Abstract
Human cytomegalovirus (HCMV) infection and reactivation are a major cause of morbidity in immune-suppressed patients. Interestingly, epidemiological studies have shown that patients administered the mammalian target of rapamycin (mTOR) inhibitor, sirolimus (rapamycin), exhibit more favourable outcomes, suggestive of activity against HCMV in vivo. Given its relative lack of activity against lytic infection, it is postulated that rapamycin inhibits HCMV reactivation. Here, we showed that rapamycin administered acutely or chronically has little impact on induction of immediate early (IE) gene expression in experimentally latent dendritic cells or cells from naturally latent individuals. Furthermore, we extended these observations to include other inhibitors of mTORC1 and mTORC 2, which similarly have minimal effects on induction of IE gene expression from latency. Taken together, these data suggest that favourable outcomes associated with sirolimus are attributable to indirect effects that influence HCMV reactivation, rather than a direct mechanistic action against HCMV itself.
Human cytomegalovirus (HCMV) reactivation is a major cause of disease in transplant recipients and critically ill patients (Legendre & Pascual, 2008; Limaye et al., 2008). Similarly, primary infection poses a major health threat to immunocompromised populations and represents the predominant viral cause of congenital disease, particularly in the developed world (Revello & Gerna, 2004). Thus, understanding the mechanisms underlying HCMV infection and pathogenesis is of significant clinical importance.
HCMV reactivation generally occurs sporadically but at subclinical levels due to the controlling presence of a robust immune response (Jackson et al., 2011; Jost & Altfeld, 2013; Rölle & Olweus, 2009), rendering immune suppression a major factor in clinical reactivation (Smith & Khanna, 2013; Watkins et al., 2012). Although studies systematically correlating HCMV disease incidence with different immune suppression methods have been performed (Chakrabarti et al., 2002, 2004; Lin et al., 2002), the majority of findings require further investigation for unequivocal interpretation. Interestingly, accumulating data have provided evidence of better outcomes upon immune suppression with sirolimus (rapamycin) following both stem cell (Marty et al., 2007) and solid organ transplantation (Demopoulos et al., 2008; Ghassemieh et al., 2013), suggesting that this immunosuppression regimen has a direct impact on HCMV.
Targets of rapamycin (Tor1 and Tor2) were originally identified as yeast proteins sensitive to a naturally occurring antifungal agent expressed by Streptomyces hygroscopicus (Heitman et al., 1991; Vézina et al., 1975), and subsequent studies revealed a mammalian target (mTOR) particularly active against the mTOR complex 1 (mTORC1) arm (Heitman et al., 1991; Sabatini et al., 1994). mTOR is a serine/threonine kinase controlling a range of cellular functions, including cell growth, proliferation and survival and affecting transcription and protein synthesis (Lamming et al., 2013). Two functional complexes exist: mTORC1, classically described as rapamycin-sensitive and important for stimulation of protein synthesis via activation of p70S6 kinase 1 and 4E-BP1, and a second less well-characterized mTORC2 complex that is generally considered rapamycin-insensitive (Loewith et al., 2002) and implicated in cytoskeletal organization as well as mediation of AKT signalling (Kim et al., 2002; Sarbassov et al., 2005).
Viral targeting of the mTOR pathways during lytic infection positively influences viral replication (Clippinger et al., 2011; Kudchodkar et al., 2004, 2006; Moorman & Shenk, 2010). Interestingly, while HCMV utilizes the mTORC1 pathway, rapamycin has a minimal impact on viral replication in fibroblasts, since HCMV activates a rapamycin-insensitive pathway (Kudchodkar et al., 2004; Moorman & Shenk, 2010). Within 12 h, phosphorylation of 4E-BP1 becomes insensitive to rapamycin, as shown by Kudchodkar et al. (2004). Reconciling these data with improved prognosis regarding HCMV infection for patients immunosuppressed with sirolimus led to the proposal that sirolimus abrogates HCMV reactivation and disease in vivo by preventing reactivation of HCMV IE gene expression (Marty et al., 2007).
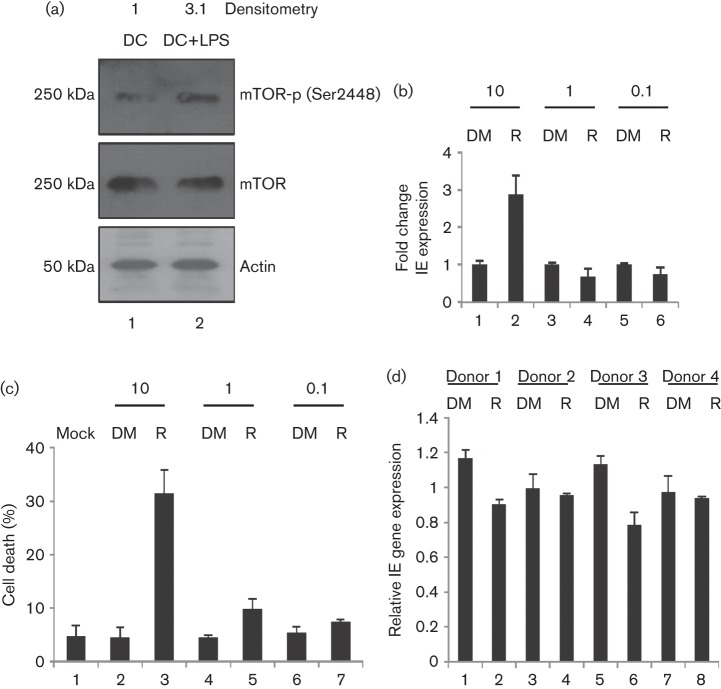
Clearly, for mTOR to be important in HCMV reactivation, it is required to be active in DC (dendritic cell) differentiation. Western blot analysis of mTOR autophosphorylation (anti-phospho-mTOR antibody (Ser2448), 1 : 750, cell signalling) in DCs derived from monocytes (isolated from healthy volunteers under ethical approval from the Cambridge Local Research Ethics committee) suggested that mTOR is active in these cells (Fig. 1a), and enhanced threefold by the addition of LPS (Fig. 1a; densitometry using Image J Software, NIH). Having established mTOR activity in DCs, we examined the effects of rapamycin on reactivation. Latently infected monocytes differentiated into immature DCs (Reeves & Compton, 2011) were treated with log dilutions of rapamycin for 1 h (10 µM–100 nM in DMSO), prior to reactivation. At 24 h post-reactivation, no overt inhibitory effect on immediate early (IE) gene expression was observed using real time qPCR (RT-qPCR) at the lower doses of rapamycin (Fig. 1b), which are higher than those achieved clinically (20–50 nM). However, we noted a trend suggesting that rapamycin has a minor inhibitory effect on IE gene expression following an analysis in cells from multiple donors, although the differences in expression when all four donors were taken together were non-significant (Fig. 1d; P = 0.16). Elevated IE gene expression at higher doses of the drug (Fig. 1b) may be linked to the observation that rapamycin at high concentrations mimics or enhances aspects of the inflammatory response, even in the absence of inflammatory cytokines (Barilli et al., 2008; Turnquist et al., 2010) or that activation of death pathways (Fig. 1c) indirectly stimulates HCMV gene expression.
Fig. 1.
Rapamycin does not inhibit reactivation of HCMV gene expression. (a) Western blot analysis of phosphorylated and total mTOR in immature DCs (1) and mature DCs (2). (b) RNA isolated from immature DCs stimulated with LPS was analysed for IE and GAPDH RNA expression using qRT-PCR. Prior to reactivation, cells were incubated for 2 h with DMSO (1, 3, 5) or rapamycin (2, 4, 6). (c) Immature DCs (1) incubated with DMSO (2, 4, 6) or rapamycin (3, 5, 7) were analysed for viability using trypan blue at 24 h post-treatment. (d) RNA isolated from MoDCs derived from four independent monocyte donors was analysed for reactivation of experimental latency via qRT-PCR post-LPS stimulation in the presence of 1 µM rapamycin (2, 4, 6, 8) or DMSO control (1, 3, 5, 7). IE gene expression was determined, relative to untreated (no solvent) LPS control. Statistical analysis was performed on the four donors combined (n = 4; t-test, P = 0.16).
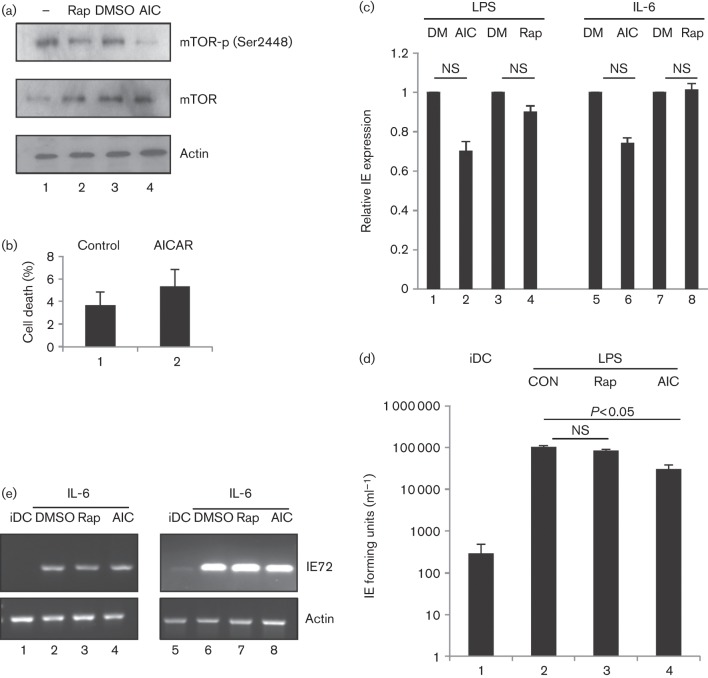
Pre-treatment of fibroblasts with another mTORC1 inhibitor, AICAR (aminoimidazole carboxamide ribonucleotide), has been shown to block MIEP (major IE promoter) activity, but only if added prior to HCMV infection (Kudchodkar et al., 2007). Furthermore, AICAR is deleterious to viral replication during lytic infection, suggesting that modulation of the cellular AMP : ATP level affects HCMV infection (Kudchodkar et al., 2007). AICAR acts as an agonist of AMP-activated protein kinase (AMPK) through modulation of cellular AMP : ATP levels (Corton et al., 1995). AICAR couples elevated AMP levels (and thus cellular energy deprivation) with increased AMPK phosphorylation and activation (Luo et al., 2005), resulting in inactivation of mTOR pathways. Accordingly, we examined whether pre-treatment of immature DCs with AICAR affects their response to reactivation stimuli (Fig. 2). Immature DCs pre-treated with rapamycin (1 µM) or AICAR (0.5 mM) at non-toxic concentrations (Figs 1c and 2b) that suppress mTOR activation (Fig. 2a) were stimulated with LPS or IL-6, and reactivation of IE gene expression measured using RT-qPCR. Again, no appreciable effect of rapamycin on HCMV reactivation was observed following IL-6 or LPS stimulation whereas a minor effect was evident with AICAR (Fig. 2c). To assess infectious virus production after reactivation, DCs were cultured on a monolayer of human foreskin fibroblasts (HFFs) at 5 days post-reactivation for a further 5 days. Co-culture supernatants were tested for the presence of infectious virus by inoculation with fresh indicator fibroblasts and subsequent scoring of IE positivity. Consistent with IE RNA expression data, no significant impact on virus production was observed in DCs cultured with rapamycin (Fig. 2d; P>0.05). However, a statistically significant (twofold to threefold) decrease in virus production was detected in AICAR-treated reactivated DCs (Fig. 2d; P<0.05). Interestingly, earlier studies on HFFs have identified a post-IE effect of AICAR on virus production during lytic infection (Kudchodkar et al., 2007). Finally, these inhibitors appeared to have no significant impact on induction of IE gene expression from latent HCMV in cells of seropositive donors (Fig. 2e), suggesting that our observations with experimental latency can be replicated in natural latency using previously defined protocols (Reeves & Compton, 2011).
Fig. 2.
Inhibition of mTORC1 using AICAR promotes a minor defect in virus reactivation. (a) Western blot analysis of mTOR phosphorylation in DCs (1) or DCs incubated with 1 µM rapamycin (2), DMSO (3) or 0.5 mM AICAR (4) for 3 h. (b) Immature DCs incubated with DMSO (1) or AICAR (2) were analysed for viability using trypan blue at 24 h post-treatment. (c) qRT-PCR analysis of RNA isolated from immature DCs pre-treated with DMSO (1, 3, 5, 7; DM), AICAR (2, 6; AIC) or rapamycin (4, 8; Rap) for 2 h, prior to LPS (1–4) or IL-6 (5–8) stimulation. (d) Supernatants from DC : HFF co-cultures at 10 days post-reactivation were used to inoculate fresh fibroblasts and scored for IE positivity as a measure of infectious virus reactivation. Quantification is shown for immature DC (iDC), immature DC+LPS (CON), immature DC+LPS+rapamycin (Rap) and immature DC+LPS+AICAR (AIC). (e) Monocytes isolated from two seropositive donors were differentiated into iDCs (1, 5), and prior to IL-6- induced reactivation (2–4; 6–8), pretreated with DMSO (2, 6), 1 µM rapamycin (3, 7) or AICAR (4, 8). RNA was analysed with nested PCR for IE72 gene expression as described previously (Reeves & Compton, 2011). NS, Non-significant by t-test (n = 3).
Whilst mTORC1 does not appear to play a major role in reactivation, a role for mTORC2 cannot be discounted. Indeed, studies on an NTera2/D1 quiescent infection model suggest that CREB (cAMP response element binding protein) and mTORC2 interactions promote HCMV MIEP gene expression in response to cAMP activators (Yuan et al., 2009). Furthermore, these studies in cell culture reflect acute exposure to rapamycin due to the nature of the drug administration whereas in vivo the exposure is more chronic due to long term use. Importantly, chronic exposure of cells to rapamycin is deleterious to the mTORC2 pathway (Lamming et al., 2012; Sarbassov et al., 2006; Vollenbröker et al., 2009) via blockade of the formation of de novo complexes following their natural turnover in the cell (Sarbassov et al., 2006). Accordingly, we addressed whether mTORC2 plays a role in reactivation.
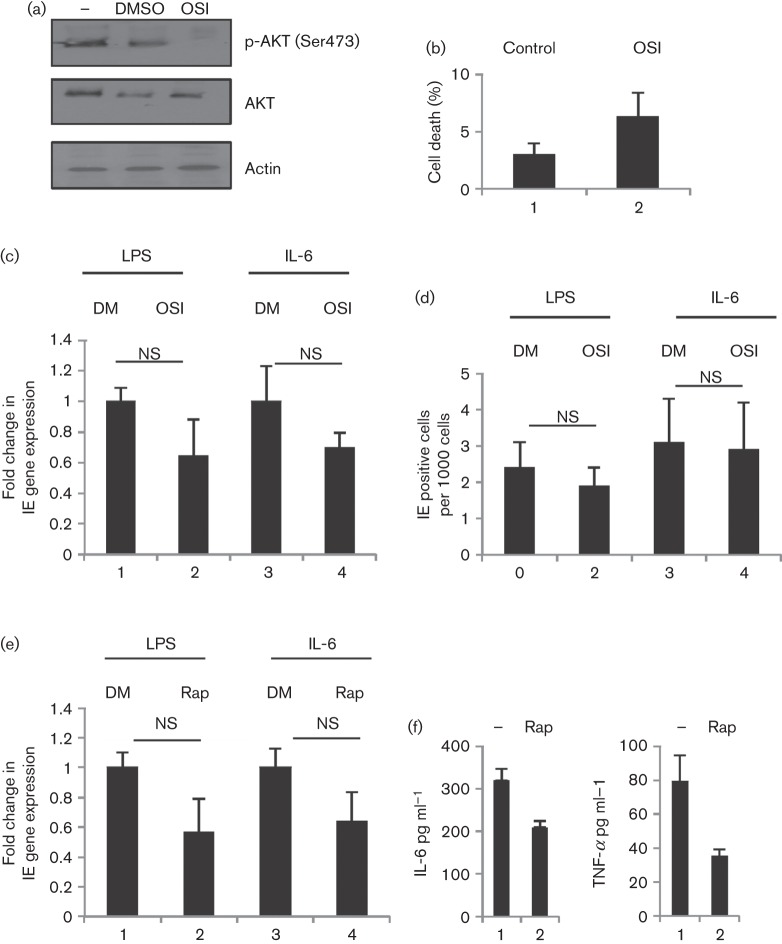
OSI-027 (Selleckchem; 10 µM in DMSO) is a well-characterized inhibitor of mTOR under trial for treatment of leukaemia due to effects on both mTORC1 and 2 in vivo (Bhagwat et al., 2011). Immature DCs derived from latently infected monocytes were treated with OSI-027 for 2 h, prior to the induction of reactivation (Fig. 3), at a concentration known to block downstream AKT activation (Fig. 3a) but not trigger cell death (Fig. 3b). However, no significant effects on RNA and protein expression levels were observed (Fig. 3c,d; P>0.05, respectively). Similarly, chronic exposure to rapamycin was ineffective at inhibiting HCMV reactivation (Fig. 3e; P>0.05), with chronic administration modelled by treating with rapamycin for the final 3 days of the 6-day differentiation period to immature DCs. The data collectively suggest that rapamycin and mTOR signalling have minor involvement in the induction of IE gene expression from DCs in vitro.
Fig. 3.
Inhibition of mTORC1 and mTORC2 does not block induction of IE gene expression (a) Western blot analysis for AKT phosphorylation in iDCs (1), iDCs plus DMSO (2) and iDCs plus OSI-027 (3). (b) Immature DCs incubated with DMSO (1) or OSI-027 (2) were analysed for viability using trypan blue at 24 h post-treatment. (c, d) qRT-PCR (c) and immunofluorescence analyses (d) of IE expression were performed on immature DCs pre-incubated with DMSO (1, 3; DM) or OSI-027 (2,4; OSI) for 2 h prior to LPS or IL-6 stimulation. (e) qRT-PCR analysis was performed on RNA isolated from immature DCs pre-incubated with DMSO (1, 3; DM) or rapamycin (2, 4; Rap) for 3 days, prior to LPS or IL-6 stimulation. (f) Immature DCs were incubated with 1 µM rapamycin (2, 4), prior to LPS stimulation and IL-6 (1, 2) and TNF-α expression measured using cytokine ELISA. NS, Non-significant with t-test (n = 3).
HCMV becomes insensitive to rapamycin within 12 h post-infection (Kudchodkar et al., 2004, 2006; Moorman & Shenk, 2010) and uses a rapamycin-insensitive pathway to maintain viral progression through its life cycle (Clippinger et al., 2011; Moorman & Shenk, 2010). However, many of these earlier studies were performed in fibroblasts (Alwine, 2008), a cell type more resistant to metabolic stress in vitro (Baines et al., 2005; Li et al., 2005). This was exemplified by studies on the role of the non-coding RNA beta 2.7 and its effects on cell viability and energy production (Reeves et al., 2007), where a growth defect was observed in neuronal but not fibroblast cells (McSharry et al., 2003; Reeves et al., 2007). Consistent with differential sensitivity, polarized M2 macrophages infected with HCMV were shown to be sensitive to rapamycin, with impaired virus production (Poglitsch et al., 2012). However, the studies presented here suggest that HCMV in DCs are similarly resistant to rapamycin during reactivation.
These observations collectively suggest that better outcomes regarding post-transplant HCMV disease associated with sirolimus-based immune suppression therapies are largely attributed to indirect effects. HCMV reactivation and disease in a clinical setting are possibly dependent on a number of factors. Firstly, there is the rapidity of reconstitution of the immune response (Smith & Khanna, 2013; Watkins et al., 2012), illustrated by the benefits associated with transplanting seropositive donor CD34+ cells to seropositive recipients in HLA-mismatched bone marrow transplants due to the transfer of pre-existing immunity (Ljungman et al., 2003). Secondly, this could affect the resolution of secondary infections potentially exacerbated by HCMV replication (Nichols et al., 2002). Inherent in these observations is the view that the nature of the reconstituted immune response dictates the ability of the host to control viral reactivation (Roux et al., 2000). Clearly, if certain immune suppressive regimens induce accelerated recovery of specific T-cell responses important for controlling HCMV, a clinical advantage exists. Thirdly, several lines of evidence, both in vitro and in vivo, suggest that inflammation exacerbates HCMV reactivation (Blankenberg et al., 2001; Hargett & Shenk, 2010; Humar et al., 1999; Prösch et al., 2002; Reeves & Compton, 2011; Söderberg-Nauclér et al., 1997). A major source of inflammation is from allogeneically stimulated T cells, an event shown to promote HCMV reactivation ex vivo (Söderberg-Nauclér et al., 1997). Indeed, reduced incidence of graft versus host disease (GvHD) observed with sirolimus (Antin et al., 2003; Armand et al., 2008) is linked with a high proportion of T regulatory cells produced in these patients (San Segundo et al., 2010). Thus, suppression of CD4+ allogeneic responses protects against GvHD (Hester et al., 2012). Clearly, in the context of HCMV, the provision of a less inflammatory environment may also affect reactivation. Consistently, in our in vitro model, the trend towards a minor decrease in IE gene expression upon stimulation with LPS may be linked with reduced inflammation in the presence of rapamycin (Fig. 3f), as measured using IL-6 and TNF-α cytokine ELISA (R&D systems, Abingdon, UK). Finally, a number of immunosuppressive regimens promote increased inflammatory responses. For example, antithymocyte G has been shown to induce inflammatory gene expression from monocytes (Rameshwar & Gascón, 1992). Among the cytokines produced in such a response, IL-6 is linked with HCMV reactivation in vitro (Hargett & Shenk, 2010; Huang et al., 2012; Reeves & Compton, 2011) and IL-1b has been shown to trigger MCMV (mouse cytomegalovirus) reactivation (Cook et al., 2006).
In summary, while these data are essentially negative, they address an important theory regarding the improved outcomes associated with sirolimus-based immune suppression. The findings imply that improved outcomes during transplant (Demopoulos et al., 2008; Ghassemieh et al., 2013; Marty et al., 2007) are associated with indirect effects on the immune system, rather than direct molecular blockade of HCMV reactivation in vivo.
Acknowledgements
We thank John Sinclair and Mark Wills for critical discussions. This work was funded by an MRC CDA Fellowship (G:0900466) to M. B. R.
References
- Alwine J. C. (2008). Modulation of host cell stress responses by human cytomegalovirus. Curr Top Microbiol Immunol 325, 263–279 [DOI] [PubMed] [Google Scholar]
- Antin J. H., Kim H. T., Cutler C., Ho V. T., Lee S. J., Miklos D. B., Hochberg E. P., Wu C. J., Alyea E. P., Soiffer R. J. (2003). Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 102, 1601–1605 10.1182/blood-2003-02-0489 [DOI] [PubMed] [Google Scholar]
- Armand P., Gannamaneni S., Kim H. T., Cutler C. S., Ho V. T., Koreth J., Alyea E. P., LaCasce A. S., Jacobsen E. D. & other authors (2008). Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol 26, 5767–5774 10.1200/JCO.2008.17.7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C. P., Kaiser R. A., Purcell N. H., Blair N. S., Osinska H., Hambleton M. A., Brunskill E. W., Sayen M. R., Gottlieb R. A. & other authors (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 10.1038/nature03434 [DOI] [PubMed] [Google Scholar]
- Barilli A., Visigalli R., Sala R., Gazzola G. C., Parolari A., Tremoli E., Bonomini S., Simon A., Closs E. I. & other authors (2008). In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc Res 78, 563–571 10.1093/cvr/cvn024 [DOI] [PubMed] [Google Scholar]
- Bhagwat S. V., Gokhale P. C., Crew A. P., Cooke A., Yao Y., Mantis C., Kahler J., Workman J., Bittner M. & other authors (2011). Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol Cancer Ther 10, 1394–1406 10.1158/1535-7163.MCT-10-1099 [DOI] [PubMed] [Google Scholar]
- Blankenberg S., Rupprecht H. J., Bickel C., Espinola-Klein C., Rippin G., Hafner G., Ossendorf M., Steinhagen K., Meyer J. (2001). Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease. Circulation 103, 2915–2921 10.1161/01.CIR.103.24.2915 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Mackinnon S., Chopra R., Kottaridis P. D., Peggs K., O’Gorman P., Chakraverty R., Marshall T., Osman H. & other authors (2002). High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood 99, 4357–4363 10.1182/blood.V99.12.4357 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Milligan D. W., Brown J., Osman H., Vipond I. B., Pamphilon D. H., Marks D. I. (2004). Influence of cytomegalovirus (CMV) sero-positivity on CMV infection, lymphocyte recovery and non-CMV infections following T-cell-depleted allogeneic stem cell transplantation: a comparison between two T-cell depletion regimens. Bone Marrow Transplant 33, 197–204 10.1038/sj.bmt.1704334 [DOI] [PubMed] [Google Scholar]
- Clippinger A. J., Maguire T. G., Alwine J. C. (2011). Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation. J Virol 85, 9369–9376 10.1128/JVI.05102-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C. H., Trgovcich J., Zimmerman P. D., Zhang Y., Sedmak D. D. (2006). Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol 80, 9151–9158 10.1128/JVI.00216-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton J. M., Gillespie J. G., Hawley S. A., Hardie D. G. (1995). 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229, 558–565 10.1111/j.1432-1033.1995.tb20498.x [DOI] [PubMed] [Google Scholar]
- Demopoulos L., Polinsky M., Steele G., Mines D., Blum M., Caulfield M., Adamkovic A., Liu Q., Harler M. B. & other authors (2008). Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc 40, 1407–1410 10.1016/j.transproceed.2008.03.084 [DOI] [PubMed] [Google Scholar]
- Ghassemieh B., Ahya V. N., Baz M. A., Valentine V. G., Arcasoy S. M., Love R. B., Seethamraju H., Alex C. G., Bag R. & other authors (2013). Decreased incidence of cytomegalovirus infection with sirolimus in a post hoc randomized, multicenter study in lung transplantation. J Heart Lung Transplant 32, 701–706 10.1016/j.healun.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Hargett D., Shenk T. E. (2010). Experimental human cytomegalovirus latency in CD14+ monocytes. Proc Natl Acad Sci U S A 107, 20039–20044 10.1073/pnas.1014509107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. (1991). Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253, 905–909 10.1126/science.1715094 [DOI] [PubMed] [Google Scholar]
- Hester J., Schiopu A., Nadig S. N., Wood K. J. (2012). Low-dose rapamycin treatment increases the ability of human regulatory T cells to inhibit transplant arteriosclerosis in vivo. Am J Transplant 12, 2008–2016 10.1111/j.1600-6143.2012.04065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. M., Kew V. G., Jestice K., Wills M. R., Reeves M. B. (2012). Efficient human cytomegalovirus reactivation is maturation dependent in the Langerhans dendritic cell lineage and can be studied using a CD14+ experimental latency model. J Virol 86, 8507–8515 10.1128/JVI.00598-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humar A., St Louis P., Mazzulli T., McGeer A., Lipton J., Messner H., MacDonald K. S. (1999). Elevated serum cytokines are associated with cytomegalovirus infection and disease in bone marrow transplant recipients. J Infect Dis 179, 484–488 10.1086/314602 [DOI] [PubMed] [Google Scholar]
- Jackson S. E., Mason G. M., Wills M. R. (2011). Human cytomegalovirus immunity and immune evasion. Virus Res 157, 151–160 10.1016/j.virusres.2010.10.031 [DOI] [PubMed] [Google Scholar]
- Jost S., Altfeld M. (2013). Control of human viral infections by natural killer cells. Annu Rev Immunol 31, 163–194 10.1146/annurev-immunol-032712-100001 [DOI] [PubMed] [Google Scholar]
- Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002). mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 10.1016/S0092-8674(02)00808-5 [DOI] [PubMed] [Google Scholar]
- Kudchodkar S. B., Yu Y., Maguire T. G., Alwine J. C. (2004). Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol 78, 11030–11039 10.1128/JVI.78.20.11030-11039.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudchodkar S. B., Yu Y., Maguire T. G., Alwine J. C. (2006). Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc Natl Acad Sci U S A 103, 14182–14187 10.1073/pnas.0605825103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudchodkar S. B., Del Prete G. Q., Maguire T. G., Alwine J. C. (2007). AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J Virol 81, 3649–3651 10.1128/JVI.02079-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D. W., Ye L., Katajisto P., Goncalves M. D., Saitoh M., Stevens D. M., Davis J. G., Salmon A. B., Richardson A. & other authors (2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D. W., Ye L., Sabatini D. M., Baur J. A. (2013). Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 123, 980–989 10.1172/JCI64099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre C., Pascual M. (2008). Improving outcomes for solid-organ transplant recipients at risk from cytomegalovirus infection: late-onset disease and indirect consequences. Clin Infect Dis 46, 732–740 10.1086/527397 [DOI] [PubMed] [Google Scholar]
- Li J., Spletter M. L., Johnson D. A., Wright L. S., Svendsen C. N., Johnson J. A. (2005). Rotenone-induced caspase 9/3-independent and -dependent cell death in undifferentiated and differentiated human neural stem cells. J Neurochem 92, 462–476 10.1111/j.1471-4159.2004.02872.x [DOI] [PubMed] [Google Scholar]
- Limaye A. P., Kirby K. A., Rubenfeld G. D., Leisenring W. M., Bulger E. M., Neff M. J., Gibran N. S., Huang M. L., Santo Hayes T. K. & other authors (2008). Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300, 413–422 10.1001/jama.2008.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. S., Zahrieh D., Weller E., Alyea E. P., Antin J. H., Soiffer R. J. (2002). Risk factors for cytomegalovirus reactivation after CD6+ T-cell-depleted allogeneic bone marrow transplantation. Transplantation 74, 49–54 10.1097/00007890-200207150-00009 [DOI] [PubMed] [Google Scholar]
- Ljungman P., Brand R., Einsele H., Frassoni F., Niederwieser D., Cordonnier C. (2003). Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood 102, 4255–4260 10.1182/blood-2002-10-3263 [DOI] [PubMed] [Google Scholar]
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. (2002). Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10, 457–468 10.1016/S1097-2765(02)00636-6 [DOI] [PubMed] [Google Scholar]
- Luo Z., Saha A. K., Xiang X., Ruderman N. B. (2005). AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26, 69–76 10.1016/j.tips.2004.12.011 [DOI] [PubMed] [Google Scholar]
- Marty F. M., Bryar J., Browne S. K., Schwarzberg T., Ho V. T., Bassett I. V., Koreth J., Alyea E. P., Soiffer R. J. & other authors (2007). Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood 110, 490–500 10.1182/blood-2007-01-069294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry B. P., Tomasec P., Neale M. L., Wilkinson G. W. (2003). The most abundantly transcribed human cytomegalovirus gene (beta 2.7) is non-essential for growth in vitro. J Gen Virol 84, 2511–2516 10.1099/vir.0.19298-0 [DOI] [PubMed] [Google Scholar]
- Moorman N. J., Shenk T. (2010). Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol 84, 5260–5269 10.1128/JVI.02733-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. G., Corey L., Gooley T., Davis C., Boeckh M. (2002). High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 185, 273–282 10.1086/338624 [DOI] [PubMed] [Google Scholar]
- Poglitsch M., Weichhart T., Hecking M., Werzowa J., Katholnig K., Antlanger M., Krmpotic A., Jonjic S., Hörl W. H. & other authors (2012). CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am J Transplant 12, 1458–1468 10.1111/j.1600-6143.2012.04002.x [DOI] [PubMed] [Google Scholar]
- Prösch S., Wuttke R., Krüger D. H., Volk H. D. (2002). NF-kappaB–a potential therapeutic target for inhibition of human cytomegalovirus (re)activation? Biol Chem 383, 1601–1609 10.1515/BC.2002.181 [DOI] [PubMed] [Google Scholar]
- Rameshwar P., Gascón P. (1992). Release of interleukin-1 and interleukin-6 from human monocytes by antithymocyte globulin: requirement for de novo synthesis. Blood 80, 2531–2538 [PubMed] [Google Scholar]
- Reeves M. B., Compton T. (2011). Inhibition of inflammatory interleukin-6 activity via extracellular signal-regulated kinase-mitogen-activated protein kinase signaling antagonizes human cytomegalovirus reactivation from dendritic cells. J Virol 85, 12750–12758 10.1128/JVI.05878-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. B., Davies A. A., McSharry B. P., Wilkinson G. W., Sinclair J. H. (2007). Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 316, 1345–1348 10.1126/science.1142984 [DOI] [PubMed] [Google Scholar]
- Revello M. G., Gerna G. (2004). Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J Clin Virol 29, 71–83 10.1016/j.jcv.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Rölle A., Olweus J. (2009). Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures. APMIS 117, 413–426 10.1111/j.1600-0463.2009.02449.x [DOI] [PubMed] [Google Scholar]
- Roux E., Dumont-Girard F., Starobinski M., Siegrist C. A., Helg C., Chapuis B., Roosnek E. (2000). Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood 96, 2299–2303 [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. (1994). RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78, 35–43 10.1016/0092-8674(94)90570-3 [DOI] [PubMed] [Google Scholar]
- San Segundo D., Fernández-Fresnedo G., Gago M., Beares I., Ruiz-Criado J., González M., Ruiz J. C., Gómez-Alamillo C., Arias M., López-Hoyos M. (2010). Number of peripheral blood regulatory T cells and lymphocyte activation at 3 months after conversion to mTOR inhibitor therapy. Transplant Proc 42, 2871–2873 10.1016/j.transproceed.2010.07.045 [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Sabatini D. M. (2005). Growing roles for the mTOR pathway. Curr Opin Cell Biol 17, 596–603 10.1016/j.ceb.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006). Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22, 159–168 10.1016/j.molcel.2006.03.029 [DOI] [PubMed] [Google Scholar]
- Smith C., Khanna R. (2013). Immune regulation of human herpesviruses and its implications for human transplantation. Am J Transplant 13 (Suppl 3), 9–23, quiz 23 10.1111/ajt.12005 [DOI] [PubMed] [Google Scholar]
- Söderberg-Nauclér C., Fish K. N., Nelson J. A. (1997). Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91, 119–126 10.1016/S0092-8674(01)80014-3 [DOI] [PubMed] [Google Scholar]
- Turnquist H. R., Cardinal J., Macedo C., Rosborough B. R., Sumpter T. L., Geller D. A., Metes D., Thomson A. W. (2010). mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood 115, 4758–4769 10.1182/blood-2009-10-251488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vézina C., Kudelski A., Sehgal S. N. (1975). Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 28, 721–726 10.7164/antibiotics.28.721 [DOI] [PubMed] [Google Scholar]
- Vollenbröker B., George B., Wolfgart M., Saleem M. A., Pavenstädt H., Weide T. (2009). mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. Am J Physiol Renal Physiol 296, F418–F426 10.1152/ajprenal.90319.2008 [DOI] [PubMed] [Google Scholar]
- Watkins R. R., Lemonovich T. L., Razonable R. R. (2012). Immune response to CMV in solid organ transplant recipients: current concepts and future directions. Expert Rev Clin Immunol 8, 383–393 10.1586/eci.12.25 [DOI] [PubMed] [Google Scholar]
- Yuan J., Liu X., Wu A. W., McGonagill P. W., Keller M. J., Galle C. S., Meier J. L. (2009). Breaking human cytomegalovirus major immediate-early gene silence by vasoactive intestinal peptide stimulation of the protein kinase A-CREB-TORC2 signaling cascade in human pluripotent embryonal NTera2 cells. J Virol 83, 6391–6403 10.1128/JVI.00061-09 [DOI] [PMC free article] [PubMed] [Google Scholar]