Abstract
Bell’s palsy is the most common cause of acute facial nerve paralysis. In China, Bell’s palsy is frequently treated with acupuncture. However, its efficacy and underlying mechanism are still controversial. In this study, we used functional MRI to investigate the effect of acupuncture on the functional connectivity of the brain in Bell’s palsy patients and healthy individuals. The patients were further grouped according to disease duration and facial motor performance. The results of resting-state functional MRI connectivity show that acupuncture induces significant connectivity changes in the primary somatosensory region of both early and late recovery groups, but no significant changes in either the healthy control group or the recovered group. In the recovery group, the changes also varied with regions and disease duration. Therefore, we propose that the effect of acupuncture stimulation may depend on the functional connectivity status of patients with Bell’s palsy.
Keywords: acupuncture, Bell’s palsy, cortical reorganization, functional connectivity, functional MRI
Introduction
Bell’s palsy, a unilateral idiopathic facial nerve palsy, is a common disorder with a fair prognosis 1. In East Asia, acupuncture is widely used to treat the disease, but the mechanism underlying its clinical efficacy is still controversial 2–4. Previous studies have demonstrated that acupuncture can alter the brain’s functional network in healthy individuals 5–7. Therefore, this study seeks to explore the mechanism of acupuncture treatment by investigating whether acupuncture changes the brain’s functional connectivity in Bell’s palsy patients.
Clinically, LI4 (or Hegu), located on the dorsum of the hand, is a commonly used acupoint in the treatment of Bell’s palsy. The theoretical basis for selecting this acupoint is that the cortical representative areas of the hand and face are adjacent in both the primary somatosensory area (SI) and the primary motor area (MI). A recent report on cortical reorganization, which demonstrated enlargement of the hand field into the site of the presumed face area in facial palsy, provided neurophysiologic support for this theory 8. Other research demonstrated that the primary somatosensory cortex is also one of the most important areas activated in response to acupuncture at Hegu 9–11. Thus, it is reasonable to select the SI as a seed region for connectivity analysis in this study.
Our previous investigation on how acupuncture aids Bell’s palsy treatment suggested that the brain’s response to acupuncture likely depends on its functional status 11. Some other functional MRI (fMRI) studies on Bell’s palsy found that cortical reorganization complemented the recovery from facial nerve palsy, with pronounced functional connectivity changes, mainly demonstrated as disruption at the early stage and enforcement at the later stage in areas related to error detection, sensorimotor integration, motor integration, and control 12–14. These results suggest that the brain’s functional connectivity status at different pathological stages has different responses to acupuncture. Therefore, in this study we hypothesized that acupuncture may modulate functional connectivity between SI and other pathologically related regions in Bell’s palsy patients, and the modulation may vary with pathological stage.
Materials and methods
Participants
This study was approved by the human research committee of the First Affiliated Hospital of Anhui University of Chinese Medicine. All participants recruited in this study were right-handed with no history of drug abuse and no mental, central nervous system, or other serious disease, and signed informed consent forms were obtained before participation in the experiment. The participants were divided into two study groups: a healthy control group (20 individuals who underwent MRI 20 times in total) and a patient group (28 patients who underwent MRI 58 times in total). All healthy individuals (20 individuals: 11 male, nine female, aged between 23 and 54 years) were either college students or staff from the First Affiliated Hospital of Anhui University of Chinese Medicine. The patient group, consisting of patients with left and right unilateral facial paresis, was further divided into three subgroups on the basis of disease duration and House–Brackmann facial nerve grading system scores (HBS; 1=normal facial movement, 6=no movement, scored by an experienced acupuncturist with no prior knowledge on the data results). The three subgroups were the early group (18 cases, nine male, nine female, aged between 19 and 70 years; duration data<14 days, HBS>I; 10 left facial pareses, eight right facial pareses), the late group (21 cases, 14 male, seven female, aged between 19 and 70 years; duration data>14 days, HBS>I; 12 left facial pareses, nine right facial pareses), and the recovered group (19 cases, 10 male, nine female, aged between 19 and 63 years; HBS=I; seven left facial paresis, 12 right facial paresis). All patients were instructed to take several scans at different pathological stages based on their willingness. Among the 28 patients in the patient group, four underwent MRI scanning only once, 18 underwent MRI scanning twice, and six underwent MRI scanning three times. Manual acupuncture was semi-individually applied to all patients three times a week at acupoints chosen by the acupuncturists on the basis of the individual symptoms during the course of acupuncture treatment.
Experimental design
All MRI examinations were conducted on the Siemens (Siemens, Erlangen, Bavaria, Germany) Symphony 1.5 T MRI whole body scanner with a standard head coil at the First Affiliated Hospital of Anhui University of Chinese Medicine. Before examination, all participants were asked to change their clothes and fully relax themselves before entering the scanning room. The participants were then instructed to lie supine with their ears packed with cotton balls, keeping their eyes closed, paying attention to acupuncture, and avoiding falling asleep or getting distracted during the scan. To avoid unwanted visual stimulation, all lights in the scanning room were turned off.
Image acquisition
A total of eight image sequences were obtained on scanning: (i) pilot images; (ii) T2-weighted images, aimed to diagnose any obvious disease of the brain; (iii) T1-weighted two-dimensional anatomical images, in the axial position parallel to the anterior commissure–posterior commissure line, with a total of 36 whole-brain slices, with TR/TE 500/12 ms, field of view 230×230 mm, slice thickness/interval 3.0/0.75 mm, and resolution 192×144; (iv) resting-state fMRI images obtained before acupuncture using an EPI BOLD sequence with TR/TE/FA 3000 ms/30 ms/90°; (v) resting-state fMRI images obtained during acupuncture with the same parameters; (vi) resting-state fMRI images obtained after acupuncture with the same parameters; (vii) task-state fMRI images obtained using the same sequence as that used in the resting state, with TR/TE/FA 4000 ms/50 ms/90°; (viii) T1-weighted three-dimensional sagittal images, with a total of 176 whole-brain slices. The spoiled gradient echo sequence was used, with TR/TE/FA 2100 mm/3.93 mm/13, field of view 250×250 mm, slice thickness/spacing 1.0/0.5 mm, and resolution 256×256. The whole scanning session took around 60 min.
Functional MRI paradigms
Resting-state fMRI data were acquired before and after acupuncture, respectively. The first resting-state data acquisition collected 200 points for 10 min before acupuncture. Thereafter, an MR-compatible stainless steel acupuncture needle was inserted into participant’s skin at Hegu on the contralateral side of paresis. When the de-qi sensation was acquired, a second fMRI data acquisition began to collect another 200 points for 10 min while the needle was being rotated bidirectionally for 10 s every 2 min. Finally, the needle was pulled out, followed by another data acquisition of 200 points for 10 min as the postacupuncture resting-state data.
Task-state fMRI data were acquired during acupuncture. The acquisition lasted 10 min 40 s, with a total of 160 points (time interval 4 s) collected during this session. The acupuncture paradigm was designed as an interleaving pattern of needle retaining and rotating, consisting of 32 points of resting state after de-qi, 32 points of stimulating state during acupuncture, 48 points of resting state after acupuncture, 32 points of stimulating state during acupuncture, and finally 16 points of resting state.
Imaging preprocessing
The fMRI data were preprocessed and analyzed using combined scripts of three software, AFNI (Medical College of Wisconsin, Milwaukee, Wisconsin, USA), FSL (Analysis Group, FMRIB, Oxford, UK), and Freesurfer (Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Boston, Massachusetts, USA). First, the anatomical and functional images were reconstructed and realigned, respectively, using a unified matrix. Thereafter, skull stripping and motion correction were performed, followed by coregistration between functional and anatomical images and normalization to the MNI152 standard brain. To remove low-frequency drift and high-frequency noises, all fMRI signals were filtered by band-pass filtering (0.007–0.1 Hz) and then spatially smoothed using a 6-mm full width at half maximum Gaussian kernel. To process unilaterally, the data on right facial paresis patients were flipped along the y-axis before processing. After preprocessing, the individual four-dimensional data were used for further group statistics and connectivity analyses.
Extraction of region of interest
The seed regions for functional connectivity analysis were extracted from task-state fMRI data using the following method. To begin, the statistical activation map of left facial paresis patients was calculated. On the basis of the map, the seed region was then extracted from the hand field in the left SI, with a diameter of 4 mm, containing 33 voxels (voxel size 2×2×2 mm). The seed region of right SI was extracted from the statistical activation map of right facial paresis patients in a similar manner.
Functional connectivity analyses
Functional connectivity of bilateral SI seed regions was computed. The temporal signal series of cerebrospinal fluid, white matter, and SI seed regions was first extracted. Thereafter, linear regression analysis was carried out to remove the six parameters of rigid transformation-based correction and the signal of cerebral spinal fluid and white matter. The individual statistical maps based on the general linear model were acquired.
Group analysis
Intergroup analysis was carried out using the paired t-test between preacupuncture and postacupuncture statistical maps. The significance threshold was set at z=2.3, P=0.01 to get the voxel size. Thereafter, the regions of interest were extracted to calculate the functional connectivity of the seed regions for all groups. The results of intergroup analysis were corrected using Monte Carlo simulation, with P=0.01, α=0.05, and cluster=68.
Results
The healthy control group
No significant changes in SI connectivity were observed.
The early group
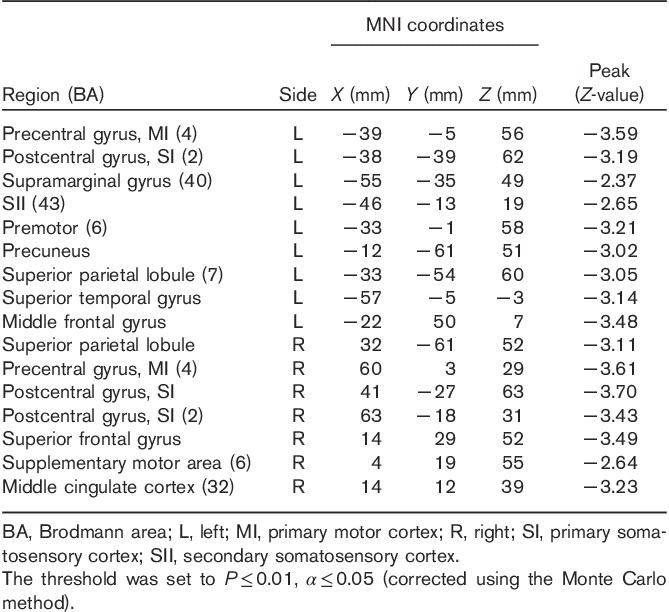
A significant decrease in right SI connectivity was observed in the bilateral SI, supramarginal gyrus (SMG), primary motor cortex (MI), left SII, middle temporal gyrus (MTG), and right superior parietal lobule (SPL; Fig. 1, Table 1). For left SI connectivity, a decrease was observed in bilateral SI, MI, SPL, left SMG, SII, premotor, precuneus, middle frontal gyrus, right superior frontal gyrus, supplementary motor area, and middle cingulate cortex (Fig. 2, Table 2).
Fig. 1.
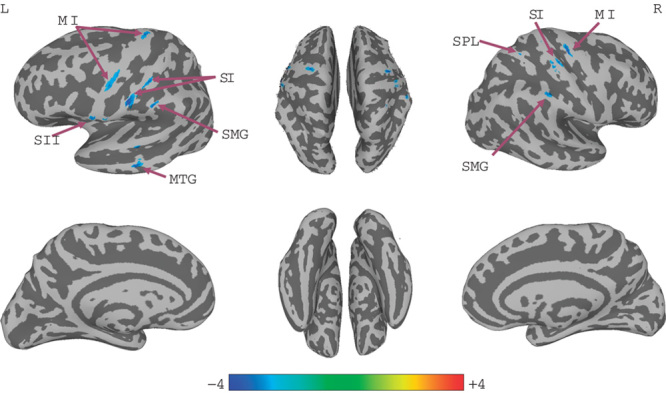
Connectivity-altered areas with right SI in the early group. P≤0.01, α≤0.05, corrected using the Monte Carlo method. MI, primary motor cortex; MTG, middle temporal gyrus; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; SMG, supramarginal gyrus; SPL, superior parietal lobule.
Table 1.
Group analysis results of areas with altered connectivity with right SI in the early group
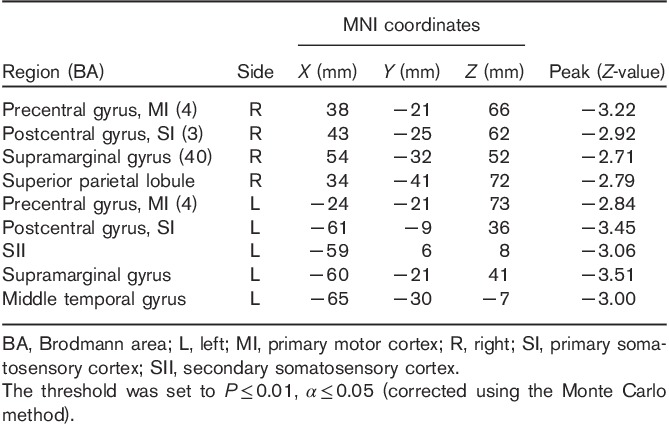
Fig. 2.
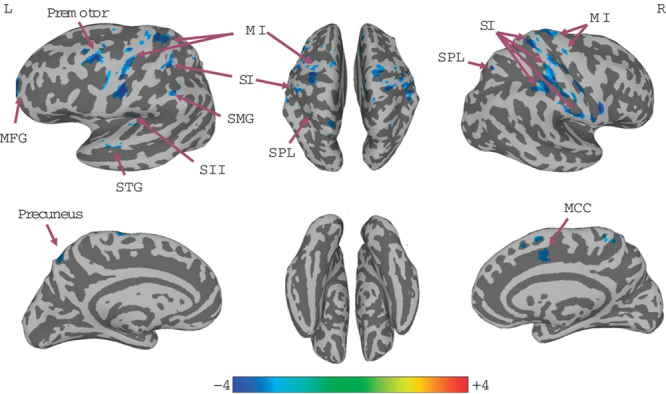
Connectivity-altered areas with left SI in the early group. P≤0.01, α≤0.05, corrected with the Monte Carlo method. MCC, middle cingulate cortex; MFG, middle frontal gyrus; MI, primary motor cortex; SFG, superior frontal gyrus; SI, primary somatosensory cortex; SMG, supramarginal gyrus; SPL, superior parietal lobule; STG, superior temporal gyrus.
Table 2.
Group analysis results of areas with altered connectivity with left SI in the early group
The late group
A significant increase in right SI connectivity was observed in the left SI, inferior parietal lobule, SII, inferior frontal gyrus, angular gyrus, precuneus, and posterior cingulate cortex (PCC; Fig. 3, Table 3). No significant changes in left SI connectivity were observed.
Fig. 3.
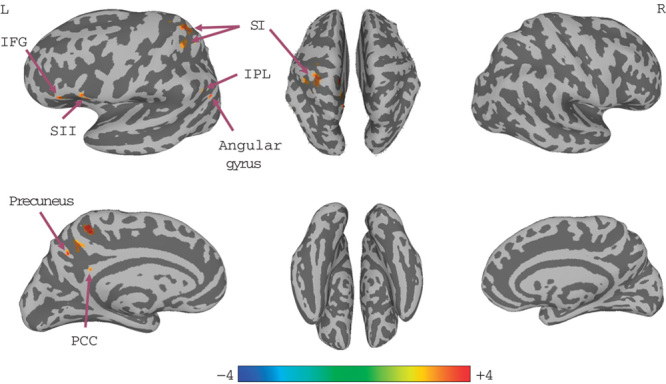
Connectivity-altered areas with right SI in the late group. P≤0.01, α≤0.05, corrected with the Monte Carlo method. IFG, inferior frontal gyrus; IPL, inferior parietal lobule; PCC, posterior cingulate cortex; SI, primary somatosensory cortex; SII, secondary somatosensory cortex.
Table 3.
Group analysis results of areas with altered connectivity with right SI in the late group
The recovered group
No significant changes in SI connectivity were observed.
Discussion
This study aims to investigate the effect of acupuncture on the functional connectivity of SI at different pathological stages of Bell’s palsy. In this part, we first discuss which areas are altered and then focus on what these changes might imply.
Overall tendency of the connectivity changes
On the basis of the results of group analysis, we found quite different SI connectivity changes at different pathological stages. Comparison of patients with healthy/healed controls showed that acupuncture did have an effect on SI connectivity. Besides, the contrast of early versus late also supported our hypothesis that acupuncture-induced connectivity changes varied with pathological stage. In the healthy control group, the brain’s connectivity status is supposed to be normal – that is, both the afferent and the efferent neuropaths in healthy individuals are intact. Therefore, as there were no pathologically affected brain regions that might have affected its functional status in healthy individuals, SI connectivity showed no significant difference in response to acupuncture. However, in the early group and the late group, considering that cortical reorganization occurred as the brain’s compensatory reaction to the loss of facial motor control 13 during recovery, it is inferred that such a process may have altered the brain’s connectivity status, leading to a different response to acupuncture compared with that in healthy individuals. In the healed group, given that brain’s functional connectivity status has generally recovered to a normal state, SI connectivity showed no significant changes as the results suggested, similar to that in the healthy control group.
Connectivity changes in specific areas in response to acupuncture
To further explain the underlying reason for the tendency, we discuss the altered areas and their pathological relation with the disease to find why the changes varied with pathological stage.
Somatosensory and motor association areas
A significant decrease in connectivity was observed in the parietal lobe (SI, SII, SPL, SMG, precuneus), frontal lobe (MI, supplementary motor area, premotor, superior frontal gyrus, middle frontal gyrus), temporal lobe (STG, middle temporal gyrus), and the middle cingulate cortex in the early recovery group. Such a decrease might have directly resulted from the imbalance caused by deefferentation without deafferentation due to Bell’s palsy. In healthy individuals, facial expressions are formed by the synergies of multiple facial muscles that are under elaborate and exact control of the brain 15. Therefore, the intactness of the sensorimotor circuit is crucial in maintaining such synergies and ensuring that the facial muscles are working properly. In patients with Bell’s palsy, however, facial nerve paresis causes the loss of movement feedback and breaks the intactness of the sensorimotor circuit, which may account for the disrupted connectivity within the affected cortical facial motor network 12,13, as well as the decreased connectivity of these somatosensory and motor-related regions in the early stage of the disease. However, as the onset duration increases, the brain’s ability to detect errors and monitor performance gradually compensates for the malfunctioning sensorimotor circuit by reorganizing the brain to increase processing between somatosensory and motor-related regions. Such increased processing may explain the increased connectivity between the somatosensory and motor-related regions including SI, SII, and the inferior frontal gyrus in the late stage. In summary, changes in both the early and the late group suggest that the functional connectivity status in patients with Bell’s palsy might differ from that in healthy participants, causing different responses to acupuncture.
DMN-related areas
Apart from sensorimotor areas, changes were also observed in precuneus, PCC, and the inferior parietal lobule (angular gyrus, SMG) during recovery from Bell’s palsy. The diversity of the altered regions suggests that the functional network of the human brain is very complicated, especially in the way in which these regions interact and are functionally connected 16. The angular gyrus, precuneus, and PCC are known to be associated with the brain’s default mode network (DMN), which is defined as a set of regions that is spontaneously active during passive moments 17. The intrinsic neural activity in these regions during a task-free period represents a complex and energy-consuming system, which consists of the prefrontal cortex, PCC, and precuneus, as well as the lateral parietal and temporal regions 18–20. Recent research on DMN in patients with diseases like Alzheimer’s disease 21 and schizophrenia 22 has suggested that exogenous and endogenous factors probably affect an individual’s inner homeostasis and his/her ability to properly regulate internal experiences like body states, feelings, and emotions by disrupting the integrity of the DMN 23. In other words, the DMN could affect homeostasis and the ability to appropriately regulate internal experiences such as body state, feelings, and emotions. A recent study found that acupuncture led to functional connectivity changes in the homeostatic afferent network only in functional diarrhea patients, possibly because of their network’s functional abnormality 24. Our results support the finding in some way, as the connectivity changes in DMN-related regions are also only observed in the recovery groups. It is inferred that acupuncture may have possibly influenced homeostasis in Bell’s palsy patients by modulating the connectivity of the brain in DMN-associated regions. Although whether such modulations would be beneficial to recovery still requires further investigations, it is to some extent in accordance with the traditional Chinese medical theory that acupuncture plays a homeostatic role 25 and its efficacy in patients and healthy individuals is divergent.
Limitations of this study
There were several limitations to this study. First, in this study, the data from right-face paralyzed patients were flipped along the y-axis before group analyses. Such a processing paradigm may cause some bias, although the factor of whether the data were flipped was considered when carrying out group analysis 13. Second, there was no sham acupuncture control used in the experiment. Thus, the changes observed in this study might not be induced by acupuncture but rather by the perception of receiving acupuncture. Therefore, further investigations with stricter control of these relevant variables are necessary to unveil the mechanism of acupuncture treatment.
Conclusion
The present study suggests that acupuncture induces functional connectivity changes in SI in patients with Bell’s palsy and that the changes vary with the pathological stage of the disease. These different changes induced by acupuncture are probably dependent on the brain’s functional connectivity status, which is affected by connectivity disruption in the acute stage and cortical reorganization during recovery. It is also inferred that acupuncture may help regulate homeostasis by modulating connectivity of DMN-associated regions in Bell’s palsy. Although further investigations are needed to confirm this inference, the finding hopes to shed light on the underlying mechanism of acupuncture for treating Bell’s palsy.
Acknowledgements
X. He and H. Wu participated in paper preparation. Y. Zhu and C. Xu participated in data processing and paper preparation. C. Li participated in experimental design, data acquisition, data processing, and paper preparation and revision. K. Park participated in experimental design. A. Mohamed participated in experimental design. W. Zhang participated in data acquisition. L. Wang participated in data acquisition. J. Yang participated in experimental design, data acquisition and paper preparation. B. Qiu participated in paper revision.
This study was supported by National Key Basic Research and Development Program (973) (2010CB530500), the National Natural Science Foundation of China (81202768, 31171083, 31230032), Anhui Provincial Natural Science Foundation (1208085MH147), and Major Scientific Projects of Anhui Provincial Education Commission (KJ2011ZD05). The authors thank Yue Qiu for her help in editing this paper.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Xiaoxuan He and Yifang Zhu contributed equally to the writing of this article.
References
- 1.Kwon HJ, Kim JI, Lee MS, Choi JY, Kang S, Chung JY, et al. Acupuncture for sequelae of Bell’s palsy: a randomized controlled trial protocol. Trials 2011; 12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandolfi M. The autumn of acupuncture. Eur J Intern Med 2012; 23:31–33 [DOI] [PubMed] [Google Scholar]
- 3.Ernst E, Lee MS, Choi TY. Acupuncture: does it alleviate pain and are there serious risks? A review of reviews. Pain 2011; 152:755–764 [DOI] [PubMed] [Google Scholar]
- 4.Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain 2011; 152:S41–S48 [DOI] [PubMed] [Google Scholar]
- 5.Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain 2008; 136:407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y, Bai L, Ren Y, Wang H, Liu Z, Zhang W, Tian J. Investigation of the large-scale functional brain networks modulated by acupuncture. Magn Reson Imaging 2011; 29:958–965 [DOI] [PubMed] [Google Scholar]
- 7.Zyloney CE, Jensen K, Polich G, Loiotile RE, Cheetham A, LaViolette PS, et al. Imaging the functional connectivity of the Periaqueductal Gray during genuine and sham electroacupuncture treatment. Mol Pain 2010; 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rijntjes M, Tegenthoff M, Liepert J, Leonhardt G, Kotterba S, Müller S, et al. Cortical reorganization in patients with facial palsy. Ann Neurol 1997; 41:621–630 [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Hao Y, Zhang Y, Liu J, Wang XY, Han JS, et al. Thirty minute transcutaneous electric acupoint stimulation modulates resting state brain activities: a perfusion and BOLD fMRI study. Brain Res 2012; 1457:13–25 [DOI] [PubMed] [Google Scholar]
- 10.Bai LJ, Qin W, Tian J, Liu P, Li L, Chen P, et al. Time-varied characteristics of acupuncture effects in fMRI studies. Hum Brain Mapp 2009; 30:3445–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Yang J, Sun J, Xu C, Zhu Y, Lu Q, et al. Brain responses to acupuncture are probably dependent on the brain functional status. Evid Based Complement Alternat Med 2013; 2013:175278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingner CM, Volk GF, Brodoehl S, Burmeister HP, Witte OW, Guntinas-Lichius O. Time course of cortical plasticity after facial nerve palsy: a single-case study. Neurorehabil Neural Repair 2012; 26:197–203 [DOI] [PubMed] [Google Scholar]
- 13.Klingner CM, Volk GF, Maertin A, Brodoehl S, Burmeister HP, Guntinas-Lichius O, Witte OW. Cortical reorganization in Bell’s palsy. Restor Neurol Neurosci 2011; 29:203–214 [DOI] [PubMed] [Google Scholar]
- 14.Smit A, van der Geest J, Metselaar M, van der Lugt A, VanderWerf F, De Zeeuw C. Long-term changes in cerebellar activation during functional recovery from transient peripheral motor paralysis. Exp Neurol 2010; 226:33–39 [DOI] [PubMed] [Google Scholar]
- 15.Cattaneo L, Pavesi G. The facial motor system. Neurosci Biobehav Rev 2014; 38:135–159 [DOI] [PubMed] [Google Scholar]
- 16.Van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20:519–534 [DOI] [PubMed] [Google Scholar]
- 17.Buckner RL. The brain’s default network: origins and implications for the study of psychosis. Dialogues Clin Neurosci 2013; 15:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003; 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 2001; 54:287–298 [DOI] [PubMed] [Google Scholar]
- 20.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common Blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 1997; 9:648–663 [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Li R, Fleisher AS, Reiman EM, Guan X, Zhang Y, et al. Altered default mode network connectivity in Alzheimer’s disease – a resting functional MRI and Bayesian network study. Hum Brain Mapp 2011; 32:1868–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ongür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 2010; 183:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008; 1124:1–38 [DOI] [PubMed] [Google Scholar]
- 24.Zhou S, Zeng F, Liu J, Zheng H, Huang W, Liu T, et al. Influence of acupuncture stimulation on cerebral network in functional diarrhea. Evid Based Complement Alternat Med 2013; 2013:975769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung L. Neurophysiological basis of acupuncture-induced analgesia--an updated review. J Acupunct Meridian Stud 2012; 5:261–270 [DOI] [PubMed] [Google Scholar]


