/j.nbd.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Phillips LK, Gould EA, Babu H, et al. Natural killer cell-activating receptor NKG2D mediates innate immune targeting of allogeneic neural progenitor cell grafts. Stem Cells. 2013;31:1829–1839. doi: 10.1002/stem.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reekmans K, De Vocht N, Praet J, et al. Spatiotemporal evolution of early innate immune responses triggered by neural stem cell grafting. Stem Cell Res Ther. 2012;3:56. doi: 10.1186/scrt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Palmer TD. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet. 2008;17:R84–92. doi: 10.1093/hmg/ddn104. [DOI] [PubMed] [Google Scholar]
- 39.Ogasawara K, Benjamin J, Takaki R, et al. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6:938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNerney ME, Lee KM, Zhou P, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 41.Feng L, Ke N, Ye Z, et al. Expression of NKG2D and its ligand in mouse heart allografts may have a role in acute rejection. Transplant Proc. 2009;41:4332–4339. doi: 10.1016/j.transproceed.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 42.Lodoen M, Ogasawara K, Hamerman JA, et al. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espinoza JL, Takami A, Trung LQ, et al. Ataxia-telangiectasia mutated kinase-mediated upregulation of NKG2D ligands on leukemia cells by resveratrol results in enhanced natural killer cell susceptibility. Cancer Science. 2013;104:657–662. doi: 10.1111/cas.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Chang CK, Hayden T, et al. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol. 2007;179:6416–6420. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- 45.Popa N, Cedile O, Pollet-Villard X, et al. RAE-1 is expressed in the adult subventricular zone and controls cell proliferation of neurospheres. Glia. 2011;59:35–44. doi: 10.1002/glia.21074. [DOI] [PubMed] [Google Scholar]
- 46.Castro RF, Evans GD, Jaszewski A, et al. Coronavirus-induced demyelination occurs in the presence of virus-specific cytotoxic T cells. Virology. 1994;200:733–743. doi: 10.1006/viro.1994.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane TE, Liu MT, Chen BP, et al. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stiles LN, Hosking MP, Edwards RA, et al. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]
- 49.Trifilo MJ, Lane TE. The CC chemokine ligand 3 regulates CD11c+CD11b+CD8alpha- dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327:8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bajenoff M, Breart B, Huang AY, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 52.Arase H, Mocarski ES, Campbell AE, et al. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 53.Brandstadter JD, Yang Y. Natural killer cell responses to viral infection. J Innate Immun. 2011;3:274–279. doi: 10.1159/000324176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feuer R, Mena I, Pagarigan RR, et al. Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. Am J Pathol. 2003;163:1379–1393. doi: 10.1016/S0002-9440(10)63496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feuer R, Pagarigan RR, Harkins S, et al. Coxsackievirus targets proliferating neuronal progenitor cells in the neonatal CNS. J Neurosci. 2005;25:2434–2444. doi: 10.1523/JNEUROSCI.4517-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsueng G, Tabor-Godwin JM, Gopal A, et al. Coxsackievirus preferentially replicates and induces cytopathic effects in undifferentiated neural progenitor cells. J Virol. 2011;85:5718–5732. doi: 10.1128/JVI.02261-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitman L, Zhou H, Perlman S, et al. IFN-gamma-mediated suppression of coronavirus replication in glial-committed progenitor cells. Virology. 2009;384:209–215. doi: 10.1016/j.virol.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marten NW, Stohlman SA, Zhou J, et al. Kinetics of virus-specific CD8+ -T-cell expansion and trafficking following central nervous system infection. J Virol. 2003;77:2775–2778. doi: 10.1128/JVI.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marten NW, Stohlman SA, Bergmann CC. Role of viral persistence in retaining CD8(+) T cells within the central nervous system. J Virol. 2000;74:7903–7910. doi: 10.1128/jvi.74.17.7903-7910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim DE, Tsuji K, Kim YR, et al. Neural stem cell transplant survival in brains of mice: assessing the effect of immunity and ischemia by using real-time bioluminescent imaging. Radiology. 2006;241:822–830. doi: 10.1148/radiol.2413050466. [DOI] [PubMed] [Google Scholar]
- 61.Jablonska A, Janowski M, Lukomska B. Different methods of immunosuppresion do not prolong the survival of human cord blood-derived neural stem cells transplanted into focal brain-injured immunocompetent rats. Acta Neurobiol Exp (Wars) 2013;73:88–101. doi: 10.55782/ane-2013-1924. [DOI] [PubMed] [Google Scholar]
- 62.Ito A, Shimura H, Nitahara A, et al. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. Int Immunol. 2008;20:1343–1349. doi: 10.1093/intimm/dxn092. [DOI] [PubMed] [Google Scholar]
- 63.Walsh KB, Lanier LL, Lane TE. NKG2D receptor signaling enhances cytolytic activity by virus-specific CD8+ T cells: evidence for a protective role in virus-induced encephalitis. J Virol. 2008;82:3031–3044. doi: 10.1128/JVI.02033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 65.Sweet TM, Del Valle L, Khalili K. Molecular biology and immunoregulation of human neurotropic JC virus in CNS. J Cell Physiol. 2002;191:249–256. doi: 10.1002/jcp.10096. [DOI] [PubMed] [Google Scholar]
- 66.Fujimoto H, Asaoka K, Imaizumi T, et al. Epstein-Barr virus infections of the central nervous system. Intern Med. 2003;42:33–40. doi: 10.2169/internalmedicine.42.33. [DOI] [PubMed] [Google Scholar]
- 67.Arthur RR, Shah KV, Charache P, et al. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988;158:563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- 68.Plaisted WC, Weinger JG, Walsh CM, et al. T cell mediated suppression of neurotropic coronavirus replication in neural precursor cells. Virology. 2013 doi: 10.1016/j.virol.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Das S, Ghosh D, Basu A. Japanese encephalitis virus induce immuno-competency in neural stem/progenitor cells. PLoS One. 2009;4:e8134. doi: 10.1371/journal.pone.0008134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-infected (N.I.) mice were transplanted with eGFP-NPCs or vehicle only at day 14 p.i. (A,B) Mice were sacrificed at days 0 and 8 p.t. and the frequency of NKG2D+ NK cells among total lymphocytes in the spinal cord (9mm rostral and caudal to transplant site) of recipient mice was determined by flow cytometry. Representative flow analysis of CD3-CD49b+ NK cells (% of total live cells, A), and NKG2D+ NK cells (% of CD3-CD49b+ cells, B) in allogeneic eGFP-NPC- and vehicle only-transplanted mice are shown. (C) Quantification of percentage of NKG2D+ NK cells in spinal cords of allogeneic eGFP-NPC- and vehicle only-transplanted mice. Data is presented as average+SEM with 4 mice per group; p<0.05.


