Abstract
Fungi are an emerging source of peptide antibiotics. With the availability of a large number of model fungal genome sequences, we can expect that more and more fungal defensin-like peptides (fDLPs) will be discovered by sequence similarity search. Here, we report a total of 69 new fDLPs encoded by 63 genes, in which a group of fDLPs derived from dermatophytes are defined as a new family (fDEF8) according to sequence and phylogenetic analyses. In the oleaginous fungus Mortierella alpine, fDLPs have undergone extensive gene expansion. Our work further enlarges the fungal defensin family and will help characterize new peptide antibiotics with therapeutic potential.
Keywords: peptide antibiotic, gene duplication, exon-intron structure, cysteine-stabilized α-helical and β-sheet motif
1. Introduction
Fungal defensin-like peptides (fDLPs) are emerging as attractive anti-infective agents due to their therapeutic efficacy, low toxicity and high serum stability [1,2]. On the basis of a combined analyses of sequence, structural, and phylogenetic data, we has identified seven fDLP families [2,3], in which three members (plectasin, micasin and eurocin), classified as ancient invertebrate-type defensins (AITDs) [1,2,4,5], have been structurally and functionally characterized. These fDLPs exhibit activity against several antibiotic-resistant clinical isolates with significant therapeutic potential [1,2,5,6]. Some efforts have been taken to improve antimicrobial efficacy and to reduce undesirable side effects of fDLPs. For example, an improved mutant of plectasin (NZ2114) is superior to two conventional antibiotics (vancomycin and daptomycin) in inhibiting methicillin-resistant Staphylococcus aureus (MRSA) with even more enhanced serum stability and extended in vivo half-life [7,8,9]. In this work, we describe 69 new fDLPs in terms of their sequences, structural characteristics, and phylogenetic relationship. This provides an array of candidates for development of new anti-infective agents against antibiotic-resistant human pathogens.
2. Discovery of New fDLPs
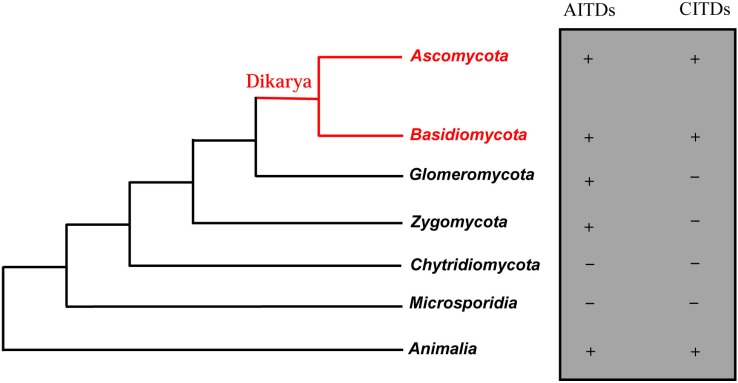
The database search strategy used here has been described previously [3]. Through an exhaustive search of 26 fungal species, we retrieved a total of 69 new fDLPs. As previously stated, overall this class of molecules exhibits a taxa-specific distribution pattern in the fungus kingdom, of which 21 fDLPs are derived from Ascomycota, 39 from Zygomycota, eight from Basidiomycota and one from Glomeromycota. In the basal fungi (Microsporidia and Chytridiomycota), no typical fDLP has been identified (Figure 1). The general features of these peptides are listed in Table 1 and Table 2. They can be grouped into six families based on sequence similarity, five of which are classified into the previously known families (fDEF1, fDEF2, fDEF3, fDEF4, and fDEF6) [3] (Figure 2 and Figure 3). This grouping is consistent with the phylogenetic analysis supported by high bootstrap values (Figure 4).
Figure 1.
Phylogenetic distribution of fDLPs. The left: A parsimony tree of fungal species, animalia is used as an outgroup. This tree is a modification of the SSU and LSU r-RNA analyses of Lutzoni et al. for the fungal kingdom [10]. The right: “+” means presence and “−” means absence.
Table 1.
Sources and characteristics of newly discovered non-Mortierella fDLPs.
| Name|Accession No. | Class | Species (phylum: subphylum: class) | Size | MW | NC |
|---|---|---|---|---|---|
| Pyronesin1|CATG01000243 (G) | fDEF1 | Pyronema omphalodes (Ascomycota: Pezizomycotina: Pezizomycetes) | 40 | 4317 | +1.2 |
| Pyronesin2|CATG01000243 (G) | P. omphalodes | 40 | 4402 | +0.2 | |
| Pyronesin3|CATG01000243 (G) | P. omphalodes | 40 | 4389 | +1.2 | |
| Pyronesin4|CATG01000243 (G) | P. omphalodes | 40 | 4416 | +2.2 | |
| Pyronesin5|CATG01000243 (G) | P. omphalodes | 40 | 4375 | +1.2 | |
| Pyronesin6|CATG01000243 (G) | P. omphalodes | 40 | 4291 | +2.4 | |
| Abisin1|AEOK01000166 (G) | Agaricus bisporus (Basidiomycota: Agaricomycotina: Agaricomycetes) | 40 | 4097 | ‒3.8 | |
| Abisin2|AEOK01000166 (G) | A. bisporus | 40 | 4097 | ‒3.8 | |
| Abisin3|AEOK01000166 | A. bisporus | 39 | 3926 | ‒2.8 | |
| Beauvesin1|ADAH01000714 (G) | Beauveria bassiana (Ascomycota: Pezizomycotina: Sordariomycetes) | 52 | 5475 | +2.9 | |
| Pyrelysin|GAJI01023341 (T) | Pyrenochaeta lycopersici (Ascomycota: Pezizomycotina: Dothideomycetes) | 55 | 5858 | +5.4 | |
| Risin|JAQX01005622 | Rhizophagus irregularis (Glomeromycota: Glomeromycetes) | 55 | 5972 | +6.1 | |
| Trimensin|FG132536 (E) | Trichophyton mentagrophytes (Ascomycota: Pezizomycotina: Eurotiomycetes) | 38 | 4156 | +2.2 | |
| Lecasin|AWYC01000479 | fDEF2 | Lecanosticta acicola (Ascomycota: Pezizomycotina: Dothideomycetes) | 42 | 4314 | ‒4.8 |
| Pochlasin1|AOSW01002431 | Pochonia chlamydosporia (Ascomycota: Pezizomycotina: Sordariomycetes) | 43 | 4339 | ‒3.5 | |
| Perisin|AFRD01000258 | Periglandula ipomoeae (Ascomycota: Pezizomycotina: Sordariomycetes) | 43 | 4080 | ‒1.5 | |
| Masysin|CANK01000016 | Malassezia sympodialis (Basidiomycota: Ustilaginomycotina: Exobasidiomycetes) | 35 | 3432 | +2.2 | |
| Maglosin1N|AAYY01000039 (G) | Malassezia globosa (Basidiomycota: Ustilaginomycotina: Exobasidiomycetes) | 40 | 3980 | +1.2 | |
| Maglosin2N|AAYY01000024 (G) | M. globosa | 40 | 4022 | +0.2 | |
| Maglosin1C|AAYY01000039 (G) | M. globosa | 41 | 3910 | +2.7 | |
| Maglosin2C|AAYY01000024 (G) | M. globosa | 40 | 3835 | +2.7 | |
| Beauvesin2C|ADAH01000123 (G) | fDEF3 | B. bassiana | 41 | 4243 | +0.9 |
| ManisinC|ADNJ01000735 | Metarhizium anisopliae (Ascomycota: Pezizomycotina: Sordariomycetes) | 41 | 4211 | ‒0.1 | |
| Pochlasin2C|AOSW01005877 | P. chlamydosporia | 41 | 4381 | +0.2 | |
| AsosinC|BACA01000303 | Aspergillus sojae (Ascomycota: Pezizomycotina: Eurotiomycetes) | 38 | 4002 | ‒1.0 | |
| Beauvesin2N|ADAH01000123 (G) | fDEF4 | B. bassiana | 48 | 5067 | +2.9 |
| ManisinN|ADNJ01000735 | M. anisopliae | 46 | 4921 | +0.2 | |
| Pochlasin2N|AOSW01005877 | P. chlamydosporia | 49 | 5185 | +2.9 | |
| AsosinN|BACA01000303 | A. sojae | 49 | 5140 | ‒1.1 | |
| Rhimisin1|ANKS01000620 | fDEF6 | Rhizopus microsporus (Zygomycota: Mucoromycotina: Mucorales) | 45 | 4867 | +10.0 |
| Rhimisin2|ANKS01000620 | R. microsporus | 44 | 4638 | +3.4 | |
| Rhimisin3|ANKS01001486 | R. microsporus | 44 | 4768 | +1.5 | |
| Rhimisin4|ANKS01001486 | R. microsporus | 45 | 4811 | +8.0 | |
| Rhidesin1|AACW02000043 | Rhizopus delemar (Zygomycota: Mucoromycotina: Mucorales) | 55 | 5885 | +10.4 | |
| Rhidesin2|AACW02000259 | R. delemar | 48 | 5270 | +0.5 | |
| Mirresin|AZYI01000143 | Mucor irregularis (Zygomycota: Mucoromycotina: Mucorales) | 60 | 6424 | +13.4 | |
| Mucisin|AOCY01001156 (G) | Mucor circinelloides (Zygomycota: Mucoromycotina: Mucorales) | 53 | 5548 | +2.2 | |
| Phycomysin|EX863311 (E) | Phycomyces blakesleeanus (Zygomycota: Mucoromycotina: Mucorales) | 50 | 5342 | +9.4 | |
| TritoDLP|ACPI01000196 (G) | fDEF8 | Trichophyton tonsurans (Ascomycota: Pezizomycotina: Eurotiomycetes) | 41 | 4323 | +3.7 |
| TrequiDLP|ABWI01000729 (G) | Trichophyton equinum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 41 | 4323 | +3.7 | |
| TriveDLP|ACYE01000402 | Trichophyton verrucosum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 42 | 4403 | +4.7 | |
| ArgyDLP|ABQE01000293 | Arthroderma gypseum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 41 | 4247 | +3.7 | |
| ArbeDLP|ABSU01000004 | Arthroderma benhamiae (Ascomycota: Pezizomycotina: Eurotiomycetes) | 42 | 4493 | +4.7 | |
| TriruDLP|ACPH01000567 (G) | Trichophyton rubrum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 42 | 4479 | +4.7 | |
| MicaDLP|ABVF01000093 | Arthroderma otae (Ascomycota: Pezizomycotina: Eurotiomycetes) | 43 | 4745 | +3.2 |
Note: MW: molecular weight; NC (net charge) is estimated at pH 7.0 with protein calculation V3.4. “E” means peptides from the Expressed Sequence Tags (EST) database and “T” means peptides from the Transcriptome Shotgun Assembly (TSA) database. “G” means proteins currently annotated in the GenBank database as hypothetical proteins (http://www.ncbi.nlm.nih.gov/) [12].
Table 2.
Sources and characteristics of the malpisin family.
| Name|Accession No. | Organism | Scaffold (Contig) | Range | Size | MW | NC |
|---|---|---|---|---|---|---|
| Malpisin1-1|AZCI01001104 | Mortierella alpina B6842 | jtg7180000084593f_7180000084594f | 55070–55405 | 41 | 4048 | ‒0.0 |
| Malpisin1-2|AZCI01001104 | 55870–56127 | 48 | 5166 | ‒3.3 | ||
| Malpisin1-3|AZCI01001104 | 56393–56635 | 45 | 5047 | +0.7 | ||
| Malpisin1-4|AZCI01001104 | 63869–64117 | 39 | 4117 | ‒1.0 | ||
| Malpisin1-5|AZCI01000882 | Contig 7180000084767 | 22045–22248 | 37 | 4259 | ‒2.8 | |
| Malpisin1-6|AZCI01000882 | 25851–26051 | 39 | 4543 | ‒3.5 | ||
| Malpisin1-7|AZCI01000882 | 42456–42677 | 33 | 3624 | ‒1.0 | ||
| Malpisin1-8|AZCI01000882 | 43573–43800 | 47 | 5078 | +1.7 | ||
| Malpisin1-9|AZCI01000882 | 45037–45261 | 48 | 5203 | +3.0 | ||
| Malpisin1-10|AZCI01000882 | 45559–45738 | 35 | 3914 | ‒0.0 | ||
| Malpisin1-11|AZCI01000882 | 46707–46913 | 43 | 4941 | +5.4 | ||
| Malpisin1-12|AZCI01001135 | jtg7180000084204f_7180000084205f_7180000084206f | 135437–135676 | 44 | 4722 | ‒0.0 | |
| Malpisin1-13|AZCI01001084 | jtg7180000084699f_7180000084700f | 362415–362627 | 47 | 4919 | ‒1.8 | |
| Malpisin1-14|AZCI01001006 | jtg7180000084769f_7180000084770f_7180000084771f_7180000084772f | 179488–179673 | 38 | 4188 | +0.2 | |
| Malpisin2-1|ADAG01001070 | Mortierella alpina ATCC 32222 | Contig 1070 | 9785–10114 | 39 | 4105 | +1.0 |
| Malpisin2-2|ADAG01001070 | 10532–10792 | 48 | 5187 | ‒2.3 | ||
| Malpisin2-3|ADAG01001070 | 11052–11297 | 44 | 4783 | ‒0.0 | ||
| Malpisin2-4|ADAG01001070 | 11773–12021 | 39 | 4052 | ‒1.8 | ||
| Malpisin2-5|ADAG01000791 | Contig 791 | 4894–5097 | 37 | 4259 | ‒2.8 | |
| Malpisin2-7|ADAG01000903 | Contig 903 | 13145–13357 | 33 | 3899 | +1.2 | |
| Malpisin2-8|ADAG01000903 | 14223–14450 | 47 | 5065 | +1.7 | ||
| Malpisin2-9|ADAG01000903 | 15634–15852 | 45 | 4917 | +4.0 | ||
| Malpisin2-10|ADAG01000903 | 16158–16337 | 35 | 3937 | +0.2 | ||
| Malpisin2-11|ADAG01000903 | 17264–17446 | 39 | 4531 | +5.0 |
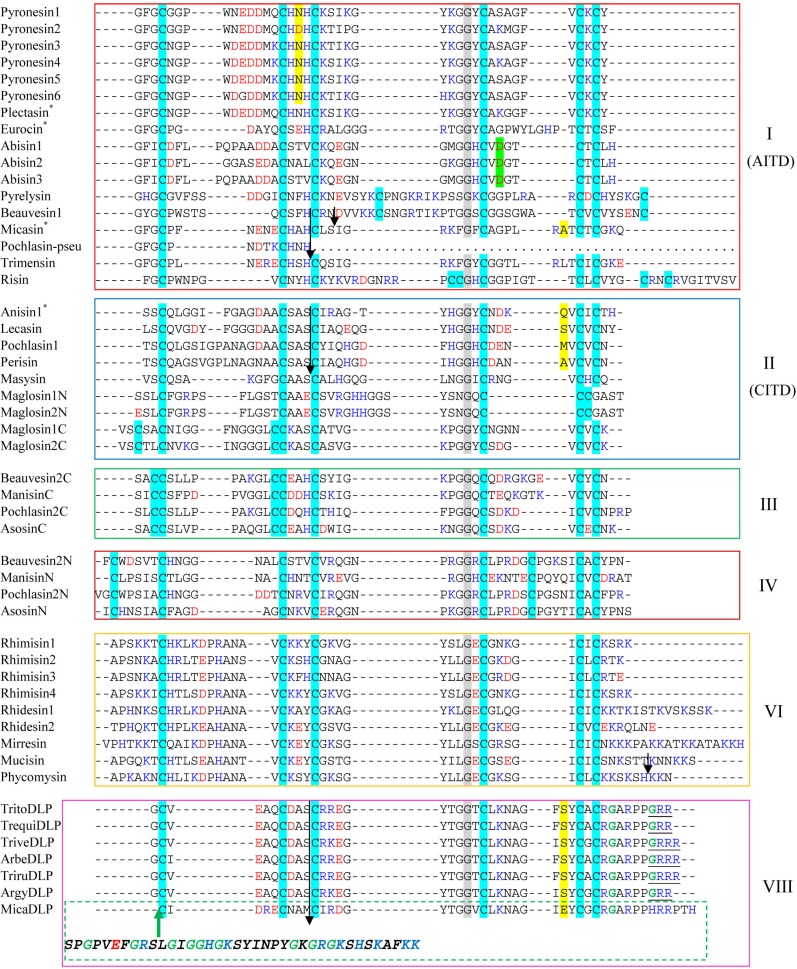
Figure 2.
Multiple sequence alignment of fDLPs. Cysteines are shadowed in cyan. Conserved glycines are highlighted in grey. Negatively (D and E) and positively (R, K and H) charged residues are boldfaced in red and blue, respectively. Introns are shown by arrows (phase 0) or small boxes (green: phase 1, yellow: phase 2). Functionally characterized fDLPs were indicated by “*”. The N-terminal extension sequence in micaDLP belonging to the family fDEF8 is italicized. Defensins from Pyronema omphalodes have been predicted and investigated by RNA-seq [13]. Extra residues for C-terminal amidation are underlined once.
Figure 3.
Multiple sequence alignment of malpisins. Color codes and symbol notes used here are the same as those in Figure 2. Pink box indicates the N-terminus of DLPs with variable length. Sequence identity (%) to micasin is shown on the right.
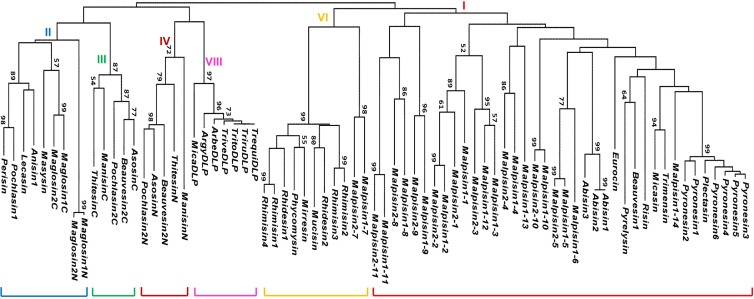
Figure 4.
Phylogenetic tree of fDLPs. The tree was constructed from the aligned amino acid sequences presented in Figure 2 and Figure 3 with the neighbor-joining method. The numbers on nodes represent bootstrap values, and only values ≥50% are shown.
All the fDLPs characterized here have a signal peptide located in the N-terminus. In comparison with fDEF1 and fDEF2 that possess a propeptide located between signal and mature peptides, fDEF6 and fDEF8 lack a propeptide. Five precursors (maglosin, beauvesin2, manisin, pochlasin2 and asosin) could release two defensins from a single precursor after the removal of a spacer propeptide (Figure 5). The malpisin family from Mortierella alpine exhibits two types of precursor organization: (1) the first type contains 10 members, all having a propeptides identified by its acidic feature and single or two basic amino acids at their ends as putative cleavage site of proprotein convertase [11]; (2) the second type contains 14 members that lack a propeptide and thus no further processing step is needed (Figure S1).
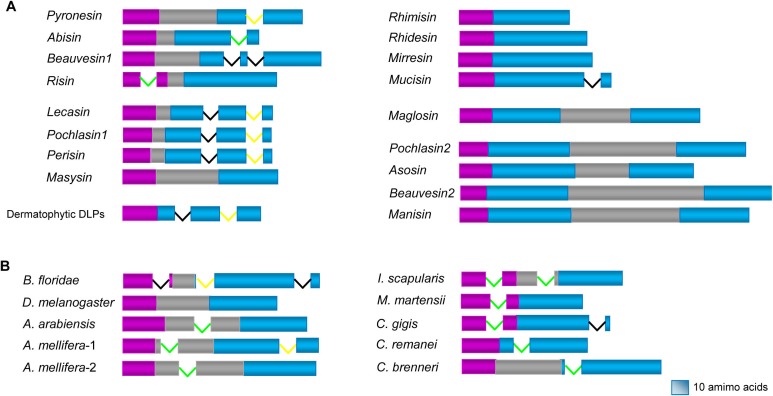
Figure 5.
Comparison of precursor organization and exon-intron structures between fDLPs and animal defensins. (A) fDLPs; (B) Animal defensins. Signal, pro- and mature peptides are shown in pink, grey and blue, respectively. Intron phases are shown in the same colors as Figure 2. Representative animal defensins are derived from Branchiostoma floridae, Drosophila melanogaster, Anopheles arabiensis, Apis mellifera, Ixodes scapularis, M. martensii, Crassostrea gigas, Caenorhabditis remanei, and C. brenneri.
Peptides in fDEF8, all derived from dermatophytes, are characterized as a new family with a short N-terminus and an extra C-terminal extension rich in arginines, prolines and glycines (Figure 2). The C-terminal extension has been considered as a common mechanism for the complexity increase of some invertebrate antimicrobial peptides (AMPs). For example, the hymenopteran defensin-1 subfamily has an extended C-terminus relative to its ancestral defensin-2 subfamily by a so-called intron exonization-mediated mechanism [14,15]. It thus appears that fungal and invertebrate defensins both convergently evolved their C-termini. The extension of a C-terminal sequence via convergent evolution was also recently observed in interleukin 6 (IL-6), a class-I helical cytokine, of two leporids (Oryctolagus and Pentalagus) [16]. The presence of C-terminal Gly-Arg or Gly-Arg-Arg in some dermatophyte-derived fDLPs suggest that they may be amidated, as previously observed in some animal toxins, e.g., the Mesobuthus α-toxins [17]. Interestingly, the mature peptide of micaDLP is larger in size than that of other members in this family, as identified by an N-terminal extension of 38 amino acids (Figure 2). High content of glycines together with a cationic characteristic hints a putative antimicrobial role of this extended unit.
M. alpine is a saprophytic species of Mucoromycotina, known as an oleaginous fungus [18]. The draft genome sequences of two M. alpina isolates (B6842 and ATCC 32222) [18,19] provide a possibility to undertake comparative study of their fDLPs. We found that the M. alpine B6842 genome encodes 14 fDLPs (Figure 3) but only 10 were found in M. alpine ATCC 32222. The failure to detect the four homologs (i.e., malpisin1-6, 1-12, 1-13, 1-14) in M. alpine ATCC 32222 could be due to the incompletely-assembled genome sequences. Our phylogenetic analysis divides all malpisins into fDEF1 and fDEF6 (Figure 4). Some malpisin members of fDEF1 extended their N-termini with diverse sequences and variable lengths (Figure 3).
3. Gene Duplication of fDLPs
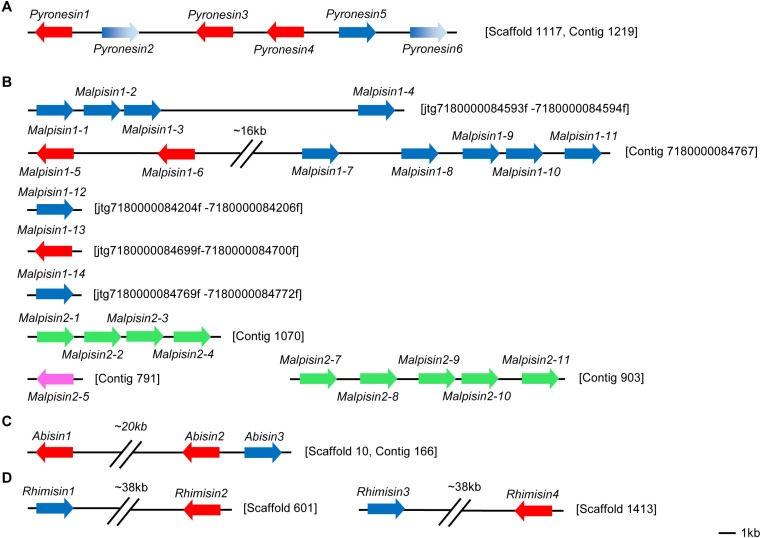
Gene duplication extensively occurs in antimicrobial peptides from insects to humans [15,20,21]. In fungi, initial annotation of defense molecules of Pyronema omphalodes also identified gene duplication as a minor multigene family of fDLPs (herein termed pyronesin1 to pyronesin6) [13]. These fDLPs are highly similar to plectasin (Figure 6A). Our studies revealed new gene duplication event in other fungal species. Malpisin is a representative example of gene duplication. As mentioned previously, there are 14 and 10 members in M. alpine B6842 and M. alpine ATCC 32222, respectively. Malpisin1-1, 1-2, 1-3 and 1-4 are tandem located on one contig (jtg7180000084593f _7180000084594f), and malpisin1-5 to malpisin1-11 on another contig (Contig 7180000084767). In addition, malpisin1-12, malpisin 1-13 and malpisin 1-14 reside on other three contigs, as shown in Figure 6B. In M. alpine ATCC 32222, malpisin2-1 to malpisin2-4 are located on contig 1070 and malpisin2-7 to malpisin2-11 on contig 903. Only malpisin2-5 is located on contig 791.
Figure 6.
The arrangement of defensin genes in chromosomes. Color arrows refer to different orientation of the genes. A to D represent the genome location of defensins in four species: Pyronema omphalodes, Mortierella alpine, Agaricus bisporus and Rhizopus microsporus. Malpisins in M. alpine B6842 is indicated in red and blue while in pink and green in M. alpine ATCC 32222. Pseudogenes of pyronesins are shown in gradient blue.
In the widely cultivated mushroom Agaricus bisporus, there are three paralogous fDLPs (abisin1 to abisin3) (Figure 6C), two of which (abisin1 and abisin3) share completely identical amino acid sequences in the mature peptide region but exhibit four synonymous substitutions at the nucleotide level. In the Pochonia chlamydosporia paralogues, pochlasin1 is highly similar to CITDs and pochlasin2 possesses two defensin-domains. In addition, a putative pseudogene (herein named pochlasin-pseu) was also identified in scaffold 1191 and assigned to AITDs in view of its high sequence similarity to micasin in the first exon. Pochlasin1 and pochlasin-pseu share a conserved phase 0 intron within the α-helical region. The loss of the last two exons (2 and 3) results in the lack the last four cysteines involved in the Csαβ folding of a mature peptide (Figure 2).
Gene duplication also occurs in the Mucorales-derived fDLPs, which leads to four and two gene copies in Rhizopus microsporus (Figure 6D) and R. delemar, respectively. In a Neighbor-Joining (NJ) tree, rhimisin1 and rhimisin4 (R. microsporus) constitutes a single clade clustering with the other three fDLPs (rhidesin1 from R. delemar, phycomycin from Phycomyces blakesleeanus and mirresin from Mucor irregularis) whereas rhimisin2 and rhimisin3 (R. microsporus) cluster with rhidesin2 (R. delemar) and mucisin (M. circinelloides) (Figure 4), suggesting that the gene duplication event could have occurred in the ancestor of the Mucorales prior to their speciation.
4. Variable Gene Structures of fDLPs
Analysis of the exon-intron structures of the newly-discovered fDLPs revealed their variability that can be described as follows: (1) all the fDLPs retain the integrity of the signal peptide except risin (Rhizophagus irregularis) and malpisin1-1 (or malpisin2-1) that have a phase 1 or phase 0 intron disrupting their signal peptides; (2) all of the genes in fDEF8 and three genes in fDEF2 (i.e., lecasin, pochlasin1 and perisin) have the same gene organization as previously identified dermatophytic defensins (micasin, arbesin, trivesin, tritosin and trirusin) and they contain two introns: the first intron (phase 0) disrupting the α-helical region; the second intron (phase 2) disrupting the c-loop; (3) the pyronesin and abisin multi-gene family in fDEF1 have only one intron disrupting either the α-helical or the c-loop region; (4) In addition to these intron-containing fDLP genes, there are some members without introns (Figure 2 and Figure 5).
The highly variable gene structures in fDLPs are reminiscent of invertebrate defensins that also exhibit diverse gene structures [22,23] (Figure 5 and Figure S2). Compared with invertebrate defensins of 5'-biased intron positions, introns of fDLPs occur preferentially in the 3'-end of the precursor-coded sequences. Because all eukaryotic Csαβ-type defensins are hypothesized to be originated from a common bacterial ancestor [24], it is reasonable to infer that considerable intron gains might have occurred in defensins from some eukaryotic lineages, and later they differentially lost in some specific species. Such a dynamic intron evolution thus shapes the biased intron location pattern between fDLPs and animal DLPs after the animal-fungi split. It is also worth mentioning that some recognizable orthologues of defensins in Branchiostoma floridae [25,26], the basal chordate amphioxus, also contain a phase 0 intron located in their c-loop (Figure 5 and Figure S2). Given a remote evolutionary distance between fungi and amphioxus, their intron position conservation could be a consequence of convergent insertion in a similar position due to the existence of “protosplice sites” [27,28]. However, the evolution via ancestral origin can be not completely ruled out in the case of the lack of gene structure information in many animal defensins from different lineages.
5. Conclusions
It is estimated that there are as many as 1.5 million species of fungi in this world. However, only a small fraction has been described and even fewer have been sequenced. To date, only about six hundred genomes were being sequenced or completely sequenced. Fungal genome project (FGP) allows us to systematically exploit peptide antibiotics instead of accidental discovery or complicated biochemical screening. This work sheds light on the persistent discovery of fDLPs from model fungal genome data. Despite this, in the lack of experimental data, it cannot be stated that all these fDLPs possess antibacterial function because in fact a classical insect-type fungal defensing - pechrysin was found to lack antibacterial activity [29] likely due to the absence of cationic residues on its molecular surface. In addition, anisin1, a DLP from Aspergillus giganteus, was found to be involved in the fitness of the species by linking stress signaling with developmental regulation [30]. Recent studies have also shown that although some peptides of fungal origin contain a similar defensin structure, they exhibit diverse or alternative biological functions beyond antimicrobial activity. An interesting overview is given by Hegedüs and Marx [31]. Therefore, further biochemical characterization of these newly-discovered fDLPs will help evaluate their potential as human medicines.
Acknowledgements
This work was supported by the National Basic Research Program of China (2010CB945300), the National Natural Science Foundation of China (31221091), and the State Key Laboratory of Integrated Management of Pest Insects and Rodents (Grant No. ChineseIPM1307).
Supplementary Files
Author Contributions
J.W. and S.Z. discovered all fDLP genes described here. J.W. and S.Z. wrote the paper. B.G. revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mygind P.H., Fischer R.L., Schnorr K.M., Hansen M.T., Sonksen C.P., Ludvigsen S., Raventos D., Buskov S., Christensen B., De Maria L., et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S., Gao B., Harvey P.J., Craik D.J. Dermatophytic defensin with antiinfective potential. Proc. Natl. Acad. Sci. USA. 2012;109:8495–8500. doi: 10.1073/pnas.1201263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu S. Discovery of six families of fungal defensin-like peptides provides insights into origin and evolution of the CSαβ defensins. Mol. Immunol. 2008;45:828–838. doi: 10.1016/j.molimm.2007.06.354. [DOI] [PubMed] [Google Scholar]
- 4.Schneider T., Kruse T., Wimmer R., Wiedemann I., Sass V., Pag U., Jansen A., Nielsen A.K., Mygind P.H., Raventos D.S., et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328:1168–1172. doi: 10.1126/science.1185723. [DOI] [PubMed] [Google Scholar]
- 5.Oeemig J.S., Lynggaard C., Knudsen D.H., Hansen F.T., Norgaard K.D., Schneider T., Vad B.S., Sandvang D.H., Nielsen L.A., Neve S., et al. Eurocin, a new fungal defensin: Structure, lipid binding, and its mode of action. J. Biol. Chem. 2012;287:42361–42372. doi: 10.1074/jbc.M112.382028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara S., Mukae H., Sakamoto N., Ishimoto H., Amenomori M., Fujita H., Ishimatsu Y., Yanagihara K., Kohno S. Plectasin has antibacterial activity and no affect on cell viability or IL-8 production. Biochem. Biophys. Res. Commun. 2008;374:709–713. doi: 10.1016/j.bbrc.2008.07.093. [DOI] [PubMed] [Google Scholar]
- 7.Xiong Y.Q., Hady W.A., Deslandes A., Rey A., Fraisse L., Kristensen H.H., Yeaman M.R., Bayer A.S. Efficacy of NZ2114, a novel plectasin-derived cationic antimicrobial peptide antibiotic, in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011;55:5325–5330. doi: 10.1128/AAC.00453-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostergaard C., Sandvang D., Frimodt-Moller N., Kristensen H.H. High cerebrospinal fluid (CSF) penetration and potent bactericidal activity in CSF of NZ2114, a novel plectasin variant, during experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 2009;53:1581–1585. doi: 10.1128/AAC.01202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andes D., Craig W., Nielsen L.A., Kristensen H.H. In vivo pharmacodynamic characterization of a novel plectasin antibiotic, NZ2114, in a murine infection model. Antimicrob. Agents Chemother. 2009;53:3003–3009. doi: 10.1128/AAC.01584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutzoni F., Kauff F., Cox C.J., McLaughlin D., Celio G., Dentinger B., Padamsee M., Hibbett D., James T.Y., Baloch E., et al. Assembling the fungal tree of life: Progress, classification, and evolution of subcellular traits. Am. J. Bot. 2004;91:1446–1480. doi: 10.3732/ajb.91.10.1446. [DOI] [PubMed] [Google Scholar]
- 11.Seidah N.G., Chrétien M. Proprotein and prohormone convertases: A family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 12.Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Wheeler D.L. GenBank. Nucleic Acids Res. 2008;36:D25–30. doi: 10.1093/nar/gkn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traeger S., Altegoer F., Freitag M., Gabaldon T., Kempken F., Kumar A., Marcet-Houben M., Poggeler S., Stajich J.E., Nowrousian M. The genome and development-dependent transcriptomes of Pyronema confluens: A window into fungal evolution. PLoS Genet. 2013;9:e1003820. doi: 10.1371/journal.pgen.1003820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian C., Gao B., Fang Q., Ye G., Zhu S. Antimicrobial peptide-like genes in Nasonia vitripennis: A genomic perspective. BMC Genomics. 2010;11:187. doi: 10.1186/1471-2164-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z., Zhu S. Comparative genomics analysis of five families of antimicrobial peptide-like genes in seven ant species. Dev. Comp. Immunol. 2012;38:262–274. doi: 10.1016/j.dci.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Neves F., Abrantes J., Pinheiro A., Almeida T., Costa P.P., Esteves P.J. Convergent evolution of IL-6 in two leporids (Oryctolagus and Pentalagus) originated an extended protein. Immunogenetics. 2014 doi: 10.1007/s00251-014-0787-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhu S., Peigneur S., Gao B., Lu X., Cao C., Tytgat J. Evolutionary diversification of Mesobuthus α-scorpion toxins affecting sodium channels. Mol. Cell. Proteomics. 2012 doi: 10.1074/mcp.M111.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Chen W., Feng Y., Ren Y., Gu Z., Chen H., Wang H., Thomas M.J., Zhang B., Berquin I.M., et al. Genome characterization of the oleaginous fungus Mortierella alpina. PLoS ONE. 2011;6:e28319. doi: 10.1371/journal.pone.0028319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etienne K.A., Chibucos M.C., Su Q., Orvis J., Daugherty S., Ott S., Sengamalay N.A., Fraser C.M., Lockhart S.R., Bruno V.M. Draft genome sequence of Mortierella alpina isolate CDC-B6842. Genome Announc. 2014;2:e01180-13. doi: 10.1128/genomeA.01180-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schutte B.C., Mitros J.P., Bartlett J.A., Walters J.D., Jia H.P., Welsh M.J., Casavant T.L., McCray P.B., Jr. Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. USA. 2002;99:2129–2133. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semple C.A., Rolfe M., Dorin J.R. Duplication and selection in the evolution of primate β-defensin genes. Genome Biol. 2003;4:R31. doi: 10.1186/gb-2003-4-5-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Froy O., Gurevitz M. Arthropod and mollusk defensins - evolution by exon-shuffling. Trends Genet. 2003;19:684–687. doi: 10.1016/j.tig.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez de la Vega R.C., Possani L.D. On the evolution of invertebrate defensins. Trends Genet. 2005;21:330–332. doi: 10.1016/j.tig.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Gao B., Rodriguez Mdel C., Lanz-Mendoza H., Zhu S. AdDLP, a bacterial defensin-like peptide, exhibits anti-Plasmodium activity. Biochem. Biophys. Res. Commun. 2009;387:393–398. doi: 10.1016/j.bbrc.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Yu J.K., Wang M.C., Shin-I T., Kohara Y., Holland L., Satoh N., Satou Y. A cDNA resource for the cephalochordate amphioxus Branchiostoma floridae. Dev. Genes Evol. 2008;218:723–727. doi: 10.1007/s00427-008-0228-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S., Peigneur S., Gao B., Umetsu Y., Ohki S., Tytgat J. Experimental conversion of a defensin into a neurotoxin: implications for origin of toxic function. Mol. Biol. Evol. 2014;31:546–559. doi: 10.1093/molbev/msu038. [DOI] [PubMed] [Google Scholar]
- 27.Sverdlov A.V., Rogozin I.B., Babenko V.N., Koonin E.V. Reconstruction of ancestral protosplice sites. Curr. Biol. 2004;14:1505–1508. doi: 10.1016/j.cub.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Rogozin I.B., Sverdlov A.V., Babenko V.N., Koonin E.V. Analysis of evolution of exon-intron structure of eukaryotic genes. Brief. Bioinformatics. 2005;6:118–134. doi: 10.1093/bib/6.2.118. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Gao B., Zhu S. Fungal defensins, an emerging source of anti-infective drugs. Chin. Sci. Bull. 2014;59:931–935. doi: 10.1007/s11434-014-0165-1. [DOI] [Google Scholar]
- 30.Eigentler A., Pocsi I., Marx F. The anisin1 gene encodes a defensin-like protein and supports the fitness of Aspergillus nidulans. Arch. Microbiol. 2012;194:427–437. doi: 10.1007/s00203-011-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegedus N., Marx F. Antifungal proteins: more than antimicrobials? Fungal Biol. Rev. 2013;26:132–145. doi: 10.1016/j.fbr.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.