Abstract
Fecal incontinence (FI) is a multifactorial disorder that imposes considerable social and economic burdens. The aim of this article is to provide an overview of current and emerging treatment options for FI. A MEDLINE search was conducted for English-language articles related to FI prevalence, etiology, diagnosis, and treatment published from January 1, 1990 through June 1, 2013. The search was extended to unpublished trials on ClinicalTrials.gov and relevant publications cited in included articles. Conservative approaches, including dietary modifications, medications, muscle-strengthening exercises, and biofeedback, have been shown to provide short-term benefits. Transcutaneous electrical stimulation was considered ineffective in a randomized clinical trial. Unlike initial studies, sacral nerve stimulation has shown reasonable short-term effectiveness and some complications. Dynamic graciloplasty and artificial sphincter and bowel devices lack randomized controlled trials and have shown inconsistent results and high rates of explantation. Of injectable bulking agents, dextranomer microspheres in non-animal stabilized hyaluronic acid (NASHA Dx) has shown significant improvement in incontinence scores and frequency of incontinence episodes, with generally mild adverse effects. For the treatment of FI, conservative measures and biofeedback therapy are modestly effective. When conservative therapies are ineffective, invasive procedures, including sacral nerve stimulation, may be considered, but they are associated with complications and lack randomized, controlled trials. Bulking agents may be an appropriate alternative therapy to consider before more aggressive therapies in patients who fail conservative therapies.
Key Words: anal incontinence, diarrhea, SNS, motility, NASHA Dx
Fecal incontinence (FI), defined as the involuntary loss of rectal contents (eg, liquid or solid stool or gas),1 is caused by disruptions in the interplay of components that help maintain fecal control. Loss of voluntary control of defecation imposes a considerable social and economic burden: patients report a severely impaired quality of life, and economic costs have been estimated at an average of $4110 per patient annually (2010 US dollars).2
Community-based US prevalence data suggest that FI affects an estimated 8.3% of the population, or approximately 20 million adults.3,4 According to a US National Health and Nutrition Examination Survey, FI affects ∼8.4% of noninstitutionalized adults.5 It is likely that FI is underestimated in the clinic because of several barriers, including misconceptions that FI is a natural part of aging, patient embarrassment, and social stigma.6 FI prevalence increases with age and regardless of ethnicity, from 2.6% in people aged 20 to 29 years to 15.3% in people aged 70 years or older.3 FI has been identified in at least 30% of residents in nursing homes7,8 and is a common reason for nursing home admissions in the elderly.9 Elderly individuals with bowel problems impose a large burden on health care resources,10 a burden that will likely increase as the population ages.11
Several risk factors for FI have been identified, and include obstetric trauma,12 anal trauma or surgery,13–15 pelvic radiotherapy for cancer,15–18 smoking,19 obesity,19 diabetes,15 and certain neurological conditions.20 There is a greater prevalence among females than males, which is attributed to maternal injuries sustained during childbirth; however, other factors may play a role in late-onset FI, such as menopause, changes in the pelvic floor due to aging, and pudendal neuropathy.3,21–24
FI is clinically subcategorized into 3 different types: (1) passive incontinence [loss of stool without the urge to defecate, mainly attributable to internal anal sphincter (IAS) dysfunction and peripheral neuropathy]; (2) urge incontinence [inability to postpone defecation urge, related mainly to external anal sphincter (EAS) dysfunction]; and (3) fecal seepage (involuntary loss of small amounts of stool), incomplete evacuation, and impaired rectal sensation).6,25
Clinical evaluation begins with a full medical history to determine the cause and severity of the FI and its impact on patients’ quality of life. Various scoring scales have been developed, including Wexner’s Cleveland Clinic Florida Fecal Incontinence Score,26 Vaizey’s (St Mark’s Incontinence Score),27 the Fecal Incontinence Severity Index (FISI),28 and the Rockwood Fecal Incontinence Quality of Life (FIQOL) Scale.29 Several methods are available to evaluate the underlying cause of FI, including anorectal manometry to assess sensation and compliance, and anal endosonography or magnetic resonance imaging to assess the thickness and integrity of the puborectalis and the IAS and EAS.6,30
Evidence suggests that the puborectalis may play an important role in anal continence.31 Although not routinely performed in clinical practice, anal electromyography and pudendal nerve terminal motor latency testing may uncover neurological damage.30 Recently, translumbar and transsacral motor-evoked potentials have provided evidence of neuropathy in patients with spinal cord injury and FI.32,33 Although these tests may help elucidate contributing factors to FI, many patients have multiple abnormalities contributing to symptoms30; therefore, FI should be recognized as a multifactorial disorder. This narrative review will focus on current and emerging treatment options for the management of FI.
METHODS
A MEDLINE search was conducted for articles related to FI treatment published from January 1, 1990 through June 1, 2013, and included the search terms “fecal incontinence” and “management”; “artificial bowel sphincter”; “biofeedback”; “bulking agent”; “conservative”; “dynamic gracilis’; “pelvic floor repair”; “levatorplasty”; “overlapping AND end-to-end”; “sacral nerve stimulation”; “SECCA”; and “radiofrequency.” The search was limited to articles published in English. Where relevant, the search was extended to unpublished trials on ClinicalTrials.gov. Bibliographies from included articles were manually reviewed for additional relevant publications.
RESULTS
Management options for FI consist of conservative approaches, surgery (minimally invasive or invasive procedures), and injectable bulking agents.
Conservative Approaches
Conservative approaches are usually first-line therapy, particularly in patients with mild symptoms, and include dietary modifications, medication, muscle-strengthening exercises (Kegel exercises), biofeedback, and nonsurgical electrical nerve stimulation.6 Dietary modification, such as avoiding caffeine, citrus fruits, spicy foods, alcohol, and dairy products (in patients with lactose intolerance) may help, but definitive evidence22 for these restrictions is lacking. Opinions differ as to whether the addition of dietary fiber is beneficial or detrimental for the treatment of FI6,34,35; however, methylcellulose is resistant to fermentation by colonic microflora and may be less likely than some other forms of fiber to exacerbate diarrhea.36
Several medications are also available to treat FI. Antidiarrheal or antimotility agents, including loperamide or diphenoxylate, may be beneficial in patients with loose stools and urgency.34 Limited evidence suggests that drugs administered to enhance sphincter tone, such as phenylepinephrine and sodium valproate, may be helpful in patients with passive FI and normal anal sphincter function.34 In 1 clinical trial, the tricyclic antidepressant amitriptyline improved FI scores (scale, 1 to 18) from a median of 16 at baseline to 3 (P<0.001) after 4 weeks of treatment.37 In an open-label uncontrolled study, clonidine, an alpha2 adrenergic agonist, improved FI after 4 weeks of therapy19; however, a randomized, placebo-controlled study showed that clonidine did not significantly improve the number of episodes of FI or quality of life.38
Anal sphincter exercises (pelvic floor muscle training) and biofeedback therapy have been used alone and in combination for the treatment of FI. Anal sphincter exercises are performed to strengthen the puborectalis muscle, which is continuous with the EAS.39 A single-center, randomized controlled study indicated that a regimen of pelvic floor exercises with biofeedback was nearly twice as effective as pelvic floor exercises alone, with 44% versus 21% of patients achieving complete continence at 3 months, respectively (P=0.008).39 In addition, symptom relief was reported for 76% of patients using biofeedback and pelvic floor exercises compared with 41% of patients performing pelvic floor exercises alone (P<0.01), and patients adjunctively using biofeedback had greater reductions in FISI scores (Fig. 1).40 In a more recent randomized study comparing 2 different pelvic floor exercise regimens, both with biofeedback, 59 of the 69 patients (86%) had improved continence with 20% fully continent, with no statistically significant differences between exercise regimens.41 A 2012 systematic review of randomized or quasirandomized controlled trials of patients performing anal sphincter exercises and/or receiving biofeedback and/or surface electrical stimulation of the anal sphincter concluded that the addition of biofeedback or electrical stimulation was superior to exercise alone in patients who had previously failed to respond to other conservative treatments.39
FIGURE 1.
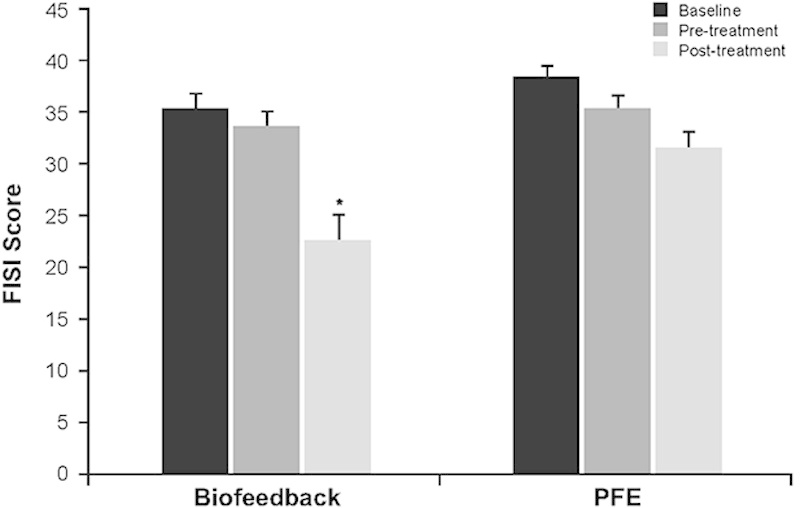
Fecal Incontinence Severity Index (FISI) scores at baseline, pretreatment (end of run-in), and at 3 months posttreatment in patients treated with biofeedback versus pelvic floor exercise (PFE). At the 3-month follow-up, patients in the biofeedback group had greater reductions in FISI scores versus patients in the PFE group (P=0.01, ANOVA). *P=0.01, biofeedback versus PFE. Adapted from Heymen et al.40
As indicated above, nonsurgical (surface) electrical stimulation, alone or in combination with biofeedback, has also proven useful. One study found the combination of electrical stimulation 20 minutes twice daily, and biofeedback was superior to electrical stimulation alone: 53.8% of 39 patients receiving the combination were continent at the end of treatment versus none of 41 patients in the electrical stimulation-alone group.42 In a small study of transcutaneous sacral nerve stimulation (SNS) applied 2 hours daily, 11 of 17 patients (69%) had improved symptoms, measured by FISI at 3 months, with improvements from a baseline of approximately 28 to 40 points as rated by both patients and the surgeon. Although the continence score improved by >50% in only 5 patients (31%) at 3 months, 2 were fully continent. Improvement continued during a mean follow-up of 19.7 months, with 53% of patients showing improvement of >50% in FISI scores.43
Transcutaneous and percutaneous posterior tibial nerve stimulation have been tried in patients with FI. In a large well-designed, multicenter, randomized, sham-controlled trial, transcutaneous posterior tibial nerve stimulation was no more efficacious than sham treatment.44 Small studies evaluating implants of percutaneous needles in the posterior tibial nerve have reported modest results.45–49 This includes results from a small, sham-controlled trial (11 treated patients vs. 8 sham-treated patients),50 but results from large, randomized controlled trials are lacking.
Surgery
Surgical options for FI may be considered in patients who fail conservative approaches. Several types of surgical management strategies are available for the treatment of FI, including direct surgical repair of defects, deformities, or obstruction; sphincter modulation; or fecal diversion. These various strategies include both invasive and less (minimally) invasive procedures.
Minimally Invasive Procedures
The SECCA procedure (or radiofrequency anal sphincter remodeling) involves delivering temperature-controlled radiofrequency energy to the anorectal junction.51 Although technically a nonsurgical procedure, the mechanism of action—tissue damage and wound healing—is considered invasive. This procedure results in tissue damage, remodeling, scarring, and contraction to potentially narrow the anal canal.52,53 Data on the SECCA procedure are variable, and results from randomized controlled trials are lacking. The technique’s pioneers reported 5-year follow-up of 19 patients, noting that 12-month improvements versus baseline in mean FI scores and FIQOL scores were sustained at 5 years.54 Other studies have also reported improvements in one or both these measures, albeit over a shorter time duration,51,55,56 and many patients continued to have moderate FI.51,56
SNS is an established, FDA-approved technique for neuromodulation in patients with FI. A low-amplitude electrical current is applied to a sacral nerve, usually S3, via an electrode in the sacral foramen. An advantage of SNS over alternative surgical techniques is the ability to evaluate patient response to SNS, via a temporary external neurostimulator, before permanent neurostimulator implantation.57 SNS must be performed in the operating room and requires general or local anesthesia.
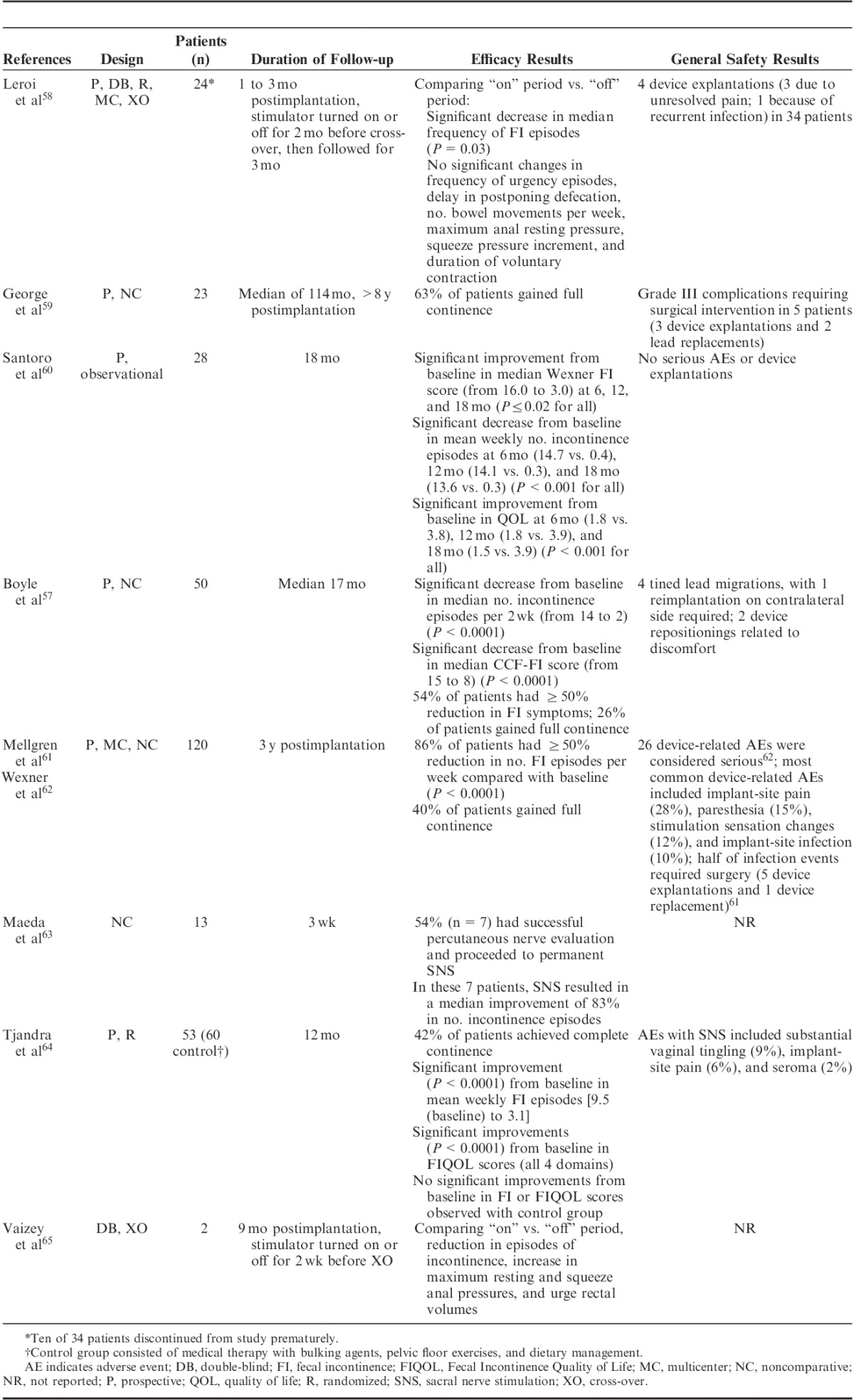
Reported efficacy of SNS has been inconsistent (Table 1).57–65 A literature review (n=14) on the clinical outcome of SNS and 9 other reports in patients with a sphincter lesion concluded that SNS has evolved to become a clinically efficient option in the treatment of FI. However, the need for long-term data has been noted with evidence of decreasing efficacy over time in more than a quarter of patients.66 A meta-analysis examined 34 studies published between 2000 and 2008 and included 790 patients, of whom 665 received a permanent implant. The analysis indicated that, compared with maximal conservative therapy, SNS significantly improved functional and quality-of-life outcomes. Improvement in weekly FI episodes and FI scores was significantly greater in patients with intact versus impaired sphincters, but those with impaired sphincters experienced a greater increase in the ability to defer defecation. However, the complication rate among the 665 patients who had permanent SNS electrode implantation was ∼15%, resulting in permanent removal of the device in 18 (2.7%) patients.67 A multicenter, prospective nonrandomized trial, not included as part of the previous meta-analysis, reported that 83% of 106 patients had ≥50% improvement in FI at 12 months and 40% became fully continent.62 Improvements were sustained for 3 years.61,62 A total of 307 adverse events (AEs, 26 serious) in 96 patients were considered device or therapy related. Authors reported that this AE rate compared favorably with those associated with artificial bowel sphincter (ABS) and dynamic graciloplasty (reviewed below).62 In 1 recent study reporting long-term benefits of SNS, 12 of 25 (48%) patients remained fully continent at the last follow-up visit (median, 114 mo; range, 96 to 164 mo). However, complications necessitated device removal in 3 (12%) patients.59
TABLE 1.
Efficacy and Safety of SNS in Patients With Chronic FI
Although SNS is expensive compared with more conservative approaches, some studies have shown SNS to be cost-effective compared with colostomy or dynamic graciloplasty.68 However, it has been suggested that the costs may be significantly higher than previously thought, given that only 1 in 4 patients achieve complete continence, and that there is no reliable way to predict which patient will respond after permanent device implantation.57 In addition, well-controlled randomized studies comparing SNS with sham treatment, particularly long-term studies, are lacking. Other forms of neurostimulation are being investigated, including pudendal nerve stimulation.69
Autologous myoblast injection is an investigational procedure in which myoblasts cultured from a striated muscle biopsy, taken surgically from a patient’s pectoralis muscle, are injected into the EAS.70 Daily anal electrical stimulation is required, both preprocedure (eg, 10 wk) and postprocedure (eg, 4 wk), to encourage myoblast integration into the tissue.70 A pilot study (n=10) showed that at 12 months, Wexner FI scores had decreased by a mean of 14 U (P<0.001) and Rockwood FIQOL scores had improved by a median of 30 U from baseline (P=0.005).70
Invasive Procedures
Invasive surgical procedures are typically reserved for patients for whom conservative or less invasive options have failed. A 2013 systematic review of randomized trials of surgery for FI (through March 2013 and excluding prolapse repair) concluded that there was little evidence for or against surgery for FI.53 However, the authors acknowledged that most of the studies evaluated were outdated and did not include more commonly used techniques.53 If medical and other surgical therapies are ineffective in treating FI or are contraindicated, a colostomy may be considered.
Colostomy is an established surgical option typically reserved for patients with FI refractory to a variety of other treatment options.6,71 Although patients are generally apprehensive about receiving a colostomy,71 survey data have noted improvement in quality of life following a colostomy compared with the FI experience before surgery,72 as well as increased scores on coping, embarrassment, and lifestyle scales of the FIQOL instrument in patients who had received a colostomy compared with patients with FI.73 Colostomy has been associated with bleeding, cardiac or respiratory events related to anesthesia, and parastomal hernia, but it remains a treatment option for patients with FI who have failed other therapies or for whom other therapies are not viable options.71
Anal sphincteroplasty involves repairing the damaged or weakened anal sphincter (using an overlapping or end-to-end technique) or creating a new functional sphincter using skeletal muscle from an adjacent site. In patients with FI of multiple etiologies, sphincter repair may be combined with pelvic floor repair.53,74 Long-term (>5 y) functional outcomes have generally been disappointing, regardless of the technique used (Fig. 2).75–85 Therefore, surgeons have turned to other surgical approaches, such as graciloplasty and artificial sphincters.
FIGURE 2.
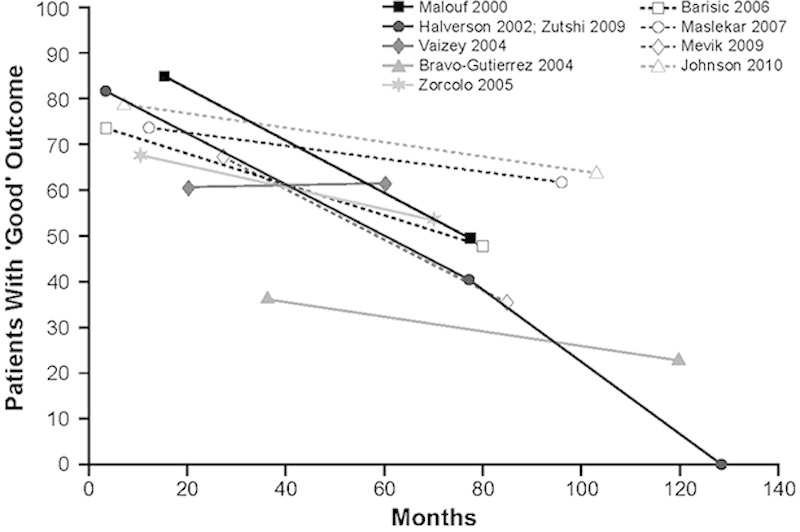
Percentage of patients with “good” long-term outcomes after anal sphincter repair for FI. “Good” outcome was determined using definitions provided by the authors of each article. Adapted from Glasgow and Lowry.75 Data from Malouf et al,76 Halverson and Hull,85 Zutshi et al,77 Vaizey et al,78 Bravo Gutierrez et al,79 Zorcolo et al,80 Barisic et al,81 Maslekar et al,82 Mevik et al,83 and Johnson et al.84
Graciloplasty uses the patient’s gracilis muscle to form a new sphincter around the anus. An electrical stimulator device may also be implanted in the abdominal wall (ie, dynamic graciloplasty) to sustain tone and help maintain continence.86 In a multicenter international trial of dynamic graciloplasty, success, defined as a ≥50% reduction in incontinent episodes, was reported in 47 of 76 (62%), 37 of 67 (55%), and 35 of 62 (56%) patients at 12 months, 18 months, and 2 years posttreatment, respectively.87 A systematic review reported dynamic graciloplasty success rates of 42% to 85%, with the most common AEs being infection (28%), stimulator malfunction (15%), and leg pain (13%).88
ABS devices comprise an inflatable cuff that acts as a new sphincter, a control pump, and a balloon that regulates the pressure and also acts as a fluid reservoir (Fig. 3).89 The device maintains continence when the cuff is inflated and the patient releases the pressure when they wish to defecate.90 Some health care providers have reportedly switched to using ABS devices rather than graciloplasty.91
FIGURE 3.
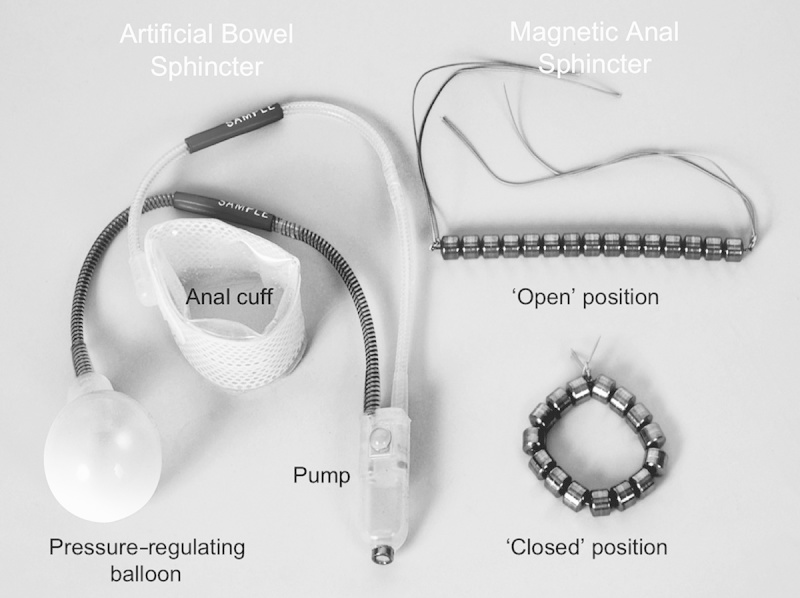
Artificial bowel sphincter (left) and the magnetic anal sphincter (right) devices. Reprinted with permission from Wong et al.89 Copyright Wolters Kluwer Health.
Reports on the efficacy and safety of ABS devices have varied (Table 2), and efficacy comparisons are confounded by the different scales used to assess improvement.91–105 The largest single-center study published (n=52 patients and 85 devices; mean follow-up, >5 y) showed that full continence is seldom achieved and that often a constant balance must be maintained between stool consistency and cuff pressures.105 In a series of 17 patients, all experienced complications, 65% needed further surgery, and 65% had the device removed.102 At 12 months, 33% and 67% of those retaining the device were completely continent to liquid and solid stools, respectively.102 In a study of 21 patients who received an ABS device at 2 French academic centers, all patients developed ≥1 complication, and 18 patients (86%) required corrective surgery. The device was permanently removed from 17 patients (81%).91
TABLE 2.
Efficacy and Safety of Artificial Bowel Sphincter Device for FI
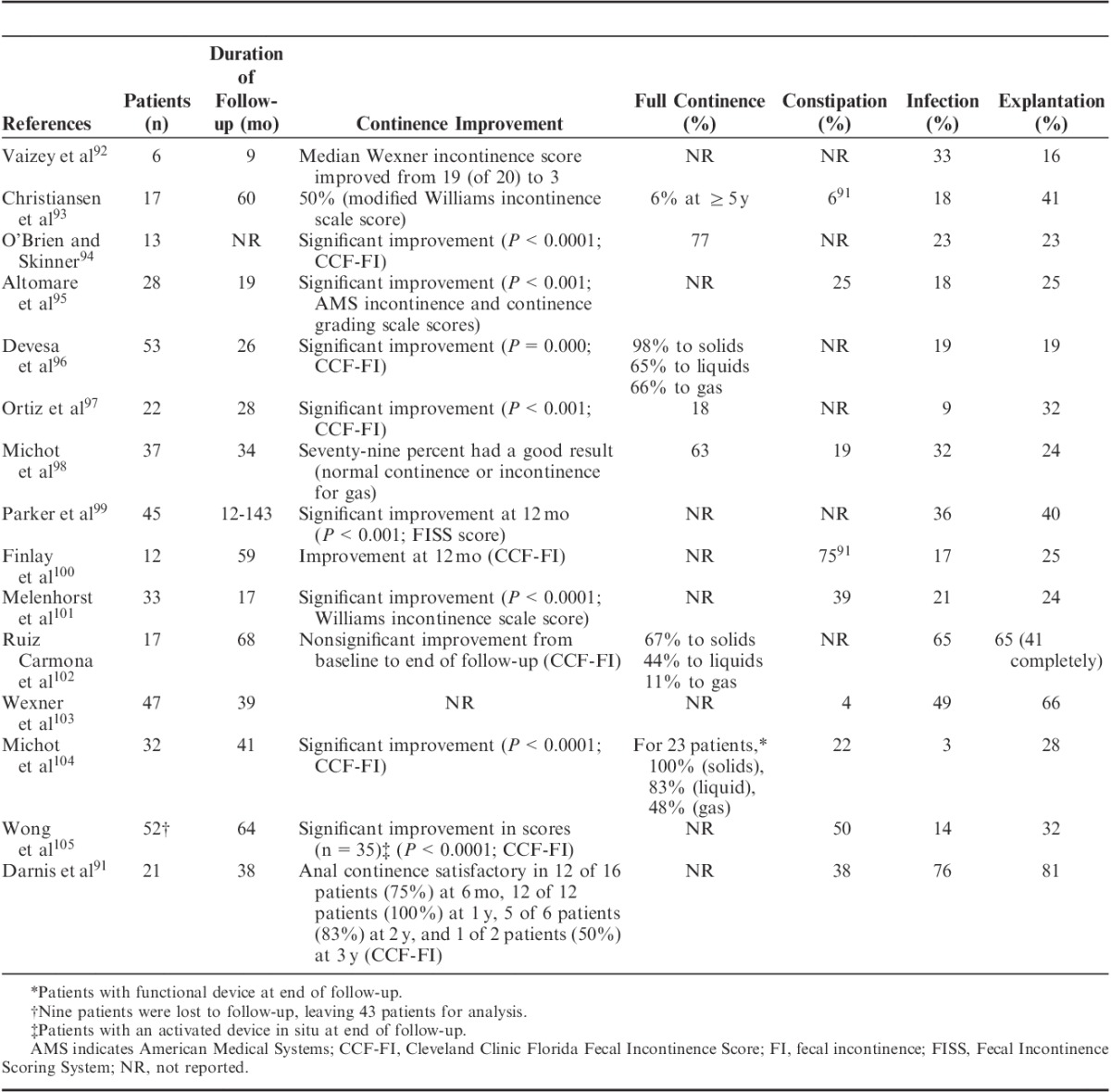
Magnetic anal sphincter (MAS) devices comprise a series of interlinked titanium beads with internal magnetic cores that form a flexible ring that is placed to encircle the EAS (Fig. 3). During the pushing process of defecation,89 the beads separate, allowing stool to pass106 through the EAS. Subsequent to completion of a feasibility study,106 a prospective, nonrandomized matched study (n=20) compared MAS and ABS devices.89 No significant differences in early postoperative complications were observed, but the MAS group had a shorter time in surgery (62 vs. 97 min; P=0.0273) and a shorter hospital stay (4.5 vs. 10 d; P<0.0001) compared with the ABS group.89 Both groups achieved significant improvements from baseline in Wexner FI scores [11-point decrease in each group; P=0.0002 (MAS), P=0.0001 (ABS)] and FIQOL scores [from 1.91 to 3.38 in the MAS group (P=0.0052) and from 1.80 to 3.55 in the ABS group (P=0.0089)].89 Four patients in the ABS group needed revisions; the device was removed in 2 patients because of pain and infection, respectively. At a mean follow-up of 8 months in the MAS group and 22.5 months in the ABS group, patients with either device still in situ had maintained initial postoperative improvements in FI scores, and similar significant improvements in FIQOL scores were observed in both the groups.89
Injectable Bulking Agents
Bulking agents vary in particle size and their capacity to migrate into the lymphatic system. Biocompatible bulking agents have been used successfully for many years for the treatment of urinary incontinence, and their potential use in FI is a logical progression.107 For FI, the mechanism of action of bulking agents is to augment the walls of the IAS to close the anal canal or raise the pressure inside the anal canal, thus preventing incontinence.1 Several bulking materials have been considered over the years. These include autologous fat, Teflon (rarely used because of safety issues), bovine glutaraldehyde cross-linked collagen, carbon-coated zirconium beads, polydimethylsiloxane elastomer (silicone), dextranomer microspheres in non-animal stabilized hyaluronic acid (NASHA Dx), hydrogel cross-linked with polyacrylamide, porcine dermal collagen, synthetic calcium hydroxyapatite ceramic microspheres, and polyacrylonitrile in cylinder form.1
NASHA Dx has been evaluated in several trials (Table 3),108–119 including a randomized, double-blind sham-controlled study in adults who had failed conservative therapies. Patients received NASHA Dx (n=136) or sham treatment (n=70) in an outpatient setting without anesthesia; patients with no persistent AEs but persistent FI after 1 month were offered 1 retreatment procedure.109 Seventy-one (52%) patients in the active treatment group versus 22 (31%) in the sham group had a treatment response (≥50% improvement from baseline in the number of FI episodes) at 6 months (odds ratio, 2.36; P=0.0089).109 There was a significant difference in the mean increase from baseline in number of incontinence-free days in the NASHA Dx group compared with sham group at month 6 (Fig. 4A)109 and a significant improvement in FIQOL, coping, and behavior scores but not lifestyle, depression and self-perception, or embarrassment at month 6 (Fig. 4B).109 Efficacy was not assessed in the sham group after 6 months; however, at 12 months, 69% of patients in the NASHA Dx group were responders.109 Efficacy and long-term durability have been reported in open-label studies110–114 and a comparative study versus biofeedback training (∼20 min daily, 5 d a week, for 6 mo; Table 3).108
TABLE 3.
Clinical Trials of Bulking Agents
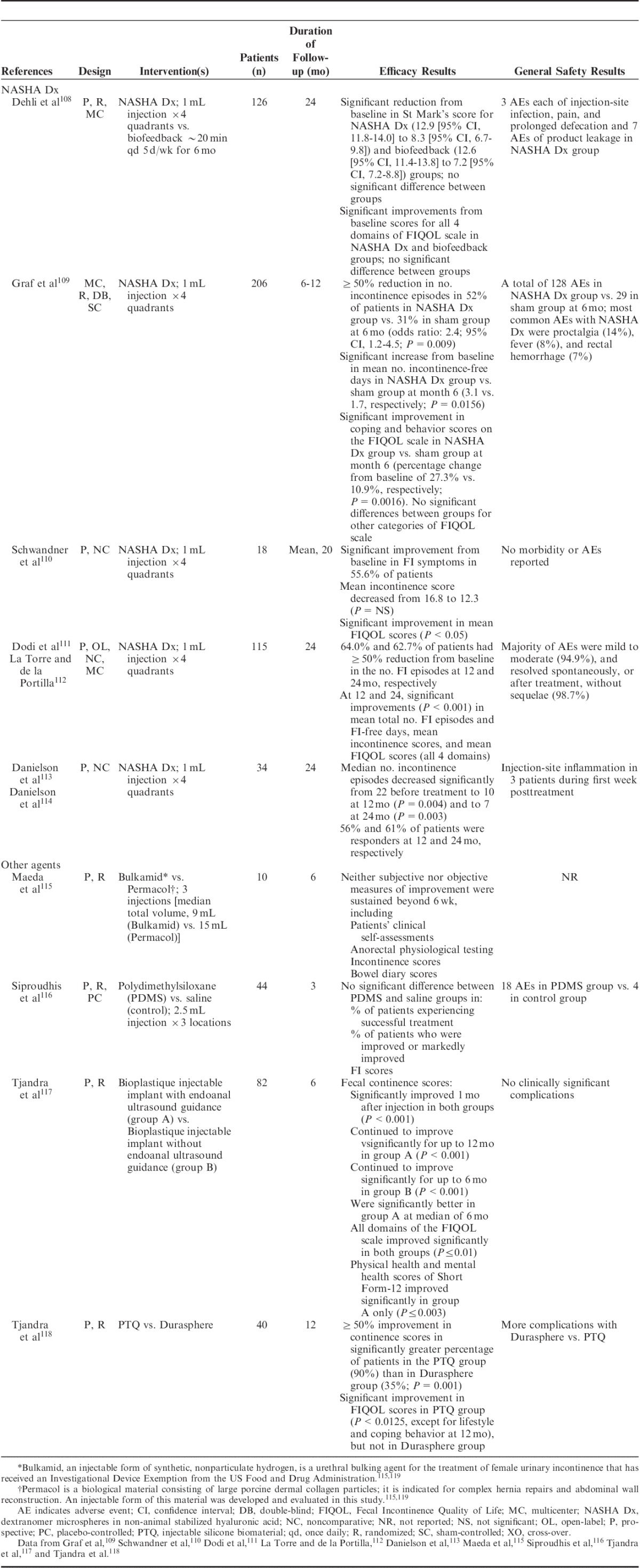
FIGURE 4.
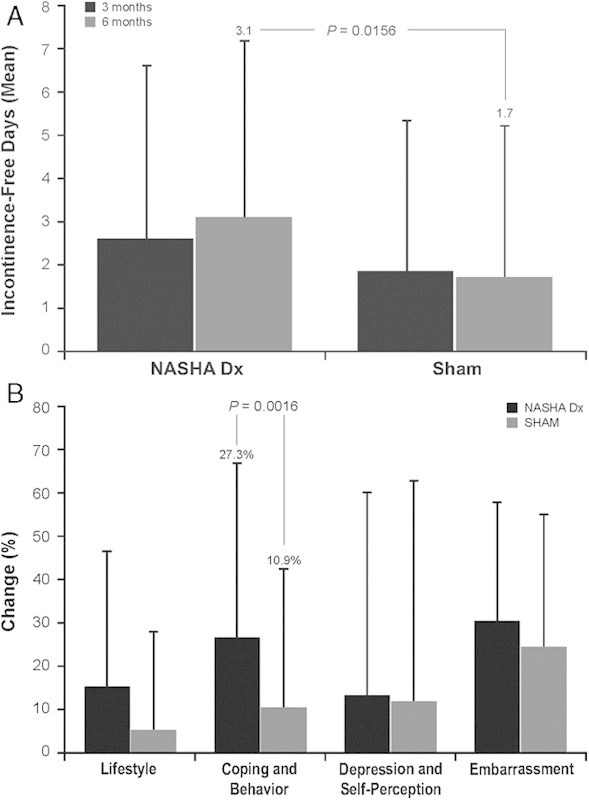
Response in patients treated with NASHA Dx (n=136) compared with sham treatment (n=70). A, The mean change from baseline in number of incontinence-free days over 2 weeks. B, The mean relative percentage change in FIQOL scores by category. FIQOL indicates Fecal Incontinence Quality of Life; NASHA Dx, dextranomer microspheres in non-animal stabilized hyaluronic acid. Adapted from Graf et al.109
The authors of a 2013 Cochrane review of 5 randomized studies of bulking agents concluded that, with the exception of the randomized NASHA Dx trial,109 although several studies of bulking agents (eg, silicone) showed short-term benefits, the quality of most trials was poor (Table 3).1
CONCLUSIONS
FI is a common and distressing problem with a significant negative impact on patients’ QOL, and conservative measures, such as lifestyle modifications and antidiarrheal agents, are considered first-line therapy for the management of FI and are generally effective in <25% of patients. Biofeedback therapy, in combination with pelvic floor exercises, may provide short-term symptom relief in ∼75% of patients, but biofeedback therapy is not widely available and lacks standardization. Although the minimally invasive surgical procedure SNS may be useful (eg, ≥50% reduction from baseline in weekly FI episodes in 54% to 86% of patients), it has been associated with complications and a failure rate of approximately 15%. Invasive surgical procedures, such as anal sphincteroplasty, are effective initially, but they may not provide long-term benefit. Success rates with surgical sphincter replacement methods, such as graciloplasty and ABS, may be limited by complication rates, including device explantation. MAS is promising and may be superior to ABS, but controlled, adequately powered studies with long-term follow-up are needed. Injectable bulking agents, such as NASHA Dx, may provide an alternative in patients for whom conservative therapies are ineffective.
ACKNOWLEDGMENTS
The authors thank Mary Beth Moncrief, PhD, and Julia Schroeder, BA, Synchrony Medical Communications, LLC, West Chester, PA for technical editorial and medical writing assistance, under the direction of S.S.C.R.
Footnotes
Statement of ethical adherence: S.S.C.R. meets the full requirements of authorship, with substantial contribution to the conception of and decision to publish this review manuscript, drafting the article or revising it critically for important intellectual content, and final approval of the version to be submitted. This manuscript has not been previously published and is not under consideration for publication elsewhere.
Funding for technical editorial and medical writing assistance was provided by Salix Pharmaceuticals Inc., Raleigh, NC.
S.S.C.R. is a member of the Data Monitoring Board for American Medical Systems Inc., but has no financial interests with any other fecal incontinence product manufacturer. Salix Pharmaceuticals did not contribute to content development of the manuscript.
REFERENCES
- 1.Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2013;2:CD007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586–598 [DOI] [PubMed] [Google Scholar]
- 3.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Census Bureau. People QuickFacts, USA. 2013. Available at: http://quickfacts.census.gov/qfd/states/00000.html. Accessed July 9, 2013
- 5.Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in US adults, 2005-2010. Clin Gastroenterol Hepatol. 2013636–643 [DOI] [PubMed] [Google Scholar]
- 6.Rao SS. Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004;99:1585–1604 [DOI] [PubMed] [Google Scholar]
- 7.Nelson R, Furner S, Jesudason V. Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum. 1998;41:1226–1229 [DOI] [PubMed] [Google Scholar]
- 8.Turnberg LA, Brocklehurst JC, Fowler CJ, et al. Incontinence. causes, management and provision of services. A Working Party of the Royal College of Physicians. J R Coll Phys Lond. 1995;29:272–274 [PMC free article] [PubMed] [Google Scholar]
- 9.Kamm MA. Faecal incontinence. BMJ. 1998;316:528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potter J. Bowel care in older people. Clin Med. 2003;3:48–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Findlay J, Maxwell-Armstrong C. Posterior tibial nerve stimulation for faecal incontinence. Br J Nurs. 2010;19:750–754 [DOI] [PubMed] [Google Scholar]
- 12.Lunniss PJ, Gladman MA, Hetzer FH, et al. Risk factors in acquired faecal incontinence. J R Soc Med. 2004;97:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad M, McCallum IJ, Mercer-Jones M. Management of faecal incontinence in adults. BMJ. 2010;340:c2964. [DOI] [PubMed] [Google Scholar]
- 14.Levin A, Cohen MJ, Mindrul V, et al. Delayed fecal incontinence following surgery for anal fissure. Int J Colorectal Dis. 2011;26:1595–1599 [DOI] [PubMed] [Google Scholar]
- 15.Rey E, Choung RS, Schleck CD, et al. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol. 2010;105:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barraclough LH, Routledge JA, Farnell DJ, et al. Prospective analysis of patient-reported late toxicity following pelvic radiotherapy for gynaecological cancer. Radiother Oncol. 2012;103:327–332 [DOI] [PubMed] [Google Scholar]
- 17.Dunberger G, Lind H, Steineck G, et al. Fecal incontinence affecting quality of life and social functioning among long-term gynecological cancer survivors. Int J Gynecol Cancer. 2010;20:449–460 [DOI] [PubMed] [Google Scholar]
- 18.Alsadius D, Hedelin M, Lundstedt D, et al. Mean absorbed dose to the anal-sphincter region and fecal leakage among irradiated prostate cancer survivors. Int J Radiat Oncol Biol Phys. 2012;84:e181–e185 [DOI] [PubMed] [Google Scholar]
- 19.Bharucha AE, Seide BM, Zinsmeister AR. The effects of clonidine on symptoms and anorectal sensorimotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laine K, Skjeldestad FE, Sanda B, et al. Prevalence and risk factors for anal incontinence after obstetric anal sphincter rupture. Acta Obstet Gynecol Scand. 2011;90:319–324 [DOI] [PubMed] [Google Scholar]
- 21.Bharucha AE. Fecal incontinence. Gastroenterology. 2003;124:1672–1685 [DOI] [PubMed] [Google Scholar]
- 22.Hannaway CD, Hull TL. Fecal incontinence. Obstet Gynecol Clin North Am. 2008;35:249–269viii [DOI] [PubMed] [Google Scholar]
- 23.Bohle B, Belvis F, Vial M, et al. Menopause and obstetric history as risk factors for fecal incontinence in women. Dis Colon Rectum. 2011;54:975–981 [DOI] [PubMed] [Google Scholar]
- 24.Kepenekci I, Keskinkilic B, Akinsu F, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum. 2011;54:85–94 [DOI] [PubMed] [Google Scholar]
- 25.Engel AF, Kamm MA, Bartram CI, et al. Relationship of symptoms in faecal incontinence to specific sphincter abnormalities. Int J Colorectal Dis. 1995;10:152–155 [DOI] [PubMed] [Google Scholar]
- 26.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97 [DOI] [PubMed] [Google Scholar]
- 27.Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the Fecal Incontinence Severity Index. Dis Colon Rectum. 1999;42:1525–1532 [DOI] [PubMed] [Google Scholar]
- 29.Rockwood TH, Church JM, Fleshman JW, et al. Fecal incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16 [DOI] [PubMed] [Google Scholar]
- 30.Rao SS. Advances in diagnostic assessment of fecal incontinence and dyssynergic defecation. Clin Gastroenterol Hepatol. 2010;8:910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matzel KE, Schmidt RA, Tanagho EA. Neuroanatomy of the striated muscular anal continence mechanism. Implications for the use of neurostimulation. Dis Colon Rectum. 1990;33:666–673 [DOI] [PubMed] [Google Scholar]
- 32.Tantiphlachiva K, Attaluri A, Valestin J, et al. Translumbar and transsacral motor-evoked potentials: a novel test for spino-anorectal neuropathy in spinal cord injury. Am J Gastroenterol. 2011;106:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao SSC, Cross-Adame E, Tantiphlachiva K, et al. Translumbar and transsacral magnetic neuro-stimulation for the assessment of neuropathy in fecal incontinence. Dis Colon Rectum. 2014;57:645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheetham M, Brazzelli M, Norton C, et al. Drug treatment for faecal incontinence in adults. Cochrane Database Syst Rev. 2003CD002116. [DOI] [PubMed] [Google Scholar]
- 35.Bliss DZ, Savik K, Jung HJ, et al. Symptoms associated with dietary fiber supplementation over time in individuals with fecal incontinence. Nurs Res. 2011;60:S58–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sze EH, Hobbs G. Efficacy of methylcellulose and loperamide in managing fecal incontinence. Acta Obstet Gynecol Scand. 2009;88:766–771 [DOI] [PubMed] [Google Scholar]
- 37.Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676–1681 [DOI] [PubMed] [Google Scholar]
- 38.Bharucha AE, Fletcher JG, Camilleri M, et al. Effects of clonidine in women with fecal incontinence. Clin Gastroenterol Hepatol. 2014;12:843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2012;7:CD002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartlett L, Sloots K, Nowak M, et al. Biofeedback for fecal incontinence: a randomized study comparing exercise regimens. Dis Colon Rectum. 2011;54:846–856 [DOI] [PubMed] [Google Scholar]
- 42.Schwandner T, Konig IR, Heimerl T, et al. Triple target treatment (3T) is more effective than biofeedback alone for anal incontinence: the 3T-AI study. Dis Colon Rectum. 2010;53:1007–1016 [DOI] [PubMed] [Google Scholar]
- 43.Chew SS, Sundaraj R, Adams W. Sacral transcutaneous electrical nerve stimulation in the treatment of idiopathic faecal incontinence. Colorectal Dis. 2011;13:567–571 [DOI] [PubMed] [Google Scholar]
- 44.Leroi AM, Siproudhis L, Etienney I, et al. Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: a randomized trial (CONSORT 1a). Am J Gastroenterol. 2012;107:1888–1896 [DOI] [PubMed] [Google Scholar]
- 45.Hotouras A, Thaha MA, Boyle DJ, et al. Short-term outcome following percutaneous tibial nerve stimulation for faecal incontinence: a single-centre prospective study. Colorectal Dis. 2012;14:1101–1105 [DOI] [PubMed] [Google Scholar]
- 46.Hotouras A, Murphy J, Allison M, et al. Prospective clinical audit of two neuromodulatory treatments for fecal incontinence: sacral nerve stimulation (SNS) and percutaneous tibial nerve stimulation (PTNS). Surg Today. 2014[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Findlay JM, Yeung JM, Robinson R, et al. Peripheral neuromodulation via posterior tibial nerve stimulation—a potential treatment for faecal incontinence? Ann R Coll Surg Engl. 2010;92:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Govaert B, Pares D, Delgado-Aros S, et al. A prospective multicentre study to investigate percutaneous tibial nerve stimulation for the treatment of faecal incontinence. Colorectal Dis. 2010;12:1236–1241 [DOI] [PubMed] [Google Scholar]
- 49.Boyle DJ, Prosser K, Allison ME, et al. Percutaneous tibial nerve stimulation for the treatment of urge fecal incontinence. Dis Colon Rectum. 2010;53:432–437 [DOI] [PubMed] [Google Scholar]
- 50.George AT, Kalmar K, Sala S, et al. Randomized controlled trial of percutaneous versus transcutaneous posterior tibial nerve stimulation in faecal incontinence. Br J Surg. 2013;100:330–338 [DOI] [PubMed] [Google Scholar]
- 51.Ruiz D, Pinto RA, Hull TL, et al. Does the radiofrequency procedure for fecal incontinence improve quality of life and incontinence at 1-year follow-up? Dis Colon Rectum. 2010;53:1041–1046 [DOI] [PubMed] [Google Scholar]
- 52.Felt-Bersma RJ, Szojda MM, Mulder CJ. Temperature-controlled radiofrequency energy (SECCA) to the anal canal for the treatment of faecal incontinence offers moderate improvement. Eur J Gastroenterol Hepatol. 2007;19:575–580 [DOI] [PubMed] [Google Scholar]
- 53.Brown SR, Wadhawan H, Nelson RL. Surgery for faecal incontinence in adults. Cochrane Database Syst Rev. 2013;7:CD001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi-Monroy T, Morales M, Garcia-Osogobio S, et al. SECCA procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51:355–359 [DOI] [PubMed] [Google Scholar]
- 55.Efron JE, Corman ML, Fleshman J, et al. Safety and effectiveness of temperature-controlled radio-frequency energy delivery to the anal canal (SECCA procedure) for the treatment of fecal incontinence. Dis Colon Rectum. 2003;46:1606–1616 [DOI] [PubMed] [Google Scholar]
- 56.Lefebure B, Tuech JJ, Bridoux V, et al. Temperature-controlled radio frequency energy delivery (SECCA procedure) for the treatment of fecal incontinence: results of a prospective study. Int J Colorectal Dis. 2008;23:993–997 [DOI] [PubMed] [Google Scholar]
- 57.Boyle DJ, Murphy J, Gooneratne ML, et al. Efficacy of sacral nerve stimulation for the treatment of fecal incontinence. Dis Colon Rectum. 2011;54:1271–1278 [DOI] [PubMed] [Google Scholar]
- 58.Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302–306 [DOI] [PubMed] [Google Scholar]
- 60.Santoro GA, Infantino A, Cancian L, et al. Sacral nerve stimulation for fecal incontinence related to external sphincter atrophy. Dis Colon Rectum. 2012;55:797–805 [DOI] [PubMed] [Google Scholar]
- 61.Mellgren A, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011;54:1065–1075 [DOI] [PubMed] [Google Scholar]
- 62.Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441–449 [DOI] [PubMed] [Google Scholar]
- 63.Maeda Y, Hoyer M, Lundby L, et al. Temporary sacral nerve stimulation for faecal incontinence following pelvic radiotherapy. Radiother Oncol. 2010;97:108–112 [DOI] [PubMed] [Google Scholar]
- 64.Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51:494–502 [DOI] [PubMed] [Google Scholar]
- 65.Vaizey CJ, Kamm MA, Roy AJ, et al. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2000;43:298–302 [DOI] [PubMed] [Google Scholar]
- 66.Matzel KE. Sacral nerve stimulation for faecal incontinence: its role in the treatment algorithm. Colorectal Dis. 2011;13suppl 210–14 [DOI] [PubMed] [Google Scholar]
- 67.Tan E, Ngo NT, Darzi A, et al. Meta-analysis: sacral nerve stimulation versus conservative therapy in the treatment of faecal incontinence. Int J Colorectal Dis. 2011;26:275–294 [DOI] [PubMed] [Google Scholar]
- 68.Hetzer FH, Bieler A, Hahnloser D, et al. Outcome and cost analysis of sacral nerve stimulation for faecal incontinence. Br J Surg. 2006;93:1411–1417 [DOI] [PubMed] [Google Scholar]
- 69.Bock S, Folie P, Wolff K, et al. First experiences with pudendal nerve stimulation in fecal incontinence: a technical report. Tech Coloproctol. 2010;14:41–44 [DOI] [PubMed] [Google Scholar]
- 70.Frudinger A, Kolle D, Schwaiger W, et al. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut. 2010;59:55–61 [DOI] [PubMed] [Google Scholar]
- 71.Van Koughnett JA, Wexner SD. Current management of fecal incontinence: choosing amongst treatment options to optimize outcomes. World J Gastroenterol. 2013;19:9216–9230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Norton C, Burch J, Kamm MA. Patients’ views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062–1069 [DOI] [PubMed] [Google Scholar]
- 73.Colquhoun P, Kaiser R, Jr, Efron J, et al. Is the quality of life better in patients with colostomy than patients with fecal incontinence? World J Surg. 2006;30:1925–1928 [DOI] [PubMed] [Google Scholar]
- 74.Steele SR, Lee P, Mullenix PS, et al. Is there a role for concomitant pelvic floor repair in patients with sphincter defects in the treatment of fecal incontinence? Int J Colorectal Dis. 2006;21:508–514 [DOI] [PubMed] [Google Scholar]
- 75.Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482–490 [DOI] [PubMed] [Google Scholar]
- 76.Malouf AJ, Norton CS, Engel AF, et al. Long-term results of overlapping anterior anal-sphincter repair for obstetric trauma. Lancet. 2000;355:260–265 [DOI] [PubMed] [Google Scholar]
- 77.Zutshi M, Tracey TH, Bast J, et al. Ten-year outcome after anal sphincter repair for fecal incontinence. Dis Colon Rectum. 2009;52:1089–1094 [DOI] [PubMed] [Google Scholar]
- 78.Vaizey CJ, Norton C, Thornton MJ, et al. Long-term results of repeat anterior anal sphincter repair. Dis Colon Rectum. 2004;47:858–863 [DOI] [PubMed] [Google Scholar]
- 79.Bravo Gutierrez A, Madoff RD, Lowry AC, et al. Long-term results of anterior sphincteroplasty. Dis Colon Rectum. 2004;47:727–731 [DOI] [PubMed] [Google Scholar]
- 80.Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum. 2005;48:524–531 [DOI] [PubMed] [Google Scholar]
- 81.Barisic GI, Krivokapic ZV, Markovic VA, et al. Outcome of overlapping anal sphincter repair after 3 months and after a mean of 80 months. Int J Colorectal Dis. 2006;21:52–56 [DOI] [PubMed] [Google Scholar]
- 82.Maslekar S, Gardiner AB, Duthie GS. Anterior anal sphincter repair for fecal incontinence: good longterm results are possible. J Am Coll Surg. 2007;204:40–46 [DOI] [PubMed] [Google Scholar]
- 83.Mevik K, Norderval S, Kileng H, et al. Long-term results after anterior sphincteroplasty for anal incontinence. Scand J Surg. 2009;98:234–238 [DOI] [PubMed] [Google Scholar]
- 84.Johnson E, Carlsen E, Steen TB, et al. Short- and long-term results of secondary anterior sphincteroplasty in 33 patients with obstetric injury. Acta Obstet Gynecol Scand. 2010;89:1466–1472 [DOI] [PubMed] [Google Scholar]
- 85.Halverson AL, Hull TL. Long-term outcome of overlapping anal sphincter repair. Dis Colon Rectum. 2002;45:345–348 [DOI] [PubMed] [Google Scholar]
- 86.Edden Y, Wexner SD. Therapeutic devices for fecal incontinence: dynamic graciloplasty, artificial bowel sphincter and sacral nerve stimulation. Expert Rev Med Devices. 2009;6:307–312 [DOI] [PubMed] [Google Scholar]
- 87.Wexner SD, Baeten C, Bailey R, et al. Long-term efficacy of dynamic graciloplasty for fecal incontinence. Dis Colon Rectum. 2002;45:809–818 [DOI] [PubMed] [Google Scholar]
- 88.Chapman AE, Geerdes B, Hewett P, et al. Systematic review of dynamic graciloplasty in the treatment of faecal incontinence. Br J Surg. 2002;89:138–153 [DOI] [PubMed] [Google Scholar]
- 89.Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773–779 [DOI] [PubMed] [Google Scholar]
- 90.Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45:1139–1153 [DOI] [PubMed] [Google Scholar]
- 91.Darnis B, Faucheron JL, Damon H, et al. Technical and functional results of the artificial bowel sphincter for treatment of severe fecal incontinence: is there any benefit for the patient? Dis Colon Rectum. 2013;56:505–510 [DOI] [PubMed] [Google Scholar]
- 92.Vaizey CJ, Kamm MA, Gold DM, et al. Clinical, physiological, and radiological study of a new purpose-designed artificial bowel sphincter. Lancet. 1998;352:105–109 [DOI] [PubMed] [Google Scholar]
- 93.Christiansen J, Rasmussen OO, Lindorff-Larsen K. Long-term results of artificial anal sphincter implantation for severe anal incontinence. Ann Surg. 1999;230:45–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Brien PE, Skinner S. Restoring control: the Acticon Neosphincter artificial bowel sphincter in the treatment of anal incontinence. Dis Colon Rectum. 2000;43:1213–1216 [DOI] [PubMed] [Google Scholar]
- 95.Altomare DF, Dodi G, La TF, et al. Multicentre retrospective analysis of the outcome of artificial anal sphincter implantation for severe faecal incontinence. Br J Surg. 2001;88:1481–1486 [DOI] [PubMed] [Google Scholar]
- 96.Devesa JM, Rey A, Hervas PL, et al. Artificial anal sphincter: complications and functional results of a large personal series. Dis Colon Rectum. 2002;45:1154–1163 [DOI] [PubMed] [Google Scholar]
- 97.Ortiz H, Armendariz P, DeMiguel M, et al. Complications and functional outcome following artificial anal sphincter implantation. Br J Surg. 2002;89:877–881 [DOI] [PubMed] [Google Scholar]
- 98.Michot F, Costaglioli B, Leroi AM, et al. Artificial anal sphincter in severe fecal incontinence: outcome of prospective experience with 37 patients in one institution. Ann Surg. 2003;237:52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum. 2003;46:722–729 [DOI] [PubMed] [Google Scholar]
- 100.Finlay IG, Richardson W, Hajivassiliou CA. Outcome after implantation of a novel prosthetic anal sphincter in humans. Br J Surg. 2004;91:1485–1492 [DOI] [PubMed] [Google Scholar]
- 101.Melenhorst J, Koch SM, van Gemert WG, et al. The artificial bowel sphincter for faecal incontinence: a single centre study. Int J Colorectal Dis. 2008;23:107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ruiz Carmona MD, Alos Company R, Roig Vila JV, et al. Long-term results of artificial bowel sphincter for the treatment of severe faecal incontinence. Are they what we hoped for? Colorectal Dis. 2009;11:831–837 [DOI] [PubMed] [Google Scholar]
- 103.Wexner SD, Jin HY, Weiss EG, et al. Factors associated with failure of the artificial bowel sphincter: a study of over 50 cases from Cleveland Clinic Florida. Dis Colon Rectum. 2009;52:1550–1557 [DOI] [PubMed] [Google Scholar]
- 104.Michot F, Lefebure B, Bridoux V, et al. Artificial anal sphincter for severe fecal incontinence implanted by a transvaginal approach: experience with 32 patients treated at one institution. Dis Colon Rectum. 2010;53:1155–1160 [DOI] [PubMed] [Google Scholar]
- 105.Wong MT, Meurette G, Wyart V, et al. The artificial bowel sphincter: a single institution experience over a decade. Ann Surg. 2011;254:951–956 [DOI] [PubMed] [Google Scholar]
- 106.Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53:1604–1610 [DOI] [PubMed] [Google Scholar]
- 107.Vaizey CJ, Kamm MA. Injectable bulking agents for treating faecal incontinence. Br J Surg. 2005;92:521–527 [DOI] [PubMed] [Google Scholar]
- 108.Dehli T, Stordahl A, Vatten LJ, et al. Sphincter training or anal injections of dextranomer for treatment of anal incontinence: a randomized trial. Scand J Gastroenterol. 2013;48:302–310 [DOI] [PubMed] [Google Scholar]
- 109.Graf W, Mellgren A, Matzel KE, et al. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet. 2011;377:997–1003 [DOI] [PubMed] [Google Scholar]
- 110.Schwandner O, Brunner M, Dietl O. Quality of life and functional results of submucosal injection therapy using dextranomer hyaluronic acid for fecal incontinence. Surg Innov. 2011;18:130–135 [DOI] [PubMed] [Google Scholar]
- 111.Dodi G, Jongen J, de la Portilla F, et al. An open-label, noncomparative, multicenter study to evaluate efficacy and safety of NASHA/Dx gel as a bulking agent for the treatment of fecal incontinence. Gastroenterol Res Pract. 2010;2010:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.La Torre F, de la Portilla F. Long-term efficacy of dextranomer in stabilized hyaluronic acid (NASHA/Dx) for treatment of faecal incontinence. Colorectal Dis. 2013;15:569–574 [DOI] [PubMed] [Google Scholar]
- 113.Danielson J, Karlbom U, Sonesson AC, et al. Submucosal injection of stabilized nonanimal hyaluronic acid with dextranomer: a new treatment option for fecal incontinence. Dis Colon Rectum. 2009;52:1101–1106 [DOI] [PubMed] [Google Scholar]
- 114.Danielson J, Karlbom U, Wester T, et al. Efficacy and quality of life 2 years after treatment for faecal incontinence with injectable bulking agents. Tech Coloproctol. 2013;17:389–395 [DOI] [PubMed] [Google Scholar]
- 115.Maeda Y, Vaizey CJ, Kamm MA. Pilot study of two new injectable bulking agents for the treatment of faecal incontinence. Colorectal Dis. 2008;10:268–272 [DOI] [PubMed] [Google Scholar]
- 116.Siproudhis L, Morcet J, Laine F. Elastomer implants in faecal incontinence: a blind, randomized placebo-controlled study. Aliment Pharmacol Ther. 2007;25:1125–1132 [DOI] [PubMed] [Google Scholar]
- 117.Tjandra JJ, Lim JF, Hiscock R, et al. Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Dis Colon Rectum. 2004;47:2138–2146 [DOI] [PubMed] [Google Scholar]
- 118.Tjandra JJ, Chan MK, Yeh HC. Injectable silicone biomaterial (PTQ) is more effective than carbon-coated beads (Durasphere) in treating passive faecal incontinence—a randomized trial. Colorectal Dis. 2009;11:382–389 [DOI] [PubMed] [Google Scholar]
- 119.US Food and Drug Administration. Tissue Science plc’s Permacol® sugical implant crosslinked porcine dermal collagen mesh—510(k) summary. December 17, 2004. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf4/K043366.pdf. Accessed August 28, 2013


