Supplemental Digital Content is available in the text.
Key Words: DRiPs-containing blebs (DRibbles), B cells, antigen cross-presentation, antitumor immunity
Abstract
Tumor-derived autophagosomes (DRibble) selectively capture tumor-specific antigens and induce a dramatic T-cell activation and expansion when injected into lymph nodes of naive mice. Both dendritic and B cells can efficiently cross-prime antigen-specific T cells. In this report, we demonstrated that a booster vaccination with naive B cells loaded with DRibbles eradicated E.G7-OVA tumors in mice that were previously treated with adoptive transfer naive OT-I T cells and intranodal immunization with DRibbles derived from E.G7 tumors. The antitumor efficacy was accompanied by a heighten number of tumor-specific interferon-γ-producing T cells and antibodies. However, the same treatment in the absence of adoptive T-cell transfer exhibited a limited efficacy. In contrast, when DRibble-loaded B cells were activated with CpG and anti-CD40 antibody before use as booster vaccines, established E.G7 tumors were completely eradicated in the absence of T-cell transfer. Therefore, our results document that B cells could efficiently cross-present tumor-specific antigens captured by DRibbles and suggest that naive B cells can be deployed as an effective and readily accessible source of antigen-presenting cells for cancer immunotherapy clinical trials.
Autophagy is a fundamental cellular process by which damaged proteins and other cellular waste are sequestered and recycled via degradation mediated by the lysosome and proteasome pathways.1 It has been shown previously by us that a broad array of tumor antigens, including long-lived proteins, short-lived proteins (SLiPs), and defective ribosomal products (DRiPs) could be sequestered into autophagosomes when function of lysosomes and proteasomes are inhibited.2–4 We termed these DRiPs-containing autophagosome-rich blebs as DRibbles—DRiPs in blebs. DRibbles are efficient carriers of tumor antigens for effective cross-presentation by dendritic cells (DCs). Indeed, DCs loaded with DRibbles could eradicate Lewis lung tumors (3LL) and significantly delay the growth of B16F10 melanoma.4
DCs have been widely used as professional antigen-presenting cells (APCs) to induce immunity against various types of cancer and pathogens.4–6 However, clinical applicability of DCs is often limited by their rarity in blood (<1% of total leukocytes in blood) and inefficient migration into lymphoid organs when in vitro monocyte-derived DCs are administrated.7 Therefore, B cells are sought after as alternative pAPC sources because they can be easily isolated and expanded on a large scale from nonstem cell sources.8,9 Although it is well known that very small of percentage of in vitro-cultured DCs migrate into lymph nodes; activated B cells readily migrate to secondary lymphoid organs with high expression of CD62L.9,10 Multiple studies have demonstrated that activated B cells can induce antigen-specific CD4+ and CD8+ T-cell responses and subsequently lead to antigen-specific antitumor activity.8,11–13 We have previously shown that B cells could directly capture DRibbles and present encapsulated antigens to activate primed T cells. DRibbles could induce B-cell activation, antibody production, and cytokine secretion in a TLR2/MyD88-dependent manner.14 In this study, we investigated whether DRibble-loaded B cells could induce antigen-specific T cells in tumor-bearing mice and sought to determine whether vaccination with DRibble-loaded B cells could mediate tumor regression in murine tumor models.
MATERIALS AND METHODS
Ethics Statement
All experimental protocols were approved by the Institution of Animal Care and Use Committee of Medical School of Southeast University.
Mice, Cell Lines, and Reagents
C57BL/6 and BALB/c female mice were purchased from the Comparative Medicine Center, Yangzhou University (Yangzhou, China). OT-I T-cell receptor transgenic mice (recognize the H-2Kb-restricted OVA257–264 peptide) were gifts from Dr Sheng Xia (Medical School of Jiangsu University, Zhenjiang, China). All mice were bred and maintained in a specific pathogen-free environment. E.G7-OVA and BNL cell line were purchased from ATCC. B16F10 melanoma cell line was gifts from Dr Dou Jun (Medical School of Southeast University, Nanjing, China). HepG2.2.15 cell line was gifts from Dr Jianqiong Zhang (Medical School of Southeast University, Nanjing, China). All tumor cells were cultured in complete medium made of RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, 10 μg/mL streptomycin (Life Technology or Beyotime Institute of Biotechnology, China). HepG2.2.15 cells were maintained in DMEM (Gibco, Grand Island, NY) complemented with 10% heat-inactivated FCS (Hyclone, Logan, UT), 100 U/mL penicillin, and 100 μg/mL streptomycin (Beyotime Institute of Biotechnology, China). No mycoplasmas or filamentous fungus were detected in all the cell lines and DRibble preparations by the Clinical Laboratory of the Affiliated Zhongda Hospital of Southeast University.
Preparation of DRibbles
Autophagosome-enriched DRibbles were prepared as described previously.3 Briefly, tumor cells were treated with 100 nM Rapamycin (Enzo Life Sciences, Shanghai, China), 100 nM Bortezomib (Millennium pharmaceuticals, Cambridge, MA), and 10 mM ammonium chloride in complete medium for 18–24 hours in a 5% CO2 incubator at 37°C. The cells were harvested and centrifuged at 1000 rpm to remove cells and large cell debris. The supernatant was then centrifuged at 12,000 rpm to collect the DRibbles secreted by tumor cells. The total protein content of DRibbles was quantified with the bicinchoninic acid method according to the manufacturer’s protocol (Beyotime Institute of Biotechnology, China).
In vitro Carboxy-Fluoresceindiacetatesuccinimidylester (CFSE) Dilution Assay
For cross-presentation assay, OT-I mice were vaccinated with DRibbles (30 μg total protein) via intranodal administration and primed CD8+ T cells were purified with anti-mouse CD8 antibody–conjugated Dynal beads according to the manufacturer’s protocols (Invitrogen, Carlsbad, CA) from injected lymph nodes 8 days after DRibble injection. Purified CD8+ OT-I T cells were used as the responder cells and they were stimulated with DRibbles alone, purified B cells (3×106/mL) loaded with DRibbles or whole tumor cell lysate (10 μg/mL total proteins). Medium alone and OT-I peptide SIINFEKL (100 ng/mL) were included as the negative and positive controls. Cross-presentation of OVA-containing DRibbles by B cells was measured by flow cytometry analysis of CFSE-labeled primed OT-I CD8+ T cells. Purified B cells were isolated by negative selection using anti-CD43 coupled magnetic beads and performed as described (Invitrogen, Carlsbad, CA).
Immunotherapy Experiments and Detection of Immune Responses
To establish tumor-bearing mice, C57BL/6 or BALB/c mice were injected subcutaneously with 5×105 E.G7-OVA tumor cells or 2×106 BNL tumor cells, respectively. On day 6, these mice were injected with DRibbles (30 μg total protein each) into both inguinal lymph nodes and some mice also received adoptive transfer of OT-I spleen cells (1×107). Two intravenous injections of 5×106 B cells or DC cells with and without loading of DRibbles or with and without combination of CpG-DNA (10 μM) and anti-CD40 (5 μg/mL; BD Bioscience) were given to mice 3 and 6 days after intranodal immunization with DRibbles. Untreated mice served as controls. Tumor volume and percentage of survival mice were determined over time. DCs were isolated from spleens of mice after sequential intravenous injections of plasmid DNA encoding murine Flt3L and GM-CSF as described previously.4
On the 10th day after intranodal immunization, lymphocytes were harvested from lymph nodes and spleens of the vaccinated mice. The lymphocytes were seeded into a 96-well round-bottomed plate at 1×106 cells/well. DRibbles (10 μg/mL) were added to each well. After 12 hours of stimulation, brefeldin A (10 μg/mL; Sigma-Aldrich, St. Louis, MO) was added to the culture for another 6 hours before cells were harvested and stained for flow cytometry analysis. After 72 hours of stimulation, the interferon(IFN)-γ concentration in the supernatants was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (eBioscience, San Diego, CA). OT-I T cells were gated after they were stained with PerCP-labeled anti-mouse T-cell receptor Vβ5.1/5.2 mAb and APC-labeled anti-mouse CD8 (eBioscience, San Diego, CA). In some experiments, the lymphocytes were stained with APC-labeled anti-mouse Thy1.1 antibody (eBioscience, San Diego, CA) and MHC Dextramer H-2Kb/SIINFEKL conjugated with PE (ImmuDEX, Copenhagen, Denmark).
On the 14th day after intranodal immunization, tumors were collected, enzymatically digested into single-cell suspension, and stained with FITC-labeled anti-mouse CD4, FITC-labeled anti-mouse CD8, or APC-labeled anti-mouse CD8 antibodies (eBioscience, San Diego, CA). Flow cytometry analysis was performed to determine tumor-infiltrating T cells.
To examine the antibody production induced by vaccines, serum samples were collected on the 14th day after the first injection of DRibbles. Ninety-six-well plates were coated with tumor lysate overnight at 4°C and ELISA was performed to detect antigen-specific IgM and IgG in serum samples according to the manufacturer’s protocol.
Antibody-dependent Cellular Cytotoxicity (ADCC) Assay
BALB/c mice were injected intravenously with DRibbles (30 μg total protein) 3 times on days 1, 2, and 3, respectively. Sera were collected from the orbital sinuses of mice on the seventh day after first injection and stored at −20°C. Splenocytes were used as effector cells after expansion with IL-2 (2000 U/mL) for 7 days. The BNL cells and HepG2.2.15 cells were used as target tumors cells. Target and effector cells were suspended in RPMI 1640 media supplemented with 1% FBS in the presence of the sera from mice immunized with BNL DRibbles. Four hours later, the cultured supernatants were harvested for analysis of the levels of released aspartate transaminase (AST). The AST detection was performed in the Clinical Laboratory of the Affiliated Zhongda Hospital of Southeast University.
Statistical Analysis
Log-rank nonparametric analysis was used to analyze the tumor-free survival data. Each group consisted of at least 5 mice, and no animal was excluded from the statistical evaluation. The mean±SEM. was determined for each treatment group in the individual experiments. Two-tailed t test was used to compare treatment groups with the control when significant differences were observed. Graphpad Prism 5.0 (Graphpad Software, San Diego, CA) was used for all statistical analysis.
RESULTS
B Cells Loaded With DRibbles were Efficient APCs at Activating Primed CD8+ T Cells
Whereas cross-priming of naive T cells is typically restricted to DCs, other APCs such as B cells and macrophages are known to efficiently restimulate primed T cells.15,16 To test whether DRibbles could stimulate antigen-specific responses of primed T cells when loaded onto B cells, we generated primed T cells by intranodal injection of DRibbles derived from E.G7-OVA tumor cells into OT-I transgenic mice. Using these primed OT-I CD8+ T cells as the responder cells in a CFSE dilution assay, we found that purified B cells (98.1% CD19+ 0.3% CD11c+, Fig. 1A) were capable of efficient restimulation of primed T cells (Fig. 1B). The proliferation of primed OT-I CD8+ Tcells induced by OVA+ DRibbles-loaded B cells (24.6% CFSE dilution) was significantly greater than that induced by DRibbles alone (3.5% CFSE dilution), B cells alone (6.6% CFSE dilution), and B cells (9.8% CFSE dilution) loaded with an equivalent amount (10 μg total protein) of tumor lysates (Figs. 1B, C). These data indicated that B cells loaded with DRibbles were efficient in activating effector CD8+ T cells in vitro, a process of being independent of other pAPCs.
FIGURE 1.
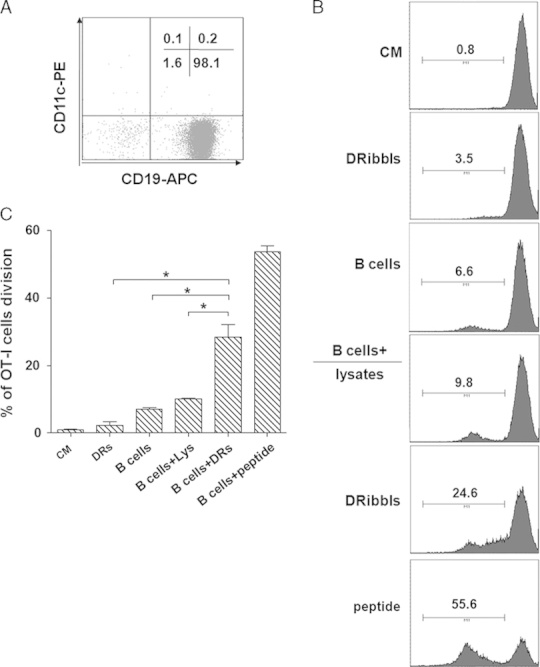
B cells loaded with DRibbles were efficient antigen-presenting cells (APCs) at restimulating primed CD8+ T cells. A, B cells purified from the C57/BL6 mice were analyzed by flow cytometry for CD19 and CD11c expression. B, Histogram and (C) bar graph were shown. DRibbles were collected from EG7-OVA tumor cells expressed OVA protein. B cells were stimulated with or without DRibbles [or whole tumor cell lysate (10 μg/mL total proteins, or 0.1 μg/mL OT-I SIINFEKL peptide)], or DRibbles alone (10 μg/mL) were then coincubated with CFSE-labeled effector OT-I CD8+ T cells. Activation of T cells was assessed by CFSE dilution on day 5. Percentage of divided OT-I T cells is shown as the mean±SEM. Data are representative of results from 2 to 4 independent experiments.
DRibble-loaded B Cells Enhanced Immune Responses and Mediated Tumor Regression When given as Booster Vaccines to Mice after Direct Intranodal DRibble Immunization
Direct intranodal injection is the most efficient route for DRibble immunization. Previously, we showed that the antitumor efficacy of DRibble vaccine in tumor-bearing mice could be enhanced by combining vaccine with treatment of T-cell costimulation antibodies.17 Here, we investigated whether DRibble-loaded B cells could also enhance the antitumor efficacy of DRibble vaccines delivered intranodally. Tumor-bearing C57BL/6 mice were established via subcutaneous injection of 5×105 E.G7-OVA lymphoma cells. Mice with palpable tumors (6 d after tumor inoculation) were immunized with intranodal injection of DRibbles along with adoptive transfer of naive OT-I T cells. Two intravenous injections of DRibbles-loaded B cells, unloaded B cells, or PBS were given at days 3 and 6 after the injection of DRibble injection (Fig. 2A). We found that vaccination with DRibbles alone slowed the tumor growth (Fig. 2B) and improved the survival of mice (53 d of median survival) (Fig. 2C) compared with the untreated control (28 d of median survival). A single DRibble immunization caused a temporary halt in tumor growth, the tumors underwent transient regression at the peak of the primary OT-I expansion, but recurred rapidly with no long-term survivors (Fig. 2C). Remarkably, booster vaccinations with DRibble-loaded B cells significantly enhanced the therapeutic efficacy of the DRibble vaccine and prolonged the median survival time to >84 days (P<0.05) compared with other groups that received booster vaccination with PBS (28 d of median survival) or unloaded B cells (56 d of median survival). Most importantly, boosting with DRibble-loaded B cells can lead to complete eradication of established tumors (Figs. 2B, C). The frequency of Vβ5.1/5.2+ CD8+ OT-I T cells in the spleen was measured by flow cytometry analysis. Results showed that the percentages of Vβ5.1/5.2+ CD8+ T cells among total splenocytes were markedly increased after administration of DRibbles-loaded B cells in comparison with other groups (Fig. 2D). In addition, flow cytometry analysis using Kb-OVA257–264 Dextramer to stain T cells that specifically recognize OVA257–264 in the context of H-2Kb revealed a 10-fold increase in the number of T cells specific for OVA257–264 when mice received additional booster vaccination with DRibble-loaded B cells (Supplementary Figs. 1A and B, Supplementary Digital Content 1, http://links.lww.com/JIT/A344). These results demonstrated that B cells can cross-present DRibble antigens and reactivate effector T-cell responses in vivo.
FIGURE 2.
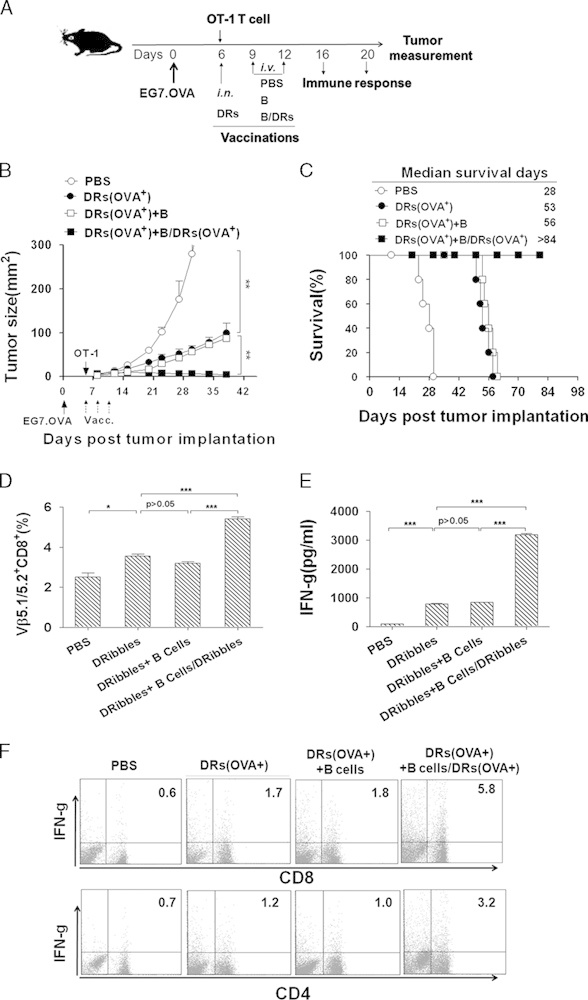
Vaccination with B cells pulsed with EG7.OVA cell-derived DRibbles and adoptively transfer of OT-I T cells eradicated E.G7-OVA tumors. A, Experimental scheme illustrates the immunotherapy protocol. C57BL/6 mice (n=5) with preestablished EG7 tumors (previously injected with 5×105 EG7 tumor cells) were treated with DRibbles injected directly into both inguinal lymph nodes and received adoptive transfer of OT-I spleen cells (1×107). Two intravenous injections of 5×106 DRibbles-loaded B cells or unloaded B cells or PBS were given 3 and 6 days after the first injection of DRibbles. Untreated mice served as the controls. Tumor volume (B) and percentage of survival (C) in mice bearing subcutaneous tumors were monitored over time. Lymphocytes were collected from lymph nodes and spleens of vaccinated mice on the 10th day after the first immunization. (D) The spleen cells were stained with antibodies against OT-1 clonal T-cell receptor Vβ antibodies and CD8. The expression of Vβ5.1/5.2+ and CD8+ on T cells was analyzed by flow cytometry. The percentage of divided OT-I T cells is shown as mean±SEM derived from 3 mice per group (n=3). E and F, The lymphocytes were restimulated with DRibbles in vitro. Supernatants were harvested for detection of secretory interferon (IFN)-γ by ELISA after 72 hours (E) or the intracellular IFN-γ staining were performed to determine the frequency of antigen-specific T cells after 12 hours (F). *P<0.05, ***P<0.001. Data are representative of 3 independent experiments with similar results.
The fact that booster vaccination with DRibble-loaded but not unloaded B cells significantly enhanced antitumor immunity suggested that DRibble-induced T-cell recall responses played an important role. Consistent with this notion, after ex vivo stimulation with DRibbles, both CD4+ and CD8+ T cells harvested from mice that received booster vaccines of DRibbles-loaded B cells produced a significantly higher level of IFN-γ compared with T cells from mice that were boosted with PBS or unloaded B cells (Figs. 2E, F). These results demonstrated that B cells could cross-present DRibble antigens and reactivate effector T-cell responses in vivo.
To examine the humoral immune response to the vaccination, serum samples were collected on the 14th day after the first injection of DRibbles. ELISA analysis showed that the levels of tumor antigen-specific serum IgM (Supplementary Fig. 2A, Supplementary Digital Content 2, http://links.lww.com/JIT/A345) and IgG (Supplementary Fig. 2B, Supplementary Digital Content 2, http://links.lww.com/JIT/A345) were significantly elevated after boosting of DRibble-loaded B cells as compared with mice that received a booster injection of unloaded B cells or PBS.
Boosting of DRibble-loaded B Cells Suppressed Tumor Growth but Generated no Cure Without Adoptive Transfer of OT-I T Cells
Next, we investigated the antitumor efficacy of DRibble vaccine in the absence of adoptive transfer of exogenous T cells. Three and 6 days after the first intranodal injection, the mice were injected with DRibble-loaded B cells or DCs, unloaded B cells, or PBS (Fig. 3A). The untreated mice served as controls. As compared with experiments shown in Figure 2, vaccination with DRibbles alone was less effective and only slightly slowed tumor growth (Fig. 3B) and improved survival (Fig. 3C, median survival of 27 vs. 21 d of untreated mice). Only boosting with DRibbles-loaded B cells significantly delayed tumor growth (Fig. 3B) and doubled the median survival (Fig. 3C, median survival of 42 vs. 21 d of untreated mice). No difference was observed when unloaded B cells were used as the boost vaccines. It is interesting to note that, the antitumor efficacy mediated by B cells and DCs appeared to be comparable, suggesting that B cells could serve as efficient APCs to boost DRibble-induced antitumor efficacy (Figs. 3B, C).
FIGURE 3.
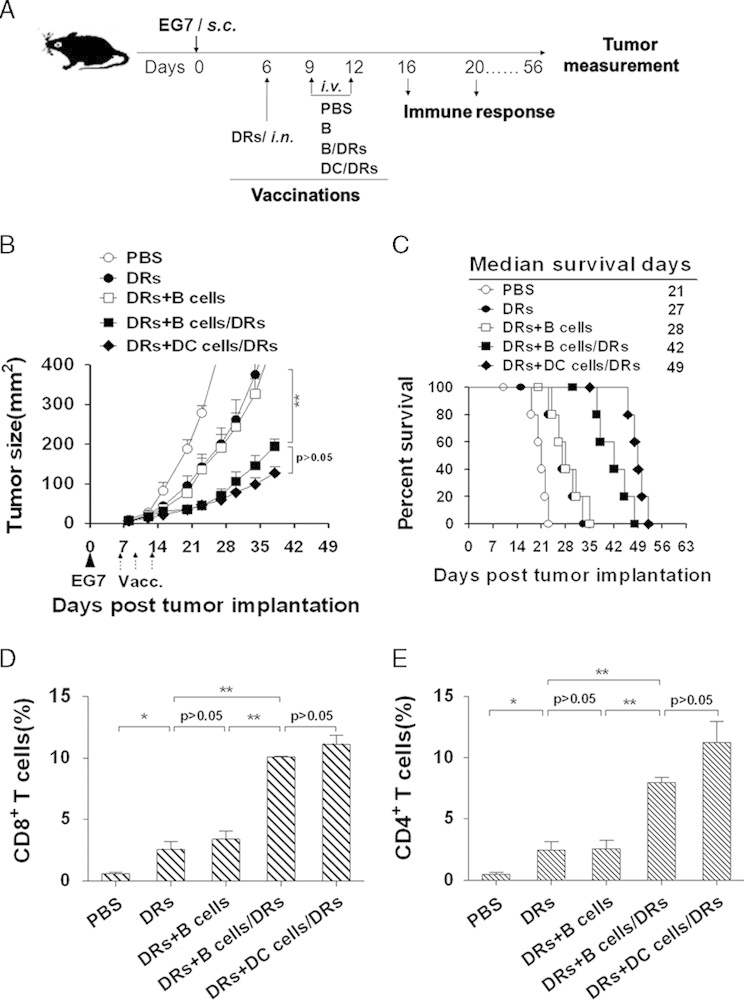
Boosting with DRibble-loaded B-cell vaccine suppressed the tumor growth but generated no cure without adoptive transfer of OT-I T cells in an established E.G7 murine thymoma model. A, Experimental scheme. C57BL/6 mice (n=5) with preestablished EG7 tumors (previously injected with 5×105 EG7 tumor cells) were first treated with DRibbles injected directly into both inguinal lymph nodes. Two intravenous injections of 5×106 DRibbles-loaded B cells or dendritic cells (DCs), unloaded B cells or PBS were given 3 and 6 days after the first injection of DRibbles. Untreated mice served as controls. Tumor volume (B) and percentage of survival (C) in mice bearing subcutaneous tumors were monitored over time. D and E, The tumor cells were stained with antibodies against CD4 and CD8. The frequency of CD8+ and CD4+ T-cell infiltrating tumors was analyzed by flow cytometry. Percentages of CD8+ (D) and CD4+ (E) T cells are shown as mean±SEM derived from 3 mice per group (n=3). *P<0.05, **P<0.01, ***P<0.001. The typical result from 3 independent experiments was shown.
To determine whether the antitumor efficacy induced by DRibble-loaded B-cell vaccines associated with T-cell infiltration into tumors, the tumor tissues were collected on the 14th day after the first injection of DRibbles, and the frequency of CD8+ and CD4+ T cells among the tumor tissues was measured by the flow cytometry analysis. Results showed that the percentages of CD8+ T cells (Fig. 3D) and CD4+ T cells (Fig. 3E) among the tumor tissues were markedly increased after the booster vaccination with DRibbles-loaded B cells or DCs when compared with other groups. Little difference was observed between B cells and DCs.
Both the lymph nodes and spleens of the mice were collected and processed into single-cell suspension on the 10th day after the first injection of DRibbles. The absolute number of spleen cells was counted. Results showed that the size of the spleen was markedly larger (Fig. 4A) and the total number of splenocytes increased significantly (Fig. 4B) if mice received DRibble-loaded B cells or DCs booster vaccines when compared with mice that received DRibbles alone, booster with unloaded B cells or PBS. After 72 hours of coincubation of lymphocytes and DRibbles in vitro, clusters of activated lymphocytes were readily observed when lymphocytes were harvested from mice that received DRibble-loaded B cells or DCs booster vaccination (Fig. 4C). Consistent with previous results, ELISA (Fig. 4D) analysis showed that lymphocytes harvested from mice that received a booster vaccine with DRibble-loaded B cells or DCs produced a significantly higher amount of IFN-γ compared with lymphocytes from other groups of mice. Similar to the results obtained from experiments in Figure 2, the levels of tumor antigen-specific serum IgM and IgG were significantly increased after boosting with DRibble-loaded B cells (data not shown).
FIGURE 4.
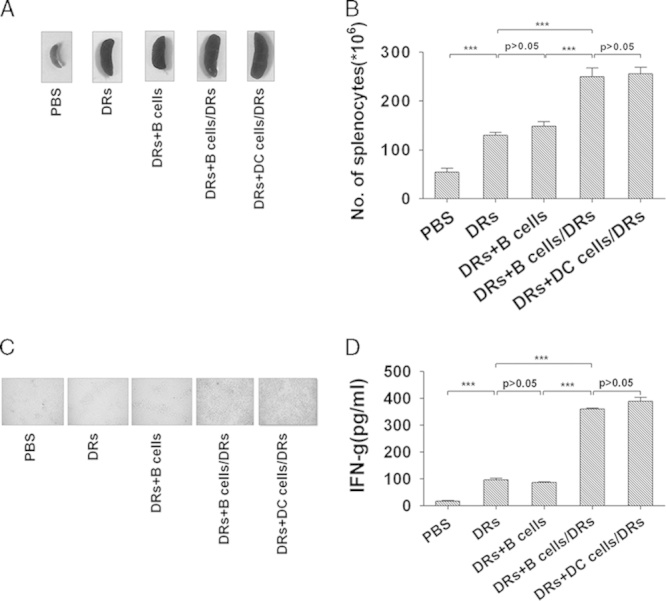
Detection of interferon-γ-producing T cells in vaccinated E.G7 tumor-bearing mice after restimulation with DRibbles. Lymphocytes were collected from lymph nodes and spleens of vaccinated mice on the 10th day after the first immunization. A, Representative spleens from each group mice were shown. B, Total number of splenocytes of each group mice was counted. The lymphocytes were coincubated with DRibbles for 72 hours. C, Images were taken after 72 hours of incubation (×200). D, Supernatants were harvested for detection of secretory interferon (IFN)-γ by ELISA. **P<0.01, ***P<0.001. Data presented were obtained as a result of triplicates.
Booster Vaccination With DRibble-loaded B Cells Delayed Tumor Growth in an Established BNL Murine Hepatocellular Carcinoma Model
To test our therapeutic vaccination strategy in a different model without the complications of model antigens such as OVA, we chose BNL hepatocellular carcinoma which originated from BALB/c mice.18 Tumor-bearing BALB/c mice were established via subcutaneous injection of mice with 2×106 BNL tumor cells, and mice with palpable tumor were vaccinated with DRibbles from BNL tumor cells directly into both inguinal lymph nodes on day 6 after tumor injection. Following the initial intranodal injection, 2 intravenous injections of DRibbles-loaded B cells or unloaded B cells were given 3 and 6 days after the first injection of DRibbles (Fig. 5A). The untreated mice served as controls. In this model, 1 single injection of DRibbles had no significant antitumor effect; however, booster vaccination with DRibble-loaded B cells elicited marginal or minimal therapeutic efficacy (Fig. 5B) and improved the survival of tumor-bearing mice (Fig. 5C) as compared with the control treatment. The flow cytometry analysis showed that the percentages of tumor-infiltrating CD8+ T cells (Fig. 5D) and CD4+ T cells (Fig. 5E) were markedly increased after boosting with DRibble-loaded B cells as compared with the other groups.
FIGURE 5.
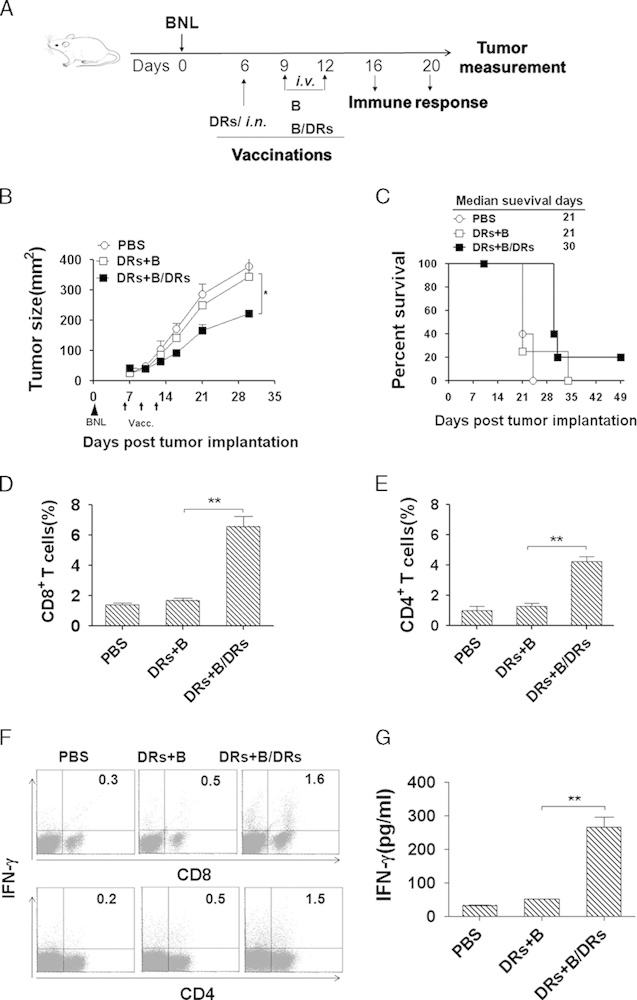
Booster vaccination with DRibble-loaded B cells delayed the tumor growth in an established BNL murine hepatocellular carcinoma model. A, B-cell therapy scheme in an established BNL murine hepatocellular carcinoma model. Immunocompetent BALB/c mice (n=5) with preestablished BNL tumors (previously injected with 2×106 BNL tumor cells) were treated with DRibbles injected directly into both inguinal lymph nodes. Two intravenous injections of 5×106 DRibbles-loaded B cells or unloaded B cells were given 3 and 6 days after the first injection of DRibbles. Untreated mice served as controls. Tumor volume (B) and percentage of survival (C) in mice bearing subcutaneous tumors were monitored over time. D and E, The frequency of CD8+ and CD4+ T cells infiltrated the tumors was analyzed by flow cytometry. Percentages of CD8+ (D) and CD4+ (E) T cells are shown as mean±SEM derived from 3 mice per group (n=3). F and G, Lymphocytes were collected from lymph nodes and spleens of vaccinated mice were restimulated with DRibbles. The frequency of interferon (IFN)-γ-producing T cells and secreted IFN-γ were detected by intracellular cytokine staining 12 hours later (F) and ELISA 72 hours later (G), respectively. *P<0.05, **P<0.01. Data are representative of 3 independent experiments with similar results.
To further determine whether boosting of DRibbles-loaded B cells could enhance both the cellular and humoral immune response in the established murine hepatocellular carcinoma model, lymphocytes were harvested from lymph nodes and spleens of BNL tumor-bearing mice and restimulated with DRibbles. The intracellular cytokine staining (Fig. 5F) and ELISA (Fig. 5G) analyses showed that lymphocytes harvested from mice that received booster vaccines with DRibbles-loaded B cells produced significantly higher levels of IFN-γ than lymphocytes from untreated mice or mice that were treated with unloaded B cells.
We found that the levels of tumor antigen-specific serum IgM and IgG (Supplementary Figs. 3A and B, Supplementary Digital Content 3, http://links.lww.com/JIT/A346) were significantly elevated after boosting of DRibble-loaded B cells. In addition, we also examined whether DRibbles-induced antibody production in vivo could mediate ADCC against the tumor cells. The results showed that BNL cells released much higher levels of AST in the presence of the antibody induced by DRibble vaccines. In contrast, the presence of the antibody had no significant effect on AST secretion when the target cells are human HepG2.2.15 tumor cells (Supplementary Fig. 3C, Supplementary Digital Content 3, http://links.lww.com/JIT/A346). These results suggested that DRibble-induced antibodies might also play an important role in the antitumor efficacy.
TLR9 and CD40 Stimulation of DRibble-loaded B Cells Enhanced their Antitumor Effect
The partial B-cell activation by DRibbles is one possible reason why a limited antitumor effect was observed in mice that received vaccines in the absence of T-cell transfer. Thus, we tested whether the antitumor efficacy of DRibble-loaded B-cell booster vaccine could be further augmented if they were activated with known B-cell stimulators, such as CpG and anti-CD40 antibody. Activation via CD40 receptor increases the antigen-presenting ability of B cells.8,12 CpG induces B cells to produce a sufficient amount of IL-12 and polarizes the immune response to Th1 type.19,20 Hence in the EG7 tumor model, we used CpG in combination with anti-CD40 antibody to treat B cells during DRibble loading to improve the efficacy of the booster vaccine. Mice bearing 6-day established EG7 tumor were first primed with DRibbles directly into both inguinal lymph nodes, and boosted with 2 intravenous injections of DRibble-loaded B cells, unloaded B cells, DRibble-loaded B cells that were activated with CpG and anti-CD40 antibody (Fig. 6A). Mice that received DRibble-loaded B cells without additional stimulation with CpG and anti-CD40 survived longer than mice in the control groups but no mice were cured. Remarkably, CpG and anti-CD40 greatly enhanced the therapeutic efficacy of the DRibble-pulsed B-cell vaccine. All of these mice experienced complete tumor regression and remained tumor free for 60 days till the experiment was terminated (Figs. 6B, C).
FIGURE 6.
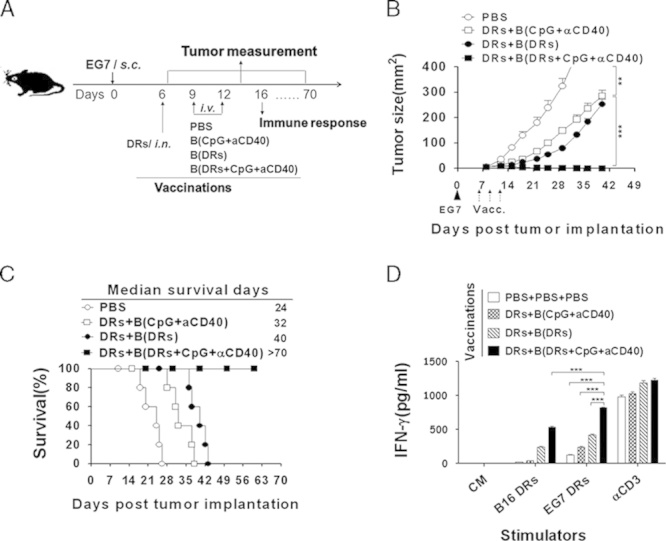
TLR9 and CD40 stimulation of DRibble-loaded B cells completely regressed established tumors in an E.G7 murine thymoma model. A, Experimental scheme. The E.G7 tumor-bearing mice were established as described in Figure 2 with without adoptive transfer of OT-I T and B cells loaded with DRibbles in the presence of CpG and anti-CD40 antibodies. Tumor volume (B) and percentage of survived mice (C) were recorded. D, Lymphocytes collected from lymph nodes and spleens of vaccinated mice were coincubated with EG7-DRibbles, B16F10-Dribbles, and anti-CD3 for 72 hours. The supernatants were harvested for the detection of secretory interferon (IFN)-γ by ELISA. ***P<0.001. A representative result from 3 independent experiments is shown.
Consistent with the remarkable antitumor efficacy, ELISA (Fig. 6D) analyses of lymphocytes from EG7-bearing mice that were restimulated with EG7-DRibbles, B16F10-Dribbles, and anti-CD3, showed that lymphocytes harvested from mice that received booster vaccinations with DRibble-loaded B cells in combination with CpG and anti-CD40 antibody produced a significantly higher level of IFN-γ than lymphocytes from mice of other treatment groups when restimulated with EG7-DRibbles. In contrast, these vaccinations did not affect the produced level of IFN-γ when restimulated with anti-CD3. Consistent to our previous results,21 we found that the autophagosome vaccine induced immune responses against heterologous tumors. Furthermore, mice cured by DRibble-loaded B cells were found to be completely resistant to a secondary tumor challenge (Supplementary Fig. 4, Supplementary Digital Content 4, http://links.lww.com/JIT/A347). These results highlighted the potential of DRibble-loaded CpG-licensed and CD40-licensed B cells as effective vaccines for cancer immunotherapy.
DISCUSSION
The concept of cancer immunotherapy can be traced back to the late 19th century22; however, cancer immunotherapy is still considered to be at an early stage, particularly for the development of therapeutic cancer vaccines. Cancer vaccines come in many varieties, from undefined antigens such as whole tumor cells, lysates, or subcellular components of tumor cells to known tumor-associated antigens in the form of peptides, proteins, DNA, and RNA, sometimes delivered in viral and bacterial vectors.23,24 Results from decades of clinical trials using vaccines to actively treat cancers only garnered limited success.25 Beside the immune suppression, we believe that the major cause of unsatisfying clinical outcomes lies in the poor cross-presentation of tumor-associated antigens that resulted in weak T-cell-mediated immune responses. We further postulated that DRiPs and SLiPs in tumor cells, which typically degraded rapidly by the proteasome and lysosome pathways, are not sampled by APCs thus not efficiently cross-presented to T cells under normal conditions. By inhibition of lysosome/proteasome activity, DRiPs and SLiPs flock to autophagosomes so that they become a readily available substrate for cross-presentation. Therefore, the autophagosome-based DRibbles vaccine can facilitate the efficient cross-presentation of multiple antigens to CD8+ T cells, a prerequisite for the development of effective antitumor response.2–4
Moreover, many DAMP molecules are present in the DRibbles vaccine, such as HSP90 (heat shock protein), HSP94,2,4 and HMGB1. HMGB1 (High Mobility Group Box 1), a chromatin-associated nuclear protein and DAMP molecule released by necrotic cells, has been shown to promote and sustain autophagy.26–30 These DAMP molecules in the DRibbles could serve as a natural adjuvant. Our preliminary data indicate that HMGB1 is involved in the DRibble-mediated B-cell activation and might suggest that HMGB1 could be active as an endogenous adjuvant that is released from tumor cells and stored in autophagosomes. Therefore, the DRibbles vaccine that incorporates SLiPs, DRiPs, DAMP molecules, and multiple epitopes or undefined antigens derived from tumor cells can elicit enhanced antitumor immune responses compared with simple peptide, protein, or tumor cell vaccines.4
Another critical step for therapeutic cancer vaccines is the requirement of DCs to elicit antitumor T-cell immune responses. The rarity of DCs in peripheral blood and inefficient trafficking of ex vivo expanded DCs into the secondary lymph tissues post critical barriers of cancer vaccines even if a large number of monocyte-derived DCs could be readily generated with recombinant cytokine in vitro.7 As another class of professional APCs, B cells can process and present exogenous antigens in the context of MHC class II molecules.31,32 B cells also express high levels of MHC class I33,34 and, in certain circumstance, they are very efficient pAPCs for antigen cross-presentation.35–37 Therefore, they can serve as an alternative source of APC cells in cell-based vaccines for immunotherapy.8,11–12,38 In contrast to DCs, B cells are relatively abundant in peripheral blood (20%–30% of blood leukocytes) and can readily be expanded in vitro from nonstem cell sources.8,9 More importantly, compared with DCs, activated B cells readily migrate to secondary lymphoid organs with high expression of CD62L.9,10 A growing body of evidence has indicated that B-cell-derived APCs are comparable with DCs regarding their ability to present antigen in vivo.39 We have previously shown that DRibble-loaded DCs vaccines could prime T cells and induce potent antitumor response.4 In this study, we demonstrated that B cells loaded with tumor cell-derived DRibbles could effectively recall antigen-specific T cells in vitro. More importantly, we further showed that boosting of DRibble-loaded B cells could markedly enhance antigen-specific immune responses in vivo and elicit protective antitumor efficacy in mice bearing EG7-OVA tumor and BNL hepatoma. It is interesting to note that, B-cell and DC antitumor efficacy in EG7-bearing mice primed with intranodal vaccination with DRibbles appeared comparable.
Although DRibbles alone could induce B-cell activation, their ability to activate could be further augmented by Toll like receptor-9 (TLR9) ligands and CD40 cross-linking. When a large number of antigen-specific T cells such as OT-I T cells were transferred into mice and primed before the DRibble-loaded B cells were administrated as a booster vaccine, DRibbles may contain sufficient activation signal to stimulate B-cell-mediated cross-presentation and T-cell activation. However, our results showed that DRibble-loaded B cells exhibited a limited antitumor efficacy in the absence of adoptive transfer of exogenous T cells and the endogenous antigen-specific T cells are typically at much lower frequency. Stronger activation signals in combination with DRibbles could overcome this limit. Indeed, DRibble-loaded B cells that were also simulated with TLR9 ligand CpG and anti-CD40 antibody, eradicated established E.G7 lymphoma in the absence of transfer of OT-I T cells. Both TLR9 and CD40 signaling are known to enhance the expression of MHC and costimulatory molecules, increase cytoskeletal activity and antigen-presenting ability, as well as IL-12 production.8–10,12,19–20 However, the underlining mechanisms by which B-cell activation by CpG and anti-CD40 increased antitumor efficacy induced by DRibbles loaded onto B cells remains to be determined.
Beside their ability to restimulate primed T cells, our experimental data suggest that DRibble-induced antibody production in vivo can mediate ADCC against tumor cells. A strong ADCC could be an important antitumor effector mechanism that is unique to B-cell-based DRibble vaccines.
In conclusion, our studies demonstrated that B cells activated by tumor-derived autophagosomes (DRibbles) together with potent B-cell activators represent a novel B-cell-based cancer immune therapy strategy for cancer treatment. Future studies will be focused to determine the underlying mechanisms of DRibble-induced B-cell activation and the requirement and relative contribution of B cells and DCs to the antitumor immune responses induced by DRibble vaccines in mice and men.
ACKNOWLEDGMENTS
The authors thank Dr SuyuShu (Cleveland Clinic, USA, Ret.) for his critical review of this manuscript.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
This project was funded by a Grant from the National Natural Science Foundation of China No. 31370895, 31170857 (L.-X.W.) and Providence Portland Medical Foundation (H.-M.H.).
H.-M.H. is the cofounder of UbiVACLLC, a small biotech company that licenses the DRibble technology for cancer immunotherapy. All authors have declared there are no financial conflicts of interest in regards to this work.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.immunotherapy-journal.com.
H.R. and S.Z. contributed equally.
REFERENCES
- 1.Abeliovich H, Dunn WAJ, Kim J, et al. Dissection of autophagosome biogenesis into distinct nucleation and expansion steps. J Cell Biol. 2000; 151:1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li YH, Wang LX, Pang P, et al. Cross presentation of tumor associated antigens through tumor-derived autophagosomes. Autophagy. 2009; 5:576–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li YH, Wang LX, Yang G, et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008; 68:6889–6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YH, Wang LX, Pang P, et al. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin Cancer Res. 2011; 17:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous anti-gen-pulsed dendritic cells. Nat Med. 1996; 2:52–58 [DOI] [PubMed] [Google Scholar]
- 6.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998; 4:328–332 [DOI] [PubMed] [Google Scholar]
- 7.Lopez JA, Bioley G, Turtle CJ, et al. Single step enrichment of blood dendritic cells by positive immunoselection. J Immunol Methods. 2003; 274:47–61 [DOI] [PubMed] [Google Scholar]
- 8.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B-cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997; 100:2757–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo E, Gryschok L, Klein-Gonzalez N, et al. CD40-activated B-cells can be generated in high number and purity in cancer patients: analysis of immunogenicity and homing potential. Clin Exp Immunol. 2009; 155:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.vonBergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, et al. CD40-activated B-cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood. 2006; 107:2786–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penafuerte C, Ng S, Bautista-Lopez N, et al. B effector cells activated by a chimeric protein consisting of IL-2 and the ectodomain of TGF-β receptor II induce potent antitumor immunity. Cancer Res. 2012; 72:1210–1220 [DOI] [PubMed] [Google Scholar]
- 12.Guo S, Xu J, Denning W, et al. Induction of protective cytotoxic T cell responses by a B cell-based cellular vaccine requires stable expression of antigen. Gene Ther. 2009; 16:1300–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Candolfi M, Curtin JF, Yagiz K, et al. B-cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011; 13:947–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Zhou M, Ren H, et al. Tumor-derived autophagosomes (DRibbles) induce B cell activation in a TLR2-MyD88 dependent manner. PLoS One. 2013; 8:e53564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Wit J, Souwer Y, Jorritsma T, et al. Antigen-specific B cells reactivate an effective cytotoxic T cell response against phagocytosed Salmonella through cross-presentation. PLoS One. 2010; 5:e13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brayer J, Cheng F, Wang H, et al. Enhanced CD8 T cell cross-presentation by macrophages with targeted disruption of STAT3. Immunol Lett. 2010; 131:126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen SM, Maston LD, Gough MJ, et al. Signaling through OX40 enhances antitumor immunity. Semin Oncol. 2010; 37:524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su S, Zhou H, Xue M, et al. Anti-tumor efficacy of a hepatocellular carcinoma vaccine based on dendritic cells combined with tumor-derived autophagosomes in murine models. Asian Pac J Cancer Prev. 2013; 14:3109–3116 [DOI] [PubMed] [Google Scholar]
- 19.Wagner M, Poeck H, Jahrsdoerfer B, et al. IL-12p70-dependent Th1 induction by human B-cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004; 172:954–963 [DOI] [PubMed] [Google Scholar]
- 20.Shirota H, Sano K, Hirasawa N, et al. B-cells capturing antigen conjugated with CpGoligodeoxy nucleotides induce Th1 cells by elaborating IL-12. J Immunol. 2002; 169:787–794 [DOI] [PubMed] [Google Scholar]
- 21.Twitty CG, Jensen SM, Hu HM, et al. Tumor-derived autophagosome vaccine: induction of cross-protective immune responses against short-lived proteins through a p62-dependent mechanism. Clin Cancer Res. 2011; 17:6467–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994; 64:529–564 [DOI] [PubMed] [Google Scholar]
- 23.Murray JL, Gillogly ME, Przepiorka D, et al. Toxicity, immunogenicity, and induction of E75-specifictumor-lytic CTLs by HER-2 peptide E75 (369-377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2002; 8:3407–3418 [PubMed] [Google Scholar]
- 24.Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004; 10:4699–4708 [DOI] [PubMed] [Google Scholar]
- 25.Curigliano G, Spitaleri G, Dettori M, et al. Vaccine immunotherapy in breast cancer treatment: promising, but still early. Expert Rev Anticancer Ther. 2007; 7:1225–1241 [DOI] [PubMed] [Google Scholar]
- 26.Ellerman JE, Brown CK, de Vera M, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007; 13:2836–2848 [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Bloom O, Zhang M, et al. HMG1 as a late mediator of endotoxin lethality in mice. Science. 1999; 285:248–251 [DOI] [PubMed] [Google Scholar]
- 28.Tang D, Kang R, Livesey KM, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010; 190:881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner M. Autophagy: in the hands of HMGB1. Nat Rev Mol Cell Biol. 2010; 11:756–757 [DOI] [PubMed] [Google Scholar]
- 30.Kang R, Livesey KM, Zeh HJ, et al. HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy. 2010; 6:1209–1211 [DOI] [PubMed] [Google Scholar]
- 31.Lanzavecchia A. Antigen-specific interaction between T and B-cells. Nature. 1985; 314:537–539 [DOI] [PubMed] [Google Scholar]
- 32.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984; 160:1102–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke Y, Kapp JA. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B-cells by receptor-mediated uptake. J Exp Med. 1996; 184:1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnaba V, Franco A, Alberti A, et al. Selective killing of hepatitis B envelope antigen-specific B-cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990; 345:258–260 [DOI] [PubMed] [Google Scholar]
- 35.de Wit J, Souwer Y, Jorritsma T, et al. Antigen-specific B-cells reactivate an effective cytotoxic T cell response against phagocytosed Salmonella through cross-presentation. PLoS One. 2010; 5:e13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grivel JC, Crook K, Leserman L. Endocytosis and presentation of liposome-associated antigens by B-cells. Immunomethods. 1994; 4:223–228 [DOI] [PubMed] [Google Scholar]
- 37.Vidard L, Kovacsovics-Bankowski M, Kraeft SK, et al. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol. 1996; 156:2809–2818 [PubMed] [Google Scholar]
- 38.Lapointe R, Bellemare-Pelletier A, Housseau F, et al. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003; 63:2836–2843 [PubMed] [Google Scholar]
- 39.Ahmadi T, Flies A, Efebera Y, et al. CD40 Ligand-activated, antigen-specific B-cells are comparable to mature dendritic cells in presenting protein antigens and major histocompatibility complex class I- and class II-binding peptides. Immunology. 2008; 124:129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]


