Abstract
This study sought to evaluate the impact of multiple negative charges on blood clearance kinetics and biodistribution properties of 99mTc-labeled RGD peptide dimers. Bioconjugates HYNIC-P6G-RGD2 and HYNIC-P6D-RGD2 were prepared by reacting P6G-RGD2 and P6D-RGD2, respectively, with excess HYNIC-OSu in the presence of diisopropylethylamine. Their IC50 values were determined to be 31 ± 5 and 41 ± 6 nM, respectively, against 125I-echistatin bound to U87MG glioma cells in a whole-cell displacement assay. Complexes [99mTc(HYNIC-P6G-RGD2)(tricine)(TPPTS)] (99mTc-P6G-RGD2) and [99mTc(HYNIC-P6D-RGD2)(tricine)(TPPTS)] (99mTc-P6D-RGD2) were prepared in high radiochemical purity (RCP > 95%) and specific activity (37–110 GBq/μmol). They were evaluated in athymic nude mice bearing U87MG glioma xenografts for their biodistribution. The most significant difference between 99mTc-P6D-RGD2 and 99mTc-P6G-RGD2 was their blood radioactivity levels and tumor uptake. The initial blood radioactivity level for 99mTc-P6D-RGD2 (4.71 ± 1.00%ID/g) was ∼5× higher than that of 99mTc-P6G-RGD2 (0.88 ± 0.05%ID/g), but this difference disappeared at 60 min p.i. 99mTc-P6D-RGD2 had much lower tumor uptake (2.20–3.11%ID/g) than 99mTc-P6G-RGD2 (7.82–9.27%ID/g) over a 2 h period. Since HYNIC-P6D-RGD2 and HYNIC-P6G-RGD2 shared a similar integrin αvβ3 binding affinity (41 ± 6 nM versus 31 ± 5 nM), the difference in their blood activity and tumor uptake is most likely related to the nine negative charges and high protein binding of 99mTc-P6D-RGD2. Despite its low uptake in U87MG tumors, the tumor uptake of 99mTc-P6D-RGD2 was integrin αvβ3-specific. SPECT/CT studies were performed using 99mTc-P6G-RGD2 in athymic nude mice bearing U87MG glioma and MDA-MB-231 breast cancer xenografts. The SPECT/CT data demonstrated the tumor-targeting capability of 99mTc-P6G-RGD2, and its tumor uptake depends on the integrin αvβ3 expression levels on tumor cells and neovasculature. It was concluded that the multiple negative charges have a significant impact on the blood clearance kinetics and tumor uptake of 99mTc-labeled dimeric cyclic RGD peptides.
Introduction
Integrin αvβ3 plays a significant role in angiogenesis and tumor metastasis,1−4 and is a receptor for extracellular matrix proteins (such as vitronectin, fibronectin, fibrinogen, laminin, collagen, Von Willebrand’s factor, and osteoponin) with the arginine-glycine-aspartic (RGD) peptide sequence.5 Over last several years, many radiolabeled (99mTc, 18F, 64Cu, 68Ga, and 111In) cyclic RGD peptides have been evaluated as radiotracers for tumor imaging by single photon emission computed tomography (SPECT) or positron emission tomography (PET),6−25 and have been reviewed extensively.26−30 The cyclic RGD peptides, such as E[c(RGDfK)]2 (RGD2), are targeting biomolecules to carry radionuclide to the integrin αvβ3 overexpressed on the tumor cells and/or tumor neovasculature. Multiple cyclic RGD peptides have been utilized to maximize their integrin αvβ3 binding affinity and the radiotracer tumor uptake. It was found that radiolabeled multimeric cyclic RGD peptides had significantly higher tumor uptake with longer tumor retention time than their monomeric counterparts.28 The linkers between the two c(RGDfK) moieties in dimeric cyclic RGD peptides (Figure 1) are also important for their tumor-targeting capability and excretion kinetics of 99mTc radiotracers.
Figure 1.
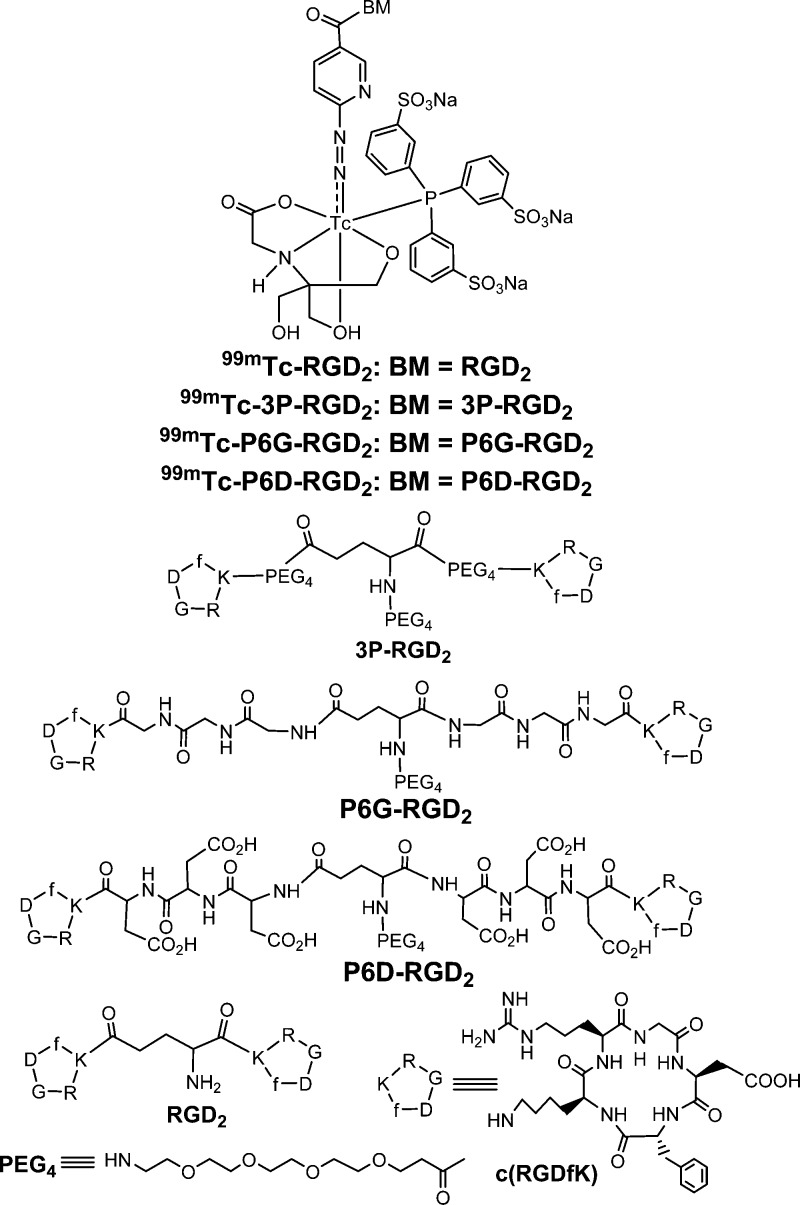
Chemdraw structures of cyclic RGD peptides (c(RGDfK), RGD2, 3P-RGD2, P6G-RGD2, P6D-RGD2, and their corresponding ternary ligand complexes [99mTc(HYNIC-BM)(tricine)(TPPTS)] (BM = biomolecule; 99mTc-RGD2: BM = RGD2; 99mTc-3P-RGD2: BM = 3P-RGD2; 99mTc-P6G-RGD2: BM = P6G-RGD2, 99mTc-P6D-RGD2: BM = P6D-RGD2).
Many water-soluble linkers have been proposed to improve pharmacokinetics of radiolabeled cyclic RGD peptides.28,31−33 For example, 7-amino-l-glycero-l-galacto-2,6-anhydro-7-deoxyheptanamide (SAA) has successfully used to minimize the liver radioactivity accumulation and increase target-to-background ratios of 18F-labeled cyclic RGD peptides.6,10 The (cysteic acid)2 dipeptide was utilized to minimize the liver uptake of radiolabeled integrin αvβ3 antagonists.34−37 The hexaethylene glycol (HEG) has been incorporated in 18F-labeled RGDfE dimers and tetramers.7−9 It was reported that the polyethylene glycol (PEG) linkers could improve not only the tumor uptake but also the pharmacokinetics of radiolabeled c(RGDyK) and 64Cu-labeled E[c(RGDyK)]2.11−13
We have been using 15-amino-4,7,10,13-tetraoxapentadecanoic acid (PEG4) and Gly-Gly-Gly (G3) as linkers to maximize the tumor-targeting capability and improve excretion kinetics of 99mTc labeled cyclic RGD peptides.38−48 In this study, we used the Asp-Asp-Asp (D3) tripeptide sequence to prepare HYNIC-P6D-RGD2 (HYNIC = 6-(2-(2-sulfonatobenzaldehyde)hydrazono)nicotinyl; P6D-RGD2 = PEG4-E[c(RGDfK(D3))]2). We were interested in P6D-RGD2 because it contains two D3 linkers with six carboxylic groups that could be deprotonated under physiological conditions (pH = 7.4). It was reported that the D3 linker was able to minimize the liver radioactivity accumulation of 99mTc-labeled c(RGDfK).49 For comparison purpose, we also prepared HYNIC-P6G-RGD2 (P6G-RGD2 = PEG4-E[c(RGDfK(G3))]2) due to the neutral charges of G3 tripeptide sequences. We evaluated the ternary ligand complexes [99mTc(HYNIC-P6G-RGD2)(tricine)(TPPTS)] (Figure 1: 99mTc-P6G-RGD2) and [99mTc(HYNIC-P6D-RGD2)(tricine)(TPPTS)] (99mTc-P6D-RGD2) in the athymic nude mice bearing U87MG glioma xenografts to explore the impact of multiple negative overall molecular charges on blood clearance kinetics and biodistribution properties of 99mTc radiotracers. SPECT/CT studies were performed using 99mTc-P6G-RGD2 in athymic nude mice bearing U87MG glioma and MDA-MB-231 breast cancer xenografts to demonstrate its utility as a radiotracer for tumor imaging.
Results
Bioconjugate Synthesis
HYNIC-P6D-RGD2 and HYNIC-P6G-RGD2 were prepared by direct conjugation of P6D-RGD2 and P6G-RGD2, respectively, with HYNIC-OSu in the presence of excess DIEA. The reaction between P6D-RGD2 and HYNIC-OSu was slow at room temperature (∼15 days to complete). The reaction between P6G-RGD2 and HYNIC-OSu was completed within 3 days at room temperature. It seems that the addition of six carboxylic groups between the two c(RGDfK) moieties make the conjugation reaction slower. HYNIC-P6D-RGD2 and HYNIC-P6G-RGD2 were purified by HPLC, and have been characterized by MALDI mass spectroscopy. The MS data were completely consistent with their proposed composition. Their HPLC purity was >95% before being used for the integrin αvβ3 binding assays and 99mTc-labeling.
Integrin αvβ3 Binding Affinity
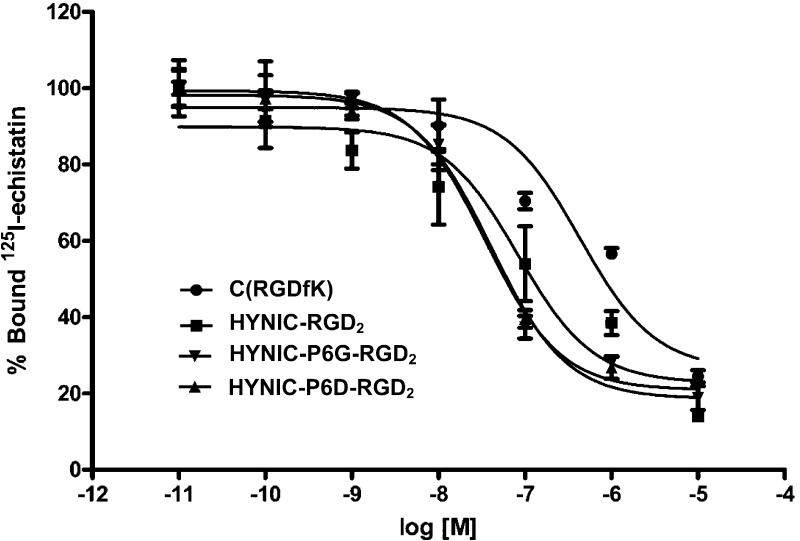
Figure 2 shows the displacement curves of 125I-echistatin bound to U87MG human glioma cells in the presence of cyclic RGD peptides. For comparison purposes, we also evaluated c(RGDyK) and HYNIC-RGD2 in the same assay. IC50 values were calculated to be 31 ± 5, 41 ± 6, 85 ± 2, and 422 ± 15 nM for HYNIC-P6G-RGD2, HYNIC-P6G-RGD2, HYNIC-RGD2, and c(RGDyK), respectively. The integrin αvβ3 binding affinity follows the order of HYNIC-P6G-RGD2 ∼ HYNIC-P6D-RGD2 > HYNIC-RGD2 ≫ c(RGDyK). The IC50 values of HYNIC-P6G-RGD2 and HYNIC-P6D-RGD2 were very close to that of HYNIC-3P-RGD2 (IC50 = 32 ± 1 nM; 3P-RGD2: PEG4-E[PEG4-c(RGKfD)]2 and PEG4 = 15-amino-4,7,10,13-tetraoxapentadecanoic acid) reported in our previous studies.39,48 Apparently, replacing the two neutral G3 or PEG4 linkers with a pair of triple-charged D3 tripeptide sequences did not significantly alter the integrin αvβ3 binding affinity of dimeric cyclic RGD peptides.
Figure 2.
Competitive displacement curves of 125I-echistatin bound to U87MG human glioma cells in the presence of cyclic RGD peptides. HYNIC-RGD2 and c(RGDyK) were used for comparison purposes. IC50 values were calculated to be 32 ± 5, 41 ± 6, 85 ± 8, and 422 ± 15 nM for HYNIC-P6G-RGD2, HYNIC-P6D-RGD2, HYNIC-RGD2, and c(RGDfK), respectively.
Radiochemistry
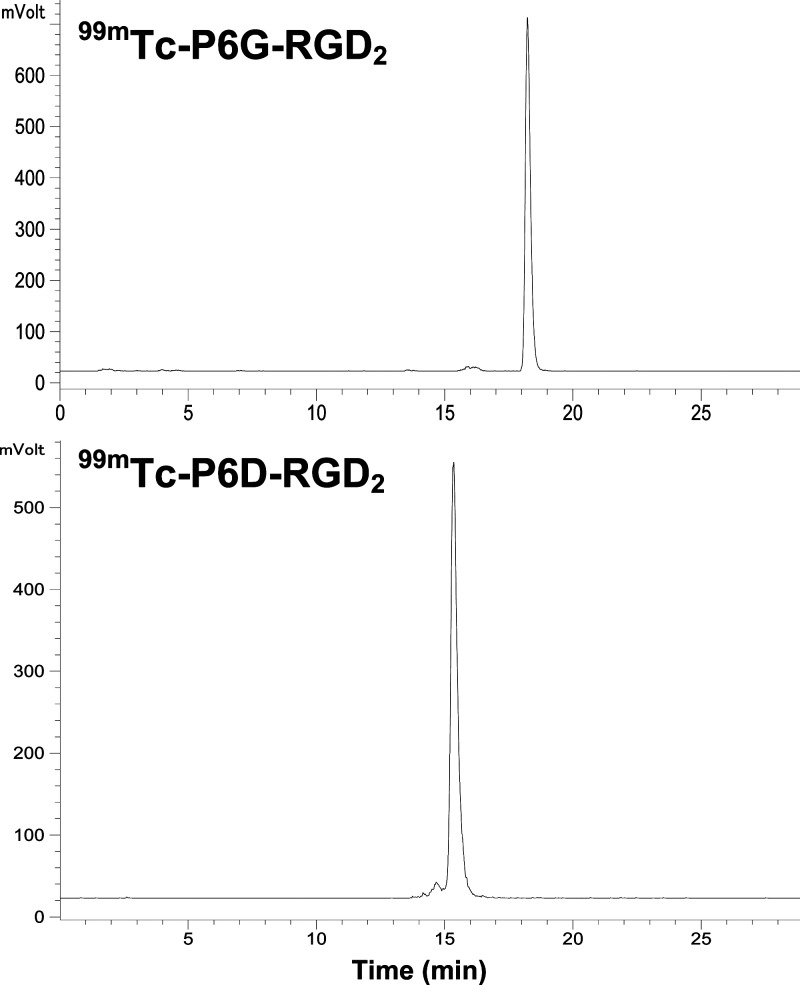
99mTc-labeling was accomplished by heating the reaction mixture at 100 °C for 15–20 min. Figure 3 shows radio-HPLC chromatograms of 99mTc-P6D-RGD2 and 99mTc-P6G-RGD2 in the kit matrix. Their RCP was >95% without postlabeling chromatographic purification. The specific activity was 37–110 GBq/μmol for 99mTc-P6D-RGD2 and 99mTc-P6G-RGD2. They both remained stable in the kit matrix for more than 6 h postlabeling.
Figure 3.
Typical radio-HPLC chromatograms of 99mTc-P6G-RGD2 and 99mTc-P6D-RGD2. Their RCP was >95% without postlabeling chromatographic purification.
Biodistribution Properties
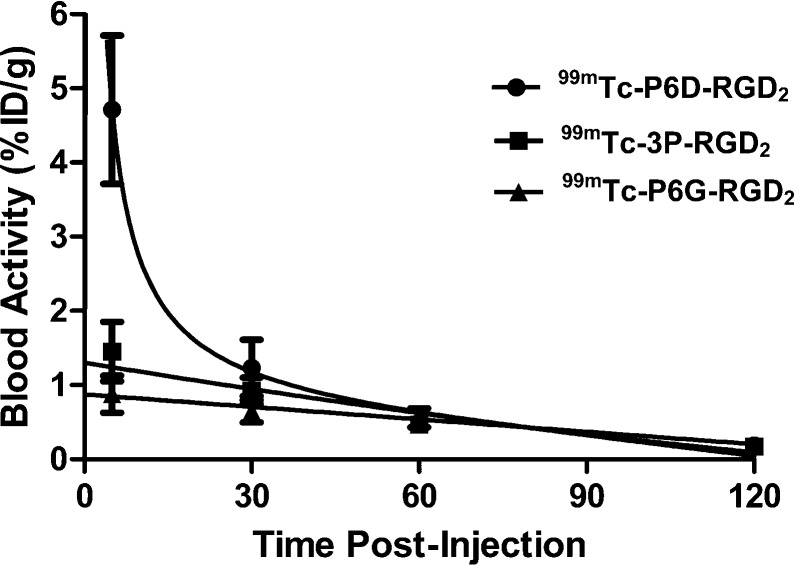
Biodistribution studies were performed on 99mTc-P6D-RGD2 and 99mTc-P6G-RGD2 to compare their blood clearance, tumor uptake, and biodistribution properties in athymic nude mice bearing U87MG glioma xenografts. Tables 1 and 2 list the selected biodistribution data for 99mTc-P6D-RGD2 and 99mTc-P6G-RGD2, respectively. For comparison purposes, we also obtained the 5 and 60 min biodistribution data of 99mTc-3P-RGD2 (Figure 1: [99mTc(HYNIC-3P-RGD2)(tricine)(TPPTS)]), which is currently under clinical evaluations as a SPECT radiotracer for imaging integrin αvβ3-positive tumors.55−57 It was found that the most significant difference between 99mTc-P6D-RGD2 and 99mTc-P6G-RGD2 was their blood activity levels and tumor uptake. The blood activity level of 99mTc-P6D-RGD2 was 4.71 ± 1.00%ID/g, ∼5× higher than that of 99mTc-P6G-RGD2 (0.88 ± 0.05%ID/g) and ∼3× higher than that of 99mTc-3P-RGD2 (1.45 ± 0.40%ID/g) at 5 min p.i., but this difference disappeared at 60 min p.i. (Figure 4). The tumor uptake of 99mTc-P6D-RGD2 (2.20 ± 0.42, 2.85 ± 0.55, 3.11 ± 0.47, and 2.45 ± 0.90%ID/g at 5, 30, 60, and 120 min p.i., respectively) was significantly lower (p < 0.01) than that of 99mTc-P6G-RGD2 (9.27 ± 0.72, 8.85 ± 0.67, 8.17 ± 1.10, and 7.82 ± 0.76%ID/g at 5, 30, 60, and 120 min p.i., respectively) over the 2 h study period. In contrast, 99mTc-P6G-RGD2 and 99mTc-3P-RGD2 shared similar blood clearance kinetics (Figure 4), and tumor uptake values (7.82–9.27%ID/g for 99mTc-P6G-RGD2; and 7.24–8.72%ID/g for 99mTc-3P-RGD2). The kidney uptake of 99mTc-P6D-RGD2 (Table 1) was also significantly higher (p < 0.01) than that of 99mTc-P6D-RGD2 (Table 2). However, 99mTc-P6D-RGD2 had the intestine uptake values of 5.86 ± 1.37, 6.58 ± 0.88, 7.08 ± 0.92, and 4.74 ± 0.33%ID/g at 5, 30, 60, and 120 min p.i., respectively, which were much lower (p < 0.01) than those of 99mTc-P6G-RGD2 (11.72 ± 2.01, 9.27 ± 1.15, 6.17 ± 1.55, and 4.74 ± 1.09%ID/g at 5, 30, 60, and 120 min p.i., respectively) over the 2 h study period. Obviously, the linker groups between two cyclic RGD moieties have a significant impact on the blood clearance kinetics (Figure 4), tumor uptake, and biodistribution properties (Figure 5) of 99mTc-labeled dimeric cyclic RGD peptides.
Table 1. Selected Biodistribution Data and Tumor-to-Background Ratios of 99mTc-P6D-RGD2 in Athymic Nude Mice (n = 5) Bearing U87MG Human Glioma Xenografts.a.
| organ | 5 min | 30 min | 60 min | 60 min (blocking) | 120 min |
|---|---|---|---|---|---|
| Blood | 4.71 ± 1.00 | 1.23 ± 0.38 | 0.49 ± 0.08 | 0.23 ± 0.01 | 0.19 ± 0.09 |
| Brain | 0.41 ± 0.09 | 0.25 ± 0.09 | 0.13 ± 0.02 | 0.02 ± 0.01 | 0.11 ± 0.01 |
| Eyes | 0.81 ± 0.36 | 0.89 ± 0.25 | 0.79 ± 0.31 | 0.23 ± 0.10 | 0.54 ± 0.15 |
| Heart | 4.23 ± 0.87 | 2.36 ± 0.24 | 1.66 ± 0.05 | 0.20 ± 0.07 | 1.11 ± 0.16 |
| Intestine | 5.86 ± 1.37 | 6.58 ± 0.88 | 7.08 ± 0.92 | 0.22 ± 0.11 | 4.74 ± 0.33 |
| Kidneys | 32.84 ± 7.81 | 24.67 ± 2.40 | 19.85 ± 0.28 | 23.94 ± 3.61 | 15.43 ± 2.30 |
| Liver | 4.26 ± 0.63 | 3.95 ± 0.11 | 3.61 ± 0.14 | 0.28 ± 0.04 | 2.85 ± 0.51 |
| Lungs | 4.46 ± 0.68 | 2.05 ± 0.28 | 1.85 ± 0.20 | 0.29 ± 0.01 | 1.11 ± 0.42 |
| Muscle | 1.63 ± 0.77 | 1.79 ± 0.20 | 1.59 ± 0.25 | 0.18 ± 0.06 | 0.79 ± 0.23 |
| Spleen | 2.70 ± 0.73 | 2.60 ± 0.37 | 2.58 ± 0.46 | 0.14 ± 0.01 | 2.40 ± 0.37 |
| Tumor | 2.20 ± 0.42 | 2.85 ± 0.55 | 3.13 ± 0.47 | 0.30 ± 0.01 | 2.45 ± 0.90 |
| Tumor/Blood | 0.49 ± 0.13 | 2.50 ± 0.33 | 6.50 ± 0.58 | 1.24 ± 0.05 | 6.98 ± 4.06 |
| Tumor/Liver | 0.52 ± 0.10 | 0.65 ± 0.15 | 0.87 ± 0.17 | 1.05 ± 0.09 | 0.84 ± 0.18 |
| Tumor/Lung | 0.49 ± 0.04 | 1.19 ± 0.35 | 1.68 ± 0.10 | 1.10 ± 0.13 | 2.41 ± 0.73 |
| Tumor/Muscle | 1.85 ± 1.08 | 1.97 ± 0.53 | 2.00 ± 0.36 | 1.80 ± 0.11 | 3.30 ± 1.24 |
The tumor uptake was expressed as an average plus/minus the standard deviation.
Table 2. Selected Biodistribution Data and Tumor-to-Background Ratios of 99mTc-P6G-RGD2 in Athymic Nude Mice (n = 5) Bearing U87MG Human Glioma Xenograftsa.
| organ | 5 min | 30 min | 60 min | 120 min |
|---|---|---|---|---|
| Blood | 0.88 ± 0.05 | 0.65 ± 0.15 | 0.56 ± 0.13 | 0.19 ± 0.02 |
| Brain | 0.22 ± 0.03 | 0.20 ± 0.08 | 0.15 ± 0.02 | 0.13 ± 0.02 |
| Eyes | 2.24 ± 0.32 | 2.04 ± 0.15 | 1.59 ± 0.17 | 1.22 ± 0.02 |
| Heart | 2.39 ± 0.25 | 1.79 ± 0.11 | 1.61 ± 0.39 | 1.02 ± 0.13 |
| Intestine | 11.72 ± 2.01 | 9.27 ± 1.15 | 6.17 ± 1.55 | 4.74 ± 1.09 |
| Kidneys | 16.62 ± 1.37 | 12.33 ± 0.86 | 10.70 ± 1.00 | 6.64 ± 0.41 |
| Liver | 3.09 ± 0.22 | 2.88 ± 0.25 | 2.77 ± 0.35 | 2.22 ± 0.30 |
| Lungs | 6.57 ± 0.90 | 4.38 ± 0.55 | 3.83 ± 0.56 | 2.81 ± 0.36 |
| Muscle | 1.99 ± 0.72 | 1.75 ± 0.24 | 1.68 ± 0.33 | 0.83 ± 0.11 |
| Spleen | 3.37 ± 0.75 | 3.08 ± 0.88 | 2.68 ± 0.57 | 2.31 ± 0.27 |
| Tumor | 9.27 ± 0.72 | 8.85 ± 0.67 | 8.17 ± 1.10 | 7.82 ± 0.76 |
| Tumor/Blood | 12.17 ± 1.12 | 14.11 ± 1.25 | 15.64 ± 2.79 | 41.76 ± 5.13 |
| Tumor/Liver | 3.04 ± 0.27 | 2.95 ± 0.33 | 2.86 ± 0.30 | 3.21 ± 0.53 |
| Tumor/Lung | 1.41 ± 0.17 | 2.07 ± 0.15 | 2.35 ± 0.24 | 2.71 ± 0.12 |
| Tumor/Muscle | 4.50 ± 1.34 | 5.04 ± 0.68 | 5.09 ± 0.51 | 9.42 ± 1.25 |
The tumor uptake was expressed as an average plus/minus the standard deviation.
Figure 4.
Comparison of the blood radioactivity accumulation of 99mTc-P6D-RGD2, 99mTc-P6G-RGD2, and 99mTc-3P-RGD2 in the athymic nude mice bearing U87MG glioma xenografts to illustrate the impact of linkers (D3 versus G3 and PEG4) between the two c(RGDfK) moieties on blood clearance kinetics of 99mTc-labeled cyclic RGD peptide dimers.
Figure 5.
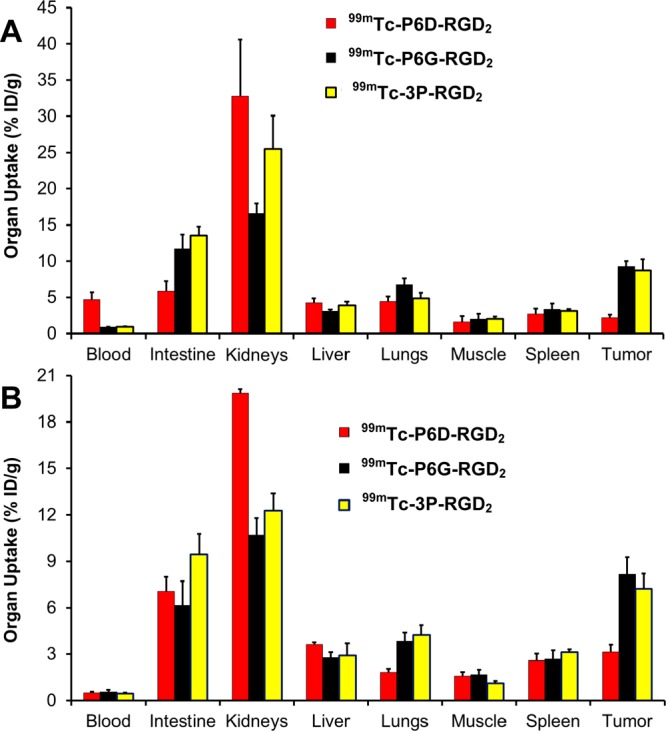
Direct comparison of the selected 5 min (A) and 60 min (B) biodistribution data between 99mTc-P6D-RGD2, 99mTc-P6G-RGD2, and 99mTc-3P-RGD2 in athymic nude mice bearing U87MG glioma xenografts to illustrate the impact of linkers (D3 versus G3 and PEG4) between the two c(RGDfK) moieties on the uptake of 99mTc-labeled cyclic RGD peptide dimers in tumor and normal organs.
Integrin αvβ3 Specificity
Figure 6A compares the 60 min biodistribution data of 99mTc-P6D-RGD2 in the athymic nude mice bearing U87MG human glioma xenografts in the absence/presence of excess RGD2. Co-injection of excess RGD2 significantly blocked its tumor uptake (0.30 ± 0.01%ID/g with RGD2 v 3.13 ± 0.47%ID/g without RGD2). There was also a significant blockage of its uptake in several integrin αvβ3-positive normal organs by coinjection of excess RGD2. For example, the 60 min uptake values of 99mTc-P6D-RGD2 in the intestine, lungs, and spleen was 7.08 ± 0.92, 1.85 ± 0.20, and 2.58 ± 0.46%ID/g, respectively, without RGD2, while its uptake in the same organs was only 0.22 ± 0.11, 0.29 ± 0.01, and 0.14 ± 0.01%ID/g, respectively, in the presence of excess RGD2. These data clearly showed that the tumor uptake of 99mTc-P6D-RGD2 is integrin αvβ3-specific. A similar conclusion could also be made on the basis of planar imaging data (Figure 6B) of the U87MG glioma-bearing mice in the absence/presence of excess RGD2.
Figure 6.
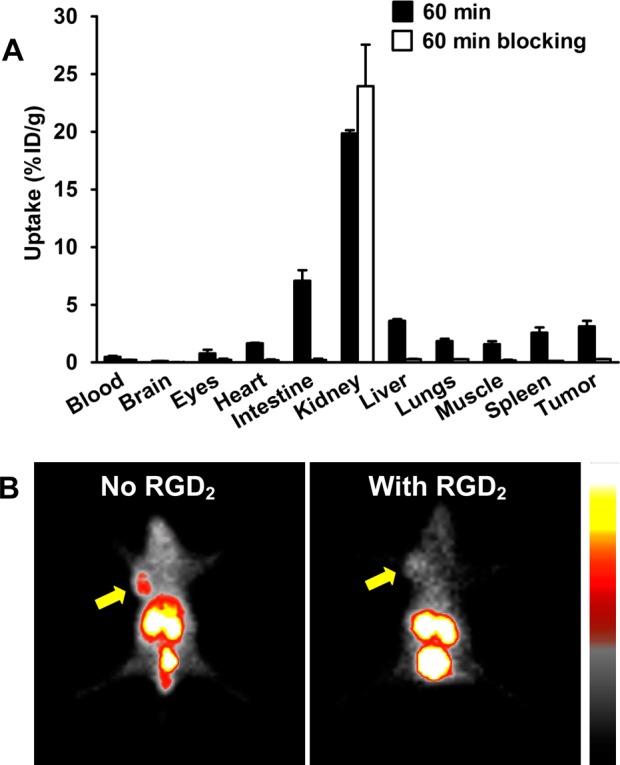
Selected 60 min biodistribution (A) and planar imaging (B) data for 99mTc-P6D-RGD2 in the athymic nude mice bearing U87MG human glioma xenografts with/without coinjection of RGD2 (350 μg/mouse or 14 mg/kg) to demonstrate its integrin αvβ3 specificity. Yellow arrows indicate the presence of U87MG glioma tumors.
SPECT/CT Imaging and Immunohistochemistry Data
We obtained the SPECT/CT images (Figure 7A) of the athymic nude mice bearing xenografted U87MG glioma and MDA-MB-231 breast tumor administered with ∼37 MBq of 99mTc-P6G-RGD2. We were interested in 99mTc-P6G-RGD2 because of its higher tumor uptake than that of 99mTc-P6D-RGD2. It was found that the tumors were clearly visualized with excellent contrast in both animal models. Its tumor uptake was ∼8.2%ID/cm3 in the U87MG glioma and 3.5%ID/cm3 in the MDA-MB-231 breast tumor on the basis of SPECT quantification. To explain the uptake difference between U87MG and MDA-MB-231 tumors, we obtained microscopic images (Figure 7B) of selected tumor slice stained with anti-integrin β3 (red color) and anti-CD31 (green color) antibody. CD31 was used as a biomarker for tumor blood vessels (both mature and neovasculature), and was visualized with FITC (green). Integrin β3 was visualized with Cy3 (red). Yellow color (red β3 staining verged with green CD31 staining) in overlay images indicates the presence of integrin αvβ3 on neovasculature. It was clear that integrin αvβ3 was highly expressed on U87MG glioma cells and neovasculature, which was in complete agreement with the high uptake of 99mTc-P6G-RGD2 in U87MG glioma tumors (Figure 7A). In contrast, the xenografted MDA-MB-231 breast tumors had relatively high expression of integrin αvβ3 on tumor cells with limited integrin αvβ3 expression on neovasculature, as indicated by the lack of yellow and orange colors in overlay images. As a result, 99mTc-P6G-RGD2 had lower uptake in the MDA-MB-231 breast tumors than that in U87MG glioma (Figure 7A).
Figure 7.
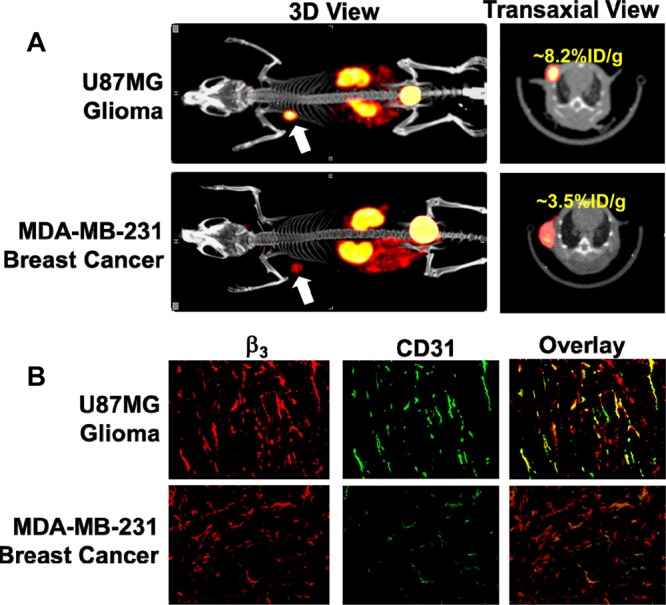
(A) 3D and transverse views of SPECT/CT images of the athymic nude mice bearing U87MG glioma and MDA-MB-231 breast tumor xenografts. Each animal was administered with ∼37 MBq of 99mTc-P6G-RGD2. SPECT/CT study was designed to illustrate their potential utility for tumor imaging. (B) Selected microscopic images (Magnification: 200×) of the tumor slice stained with hamster anti-integrin β3 (red) and rat anti-CD31 (green) antibodies. Yellow or orange color (red integrin β3 staining merged with green CD31 staining) indicates the presence of integrin αvβ3 on the tumor neovasculature.
Discussion
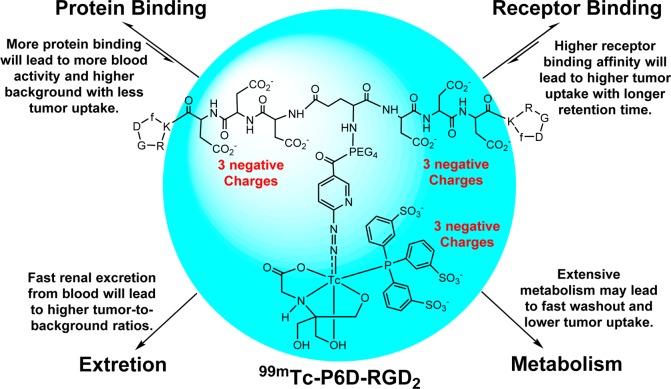
There are two biologically important interactions (Chart 1: receptor binding and protein binding) once a radiotracer is injected into the blood circulation. Receptor binding is necessary for the radiotracer to selectively localize in the targeted organ or tissue (e.g., tumor). Higher receptor binding affinity will lead to more radiotracer initial tumor uptake with a longer tumor retention time. Protein bonding is often detrimental because it will reduce the number of radiotracer molecules available for receptor binding, and result in more initial blood radioactivity accumulation (Chart 1). Therefore, the protein bonding should be minimized for the receptor-based target-specific radiotracers. In addition, more hydrophilic radiotracers tend to increase renal excretion, which will lead to lower background radioactivity in the blood pool and normal organs (e.g., liver, lungs, and muscle) with better target-to-background ratios. In contrast, more lipophilic radiotracers tend to have extensive metabolism, which often results in lower tumor uptake with poorer target-to-background ratios.
Chart 1. Schematic Illustration of Biological Interactions and Elimination Routes of 99mTc-P6D-RGD2.
In this study, we found that the negative charges had very little impact on integrin αvβ3 binding affinity of HYNIC-conjugated cyclic RGD dimers (Figure 2: IC50 = 40 ± 6 nM for HYNIC-P6D-RGD2; and IC50 = 32 ± 5 nM for HYNIC-P6G-RGD2). However, they have a dramatic impact on the blood clearance kinetics (Figure 4), tumor uptake, and biodistribution (Figure 5) of their 99mTc radiotracers (99mTc-P6D-RGD2 versus 99mTc-P6G-RGD2). Therefore, this difference is most likely related to their overall molecular charge and their protein binding capability.
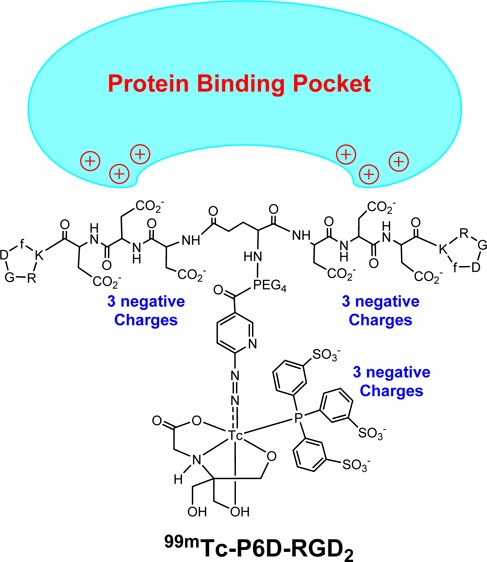
P6D-RGD2 contains two D3 tripeptide sequences with six carboxylic groups. Due to their low pKa values (4.5–5.0 for aliphatic acids), they are all expected to be deprotonated under physiological conditions (pH = 7.4). 99mTc-P6D-RGD2 has a total of nine negative molecular charges (Chart 1), which provide a strong driving force to interact with the positively charged amino residues, such as arginine and lysine. The stronger protein binding leads to higher initial blood activity level (Figure 4), lower tumor uptake and higher kidney uptake of 99mTc-P6D-RGD2 than those of both 99mTc-P6G-RGD2 and 99mTc-3P-RGD2 (Figure 5), in which the G3 and PEG4 linkers are neutral under physiological conditions.
One might ask why one D3 linker is able to minimize the liver uptake of 99mTc-labeled c(RGDfK),49 while 99mTc-P6G-RGD2 has such a high initial blood activity level (Figure 4). The answer lies in the differences of their overall negative molecular charges. In 99mTc-labeled c(RGDfK), the D3 linker offers only three negative charges, which was not sufficient for strong protein binding. In 99mTc-P6D-RGD2, the combination of two D3 linkers with the triple-charged TPPTS yields a total of nine negative charges. As a result, 99mTc-P6D-RGD2 is able to form a stronger protein binding (Chart 1), leading to high initial blood radioactivity (Figure 4) and low tumor uptake (Figure 5). It must be noted that protein binding is reversible, and the protein-bound 99mTc-P6D-RGD2 became dissociated. It is not surprising that all three radiotracers shared almost identical blood radioactivity levels at 60 min p.i. (Figure 4).
It remains unknown which protein 99mTc-P6D-RGD2 is bonded to. However, albumin is the most abundant protein in the blood plasma.58−60 The normal concentration range is 35–50 g/L for human serum albumin (HSA). HSA consists of three homologous domains (I, II, and III) and each domain is formed by two subdomains (A and B).58,59 The results from X-ray analysis of different ligand–HSA complexes showed the existence of two binding sites in the II and III domains. Subdomains IIA and IIIA contain deep pockets, the entrance of which is surrounded by positively charged amino acid residues.59 Thus, these pockets are potential binding sites for 99mTc-P6D-RGD2. If the radiotracer contains multiple negative charges (e.g., two D3 linkers in 99mTc-P6D-RGD2), the interaction between radiotracer and albumin may become significant. This might explain why 99mTc-P6D-RGD2 has much higher initial blood radioactivity than 99mTc-P6G-RGD2 and 99mTc-3P-RGD2 (Figure 4) while the D3 linker is able to reduce the liver uptake for 99mTc-labeled c(RGDfK).49
99mTc-P6D-RGD2 is not a good radiotracer for tumor imaging due to its high protein binding and low tumor uptake. However, this finding may have important applications in the design of albumin-targeted contrast agents for magnetic resonance imaging (MRI) angiography.61−63 For the receptor-based radiotracers, protein binding will reduce the number of radiolabeled biomolecules for receptor binding and the radiotracer uptake in the targeted tissues. For albumin-targeted contrast agents, albumin binding will reduce the tumbling rate of Gd(III) chelates, increase the relaxivity of Gd(III) contrast agents, and decrease the doses administered to each subject.63
Despite its low uptake in U87MG glioma tumors, the tumor uptake of 99mTc-P6D-RGD2 was integrin αvβ3-specific, as illustrated by the blocking experiments (Figure 6). SPECT/CT data clearly shows the tumor-targeting capability of 99mTc-P6G-RGD2, and its uptake is dependent on the integrin αvβ3 expression levels on tumor cells and neovasculature (Figure 7). If the integrin αvβ3 expression level is high on both tumor cells and neovasculature, 99mTc-P6G-RGD2 will have high tumor uptake. If the integrin αvβ3 expression level is low on either tumor cells or tumor neovasculature, the tumor uptake of 99mTc-P6G-RGD2 will be lower. Therefore, 99mTc-P6G-RGD2 has the potential as a screening tool for cancer patients before the anti-αvβ3 treatment.
Conclusion
The most important finding of this study is that the multiple negative charges have a significant detrimental effect on the blood clearance kinetics and tumor uptake of 99mTc-labeled dimeric cyclic RGD peptides. As a result of its higher protein binding, 99mTc-P6D-RGD2 had much lower tumor uptake than 99mTc-P6G-RGD2.
Experimental Section
Materials and Instruments
Common chemicals and solvents were purchased from Sigma/Aldrich (St. Louis, MO), and were used without further purification. Cyclic peptides, c(RGDyK), E[c(RGDfK)]2 (RGD2), P6D-RGD2 (PEG4-E[c(RGDfK(D3))]2), and P6G-RGD2 (PEG4-E[c(RGDfK(G3))]2), and 3P-RGD2 (PEG4-E[c(RGDfK(PEG4))]2) were purchased from Peptides International, Inc. (Louisville, KY). Sodium succinimidyl 6-(2-(2-sulfonatobenzaldehyde)hydrazono)nicotinate (HYNIC-NHS), HYNIC-RGD2, HYNIC-3P-RGD2, and [99mTc(HYNIC-3P-RGD2)(tricine)(TPPTS)] (99mTc-3P-RGD2) were prepared according to the literature methods.38,50 Na99mTcO4 was obtained from Cardinal HealthCare (Chicago, IL). The MALDI (matrix-assisted laser desorption ionization) data were collected on an Applied Biosystems Voyager DE PRO mass spectrometer (Framingham, MA), the Department of Chemistry, Purdue University.
HPLC Methods
The semiprep HPLC method (Method 1) used a LabAlliance HPLC system (Scientific Systems, Inc., State College, PA) equipped with a UV/vis detector (λ = 254 nm) and Zorbax C18 column (9.4 mm × 250 mm, 100 Å pore size; Agilent Technologies, Santa Clara, CA). The flow rate was 2.5 mL/min with a mobile phase being isocratic with 90% A (0.1% TFA in acetonitrile) and 10% B (0.1% TFA in water) over the first 5 min, followed by a gradient mobile phase going from 90% A and 10% B at 5 min, and to 60% A and 40% B at 20 min. The radio-HPLC method (Method 2) used the LabAlliance HPLC system equipped with a β-ram IN/US detector (Tampa, FL) and Zorbax C18 column (4.6 mm × 250 mm, 300 Å pore size; Agilent Technologies, Santa Clara, CA). The flow rate was 1 mL/min. The mobile phase was isocratic for the first 5 min with 90% A (25 mM NH4OAc, pH = 6.8) and 10% B (acetonitrile), followed by a gradient mobile phase going from 90% A and 10% B at 5 min to 60% A and 60% B at 20 min.
HYNIC-PEG4-E[c(RGDfK(D3))]2 (HYNIC-P6D-RGD2)
HYNIC-OSu (5.4 mg, 12 μmol) and P6D-RGD2 (4.5 mg, 2 μmol) were dissolved in anhydrous DMF (1.5 mL). Upon addition of excess diisopropylethylamine (DIEA: 50 μmol), the reaction mixture was stirred for ∼15 days at room temperature. To the reaction mixture was added 2 mL of water. The pH value was adjusted to 3–4 using TFA. The product was separated from the reaction mixture by HPLC (Method 1). Fractions at ∼18 min were collected. Lyophilization of the combined fractions afforded HYNIC-P6D-RGD2 as a white powder. The yield was 2.5 mg (∼48%) with >95% HPLC purity. MALDI-MS: m/z = 2557.2 for [M + H]+ (exact mass = 2557.99 calcd. for [C107H147N29O43S]).
HYNIC-PEG4-E[c(RGDfK(G3))]2 (HYNIC-P6G-RGD2)
HYNIC-NHS (4.6 mg, 11 μmol) and P6G-RGD2 (5 mg, 2.6 μmol) were dissolved in 2.0 mL of anhydrous DMF. After addition of excess DIEA (50 μmol), the reaction mixture was stirred at room temperature for 3 days. To the reaction mixture was added 2 mL of water after completion of conjugation. The pH value was adjusted to 3–4 using neat TFA. The product was separated from reaction mixture by HPLC (Method 1). The fraction at 20.5 min was collected. Lyophilization of combined fractions afforded HYNIC-P6G-RGD2 as a white powder. The yield was 2.0 mg (∼34%) with >95% HPLC purity. MALDI-MS: m/z = 2209.5 for [M + H]+ (exact mass = 2209.96 calcd. for [C95H135N29O31S]).
99mTc-Labeling
99mTc-P6G-RGD2 and 99mTc-P6D-RGD2 were prepared using a kit formulation according to the literature method.38,39 To a lyophilized vial containing 25 μg of HYNIC-P6G-RGD2 or HYNIC-P6D-RGD2, 7 mg TPPTS, 6.5 mg tricine, 40 mg mannitol, 38.5 mg disodium succinate hexahydrate, and 12.7 mg succinic acid was added 1.0–1.5 mL of Na99mTcO4 solution (370–1110 MBq). The reconstituted vial was heated in a boiling water bath for 10–20 min. After radiolabeling, a sample of resulting solution was analyzed by radio-HPLC (Method 2). The radiochemical purity (RCP) was >95% for both 99mTc-P6D-RGD2 and 99mTc-P6G-RGD2 before being used for imaging and biodistribution studies. The solution stability was monitored by radio-HPLC for 6 h.
Dose Preparation
For biodistribution, doses were prepared by dissolving 99mTc radiotracer (no HPLC purification) in saline to a concentration of ∼1 MBq/mL. Each animal was injected with ∼0.1 mL of the dose solution. For SPECT/CT imaging, doses were prepared by dissolving 99mTc-P6G-RGD2 (no HPLC purification) in saline to ∼370 MBq/mL. In the blocking experiment, RGD2 was dissolved in the dose solution to 3.5 mg/mL. The resulting dose solution was filtered with a 0.20 μm Millex-LG filter before being injected into animals. Each animal was injected with ∼0.2 mL of the dose solution.
Tumor Cell Culture
The U87MG cell line was obtained from ATCC (American Type Culture Collection, Manassas, VA). U87MG cells were cultured in the Minimum Essential Medium, Eagle with Earle’s Balanced Salt Solution (nonessential amino acids sodium pyruvate), and were supplemented with 10% fetal bovine serum (FBS, ATCC) and 1% penicillin and streptomycin solution at 37 °C in a humidified atmosphere of 5% CO2 in air. Cells were grown as monolayers and were harvested or split when they reached 90% confluence to maintain exponential growth.
Integrin αvβ3 Binding Assay
The integrin binding affinity of cyclic RGD peptides was assessed via a displacement assay using 125I-echistatin (PerkinElmer, Branford, CT) as the integrin-specific radioligand. Briefly, the filter multiscreen DV plates (Millipore, Billerica, MA) were seeded with 1 × 105 U87MG cells in binding buffer (20 mM Tris, 150 mM NaCl, 2 mM CaCl2, 1 mM MnCl2, 1 mM MgCl2, 0.1% (wt/vol) bovine serum albumin; pH 7.4) and 125I-echistatin (0.75–1.0 kBq) in the presence of increasing concentrations of the cyclic RGD peptide, incubated for 2 h at room temperature. After removing unbound 125I-echistatin, the hydrophilic PVDF filters were washed 3× with the binding buffer, and then collected. Radioactivity was determined using a PerkinElmer Wizard 1480 γ-counter (Shelton, CT). Experiments were carried out twice in triplicate. IC50 values were calculated by fitting experimental data with nonlinear regression using GraphPad Prism (GraphPad Software, Inc., San Diego, CA), and were reported as an average plus/minus standard deviation.
Animal Models
Biodistribution and imaging studies were performed in compliance with the NIH animal experimentation guidelines (Principles of Laboratory Animal Care, NIH Publication No. 86–23, revised 1985). The protocol was approved by the Purdue University Animal Care and Use Committee (PACUC). Female athymic nu/nu mice (4–5 weeks) were purchased from Harlan (Indianapolis, IN), and were inoculated subcutaneously with 5 × 106 U87MG cells into the shoulder flank of each animal. Four weeks after inoculation, animals were used for biodistribution and imaging studies.
Biodistribution Protocol
Twenty tumor-bearing mice (20–25 g) were randomly selected, and divided into four groups. Each animal was administered ∼0.1 MBq of 99mTc radiotracer by tail vein injection. Five animals were sacrificed by sodium pentobarbital overdose (∼200 mg/kg) at 5, 30, 60, and 120 min postinjection (p.i.). Blood, tumors, and normal organs (brain, eyes, heart, spleen, lungs, liver, kidneys, muscle, and intestine) were harvested, washed with saline, dried with absorbent tissue, weighed, and counted on a PerkinElmer Wizard 1480 γ-counter. The blocking experiment was performed using RGD2 as the blocking agent. Each animal was administered ∼0.1 MBq of 99mTc-P6D-RGD2 along with ∼350 μg (∼14 mg/kg) of RGD2. Biodistribution data (%ID/g) and tumor-to-background (T/B) ratios were all expressed as the average plus/minus standard deviation standard deviation from five animals. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Newman-Keuls test for multiple comparisons. The level of significance was set at p < 0.05.
Planar Imaging Protocol
Three animals bearing U87MG glioma xenografts were used for the whole-body planar imaging with 99mTc-P6D-RGD2. Tumor-bearing mice were anesthetized with intraperitoneal injection of Ketamine (40–100 mg/kg) and Xylazine (2–5 mg/kg). Each animal was administered ∼37 MBq of 99mTc-P6D-RGD2 in 0.1 mL of saline solution. Animals were then placed on a custom-made single head gamma camera (Diagnostic Services Inc., NJ) equipped with a parallel-hole, medium-energy, and high-resolution collimator. Static planar images were acquired at 30, 60, and 120 min p.i. and stored digitally in a 128 × 128 matrix. The acquisition count limits were set at 300 K. In the blocking experiment, 99mTc-P6D-RGD2 was coinjected with RGD2 (∼14 mg/kg or ∼350 μg per 25 g tumor-bearing mouse). Such a large excess of RGD2 was used to make sure that the integrin αvβ3 binding sites are completely blocked. After planar imaging, the tumor-bearing animals were euthanized by sodium pentobarbital overdose (100–200 mg/kg).
SPECT/CT Imaging
SPECT/CT images were obtained using a u-SPECT-II/CT scanner (Milabs, Utrecht, The Netherlands) equipped with a 0.6 mm multi-pinhole collimator. The glioma-bearing mouse was injected with ∼37 MBq of 99mTc-P6G-RGD2 in 0.1 mL saline via the tail vein. At 60 min p.i., the animal was placed into a shielded chamber connected to an isoflurane anesthesia unit (Univentor, Zejtun, Malta). Anesthesia was induced using an air flow rate of 350 mL/min and ∼3.0% isoflurane. After induction of anesthesia, the animal was immediately placed supine on the scanning bed. The air flow rate was then reduced to ∼250 mL/min with ∼2.0% isoflurane. Rectangular scans in the regions of interest (ROIs) from SPECT and CT were selected on the basis of orthogonal optical images provided by the integrated webcams. After SPECT (75 projections over 30 min per frame, 2 frames), the animal was transferred into the CT scanner and imaged using “normal” acquisition settings (2° intervals) at 45 kV and 500 μA. After CT acquisition, the animal was allowed to recover in a lead-shielded cage.
Image Reconstruction and Data Processing
SPECT reconstruction was performed using a POSEM (pixelated ordered subsets by expectation maximization) algorithm with 6 iterations and 16 subsets. CT data were reconstructed using a cone-beam filtered back-projection algorithm (NRecon v 1.6.3, Skyscan). After reconstruction, the SPECT and CT data were automatically coregistered according to the movement of the robotic stage, and then resampled to equivalent voxel sizes. Co-registered images were further rendered and visualized using the PMOD software (PMOD Technologies, Zurich, Switzerland). A 3D-Guassian filter (0.8 mm fwhm) was applied to smooth noise, and the LUTs (look up tables) were adjusted for good visual contrast. The reconstructed images were visualized as both orthogonal slices and maximum intensity projections.
Radioactivity Quantification
Radiation sources of a known amount of radioactivity were imaged and reconstructed using the same scanning protocol above. A standard curve was generated to correlate the pixel intensities in reconstructed images with the radioactivity measured by a γ-counter. Tumor delineation was performed on CT and SPECT images according to the literature method.51−54 The amount of radioactivity in each tumor was calculated according to the above-mentioned standard curve. The tumor uptake of 99mTc-P6G-RGD2 was expressed as the percentage of injected dose per unit volume (%ID/cm3).
Tumor Tissue Immunohistochemistry
The U87MG tumors were immediately snap-frozen in the OCT (optical cutting temperature) solution, and were then cut into slices (5 μm). After thorough drying, slices were fixed with ice-cold acetone for 10 min, and dried in the air for 20 min. Sections were blocked with 10% goat serum for 30 min, and then were incubated with the hamster anti-integrin β3 antibody (1:100, BD Biosciences, San Jose, CA) and rat anti-CD31 antibody (1:100, BD Biosciences) for 1 h at room temperature. After incubating with Cy3-conjugated goat anti-hamster and fluorescein isothiocyanate (FITC)-conjugated goat anti-rat secondary antibodies (1:100, Jackson ImmunoResearch Inc., West Grove, PA) and washing with PBS, the fluorescence was visualized with an Olympus fluorescence microscope (Olympus America Inc., Center Valley, PA). All the pictures were taken under 200× magnification. Brightness and contrast adjustments were made equally to all images.
Acknowledgments
This work was supported, in part, by Purdue University, the Challenge Research Award from Purdue Cancer Center, the Indiana Clinical and Translational Sciences Institute funded in part by grant Number TR000006 (Clinical and Translational Award) from the National Institutes of Health, National Center for advancing Translational Science, and R01 CA115883 (S.L.) from the National Cancer Institute.
Glossary
Abbreviations
- 3P-RGD2
PEG4-E[PEG4-c(RGKfD)]2 = PEG4-Glu[cyclo[Arg-Gly-Asp-d-Phe-Lys(PEG4)]]2 (PEG4 = 15-amino-4,7,10,13-tetraoxapentadecanoic acid)
- HYNIC-OSu
sodium succinimidyl 6-(2-(2-sulfonatobenzaldehyde)hydrazono)nicotinate
- MALDI
matrix-assisted laser desorption ionization
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- P6D-RGD2
PEG4-E[c(RGDfK(D3))]2 = PEG4-Glu[cyclo[Arg-Gly-Asp-d-Phe-Lys(Asp-Asp-Asp)]]2)
- P6G-RGD2
PEG4-E[c(RGDfK(G3))]2 = PEG4-Glu[cyclo[Arg-Gly-Asp-d-Phe-Lys(Gly-Gly-Gly)]]2)
- RGD2
E[c(RGDfK)]2 = Glu[cyclo(Arg-Gly-Asp-d-Phe-Lys)]2
- SPECT
single photon emission computed tomography
- 99mTc-3P-RGD2
[99mTc(HYNIC-3P-RGD2)(tricine)(TPPTS)] (HYNIC = 6-hydrazinonicotinyl; and TPPTS = trisodium triphenylphosphine-3,3′,3″-trisulfonate)
- 99mTc-P6D-RGD2
[99mTc(HYNIC-P6D-RGD2)(tricine)(TPPTS)]
- 99mTc-P6G-RGD2
[99mTc(HYNIC-P6G-RGD2)(tricine)(TPPTS)]
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Folkman J. (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31. [DOI] [PubMed] [Google Scholar]
- Bello L.; Francolini M.; Marthyn P.; Zhang J.; Carroll R. S.; Nikas D. C.; Strasser J. F.; Villani R.; Cheresh D. A.; Black P. M. (2001) αvβ3 and αvβ5 integrin expression in glioma periphery. Neurosurgery 49, 380–389. [DOI] [PubMed] [Google Scholar]
- Hwang R.; Varner J. (2004) The role of integrins in tumor angiogenesis. Hematol. Oncol. Clin. North Am. 18, 991–1006. [DOI] [PubMed] [Google Scholar]
- Jin H.; Varner J. (2004) Integrins: roles in cancer development and as treatment targets. Br. J. Cancer 90, 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann S.; Ehemann V.; Schwab M. (2002) Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res. 62, 5139–5143. [PubMed] [Google Scholar]
- Haubner R.; Wester H. J.; Weber W. A.; Mang C.; Ziegler S. I.; Goodman S. L.; Senekowitsch-Schmidtke R.; Kessler H.; Schwaiger M. (2001) Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 61, 1781–1785. [PubMed] [Google Scholar]
- Thumshirn G.; Hersel U.; Goodman S. L.; Kessler H. (2003) Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chem.—Eur. J. 9, 2717–2725. [DOI] [PubMed] [Google Scholar]
- Poethko T.; Schottelius M.; Herz M.; Haubner R.; Henriksen G.; Schwaiger M.; Wester H. j.; Thumshirn G.; Kessler H. (2004) Chemoselective pre-conjugate radiohalogenation of unprotected mono- and multimeric peptides via oxime formation. Radiochimica. Acta 92, 317–327. [Google Scholar]
- Poethko T.; Schottelius M.; Thumshirn G.; Hersel U.; Herz M.; Henriksen G.; Kessler H.; Schwaiger M.; Wester H. J. (2004) Two-step methodology for high-yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J. Nucl. Med. 45, 892–902. [PubMed] [Google Scholar]
- Haubner R.; Kuhnast B.; Mang C.; Weber W. A.; Kessler H.; Wester H. J.; Schwaiger M. (2004) [18F] Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjugate Chem. 15, 61–69. [DOI] [PubMed] [Google Scholar]
- Chen X.; Park R.; Shahinian A. H.; Tohme M.; Khankaldyyan V.; Bozorgzadeh M. H.; Bading J. R.; Moats R.; Laug W. E.; Conti P. S. (2004) 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl. Med. Biol. 31, 179–189. [DOI] [PubMed] [Google Scholar]
- Chen X.; Park R.; Shahinian A. H.; Bading J. R.; Conti P. S. (2004) Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl. Med. Biol. 31, 11–19. [DOI] [PubMed] [Google Scholar]
- Chen X.; Sievers E.; Hou Y.; Park R.; Tohme M.; Bart R.; Bremner R.; Bading J. R.; Conti P. S. (2005) Integrin αvβ3–targeted imaging of lung cancer. Neoplasia 7, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Zhang X.; Xiong Z.; Cheng Z.; Fisher D. R.; Liu S.; Gambhir S. S.; Chen X. (2005) MicroPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J. Nucl. Med. 46, 1707–1718. [PubMed] [Google Scholar]
- Li Z. B.; Cai W.; Cao Q.; Chen K.; Wu Z.; He L.; Chen X. (2007) 64Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor αvβ3 integrin expression. J. Nucl. Med. 48, 1162–1171. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Li Z. B.; Chen K.; Cai W.; He L.; Chin F. T.; Li F.; Chen X. (2007) MicroPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4). J. Nucl. Med. 48, 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Niu G.; Shi J.; Liu S.; Wang F.; Liu S.; Chen X. (2009) 68Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin αvβ3 PET imaging. Eur. J. Nucl. Med. Mol. Imaging 36, 947–957. [DOI] [PubMed] [Google Scholar]
- Li Y.; Guo J.; Tang S.; Lang L.; Chen X.; Perrin D. M. (2013) One-step and one-pot-two-step radiosynthesis of cyclo-RGD-18F-aryltrifluoroborate conjugates for functional imaging. Am. J. Nucl. Med. Mol. Imaging 3, 44–56. [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Liu S.; Wang F.; Liu S.; Chen X. (2009) Noninvasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur. J. Nucl. Med. Mol. Imaging 36, 1296–1307. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf I.; Liu S.; Kruijtzer J. A.; Soede A. C.; Oyen W. J.; Liskamp R. M.; Corstens F. H.; Boerman O. C. (2007) Effect of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD Peptide. Nucl. Med. Biol. 34, 29–35. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf I.; Kruijtzer J. A.; Liu S.; Soede A. C.; Oyen W. J.; Corstens F. H.; Liskamp R. M.; Boerman O. C. (2007) Improved targeting of the αvβ3 integrin by multimerization of RGD peptides. Eur. J. Nucl. Med. Mol. Imaging 34, 267–273. [DOI] [PubMed] [Google Scholar]
- Doss M.; Kolb H. C.; Zhang J. J.; Bélanger M. J.; Stubbs J. B.; Stabin M. G.; Hostetler E. D.; Alpaugh R. K.; Von Mehren M.; Walsh J. C.; Haka M.; Mocharla V. P.; Yu J. Q. (2012) Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J. Nucl. Med. 53, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwe K.; Kim Y. S.; Milenic D. E.; Baidoo K. E.; Brechbiel M. W. (2012) 111In- and 203Pb-labeled cyclic arginine-glycine-aspartic acid peptide conjugate as an αvβ3 integrin-binding radiotracer. J. Label Compd. Radiopharm. 55, 423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohle K.; Notni J.; Bussemer J.; Kessler H.; Schwaiger M.; Beer A. J. (2012) 68Ga-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl. Med. Biol. 39, 777–784. [DOI] [PubMed] [Google Scholar]
- Tsiapa I.; Loudos G.; Varvarigou A.; Fragogeorgi E.; Psimadas D.; Tsotakos T.; Xanthopoulos S.; Mihailidis D.; Bouziotis P.; Nikiforidis G. C.; Kagadis G. C. (2013) Biological evaluation of an ornithine-modified 99mTc-labeled RGD peptide as an angiogenesis imaging agent. Nucl. Med. Biol. 40, 262–272. [DOI] [PubMed] [Google Scholar]
- Haubner R.; Wester H. J. (2004) Radiolabeled tracers for imaging of tumor angiogenesis and evaluation of anti-angiogenic therapies. Curr. Pharm. Des. 10, 1439–1455. [DOI] [PubMed] [Google Scholar]
- Meyer A.; Aurenheimer J.; Modlinger A.; Kessler H. (2006) Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr. Pharm. Des. 12, 2723–2747. [DOI] [PubMed] [Google Scholar]
- Liu S. (2009) Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjugate Chem. 20, 2199–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkgraaf I.; Boerman O. C. (2010) Molecular imaging of angiogenesis with SPECT. Eur. J. Nucl. Med. Mol. Imaging 37(Suppl 1), S104–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi U.; Oka T.; Inoue T. (2012) Radiolabeled RGD peptides as integrin αvβ3–targeted PET tracers. Curr. Med. Chem. 19, 3301–3309. [DOI] [PubMed] [Google Scholar]
- Liu S.; Edwards D. S. (2001) Bifunctional chelators for therapeutic lanthanide radiopharmaceuticals. Bioconjugate Chem. 12, 7–34. [DOI] [PubMed] [Google Scholar]
- Liu S. (2004) The role of coordination chemistry in development of target-specific radiopharmaceuticals. Chem. Soc. Rev. 33, 445–461. [DOI] [PubMed] [Google Scholar]
- Liu S. (2008) Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv. Drug Delivery Rev. 60, 1347–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. D.; Kalogeropoulos S.; Nguyen T.; Liu S.; Bartis J.; Ellars C.; Edwards S.; Onthank D.; Yalamanchili P.; Robinson S.; Lazewatsky J.; Barrett J.; Bozarth J. (2003) Design, synthesis and evaluation of radiolabeled integrin αvβ3 receptor antagonists for tumor imaging and radiotherapy. Cancer Biother. Radiopharm. 18, 627–641. [DOI] [PubMed] [Google Scholar]
- Onthank D. C.; Liu S.; Silva P. J.; Barrett J. A.; Harris T. D.; Robinson S. P.; Edwards D. S. (2004) 90Y and 111In complexes of DOTA-conjugated integrin αvβ3 receptor antagonist: different but biologically equivalent. Bioconjugate Chem. 15, 235–241. [DOI] [PubMed] [Google Scholar]
- Harris T. D.; Cheesman E.; Harris A. R.; Sachleben R.; Edwards D. S.; Liu S.; Bartis J.; Ellars C.; Onthank D.; Yalamanchili P.; Heminway S.; Silva P.; Robinson S.; Lazewatsky J.; Rajopadhye M.; Barrett J. (2007) Radiolabeled divalent peptidomimetic vitronectin receptor antagonists as potential tumor radiotherapeutic and imaging agents. Bioconjugate Chem. 18, 1266–1279. [DOI] [PubMed] [Google Scholar]
- Harris T. D.; Kalogeropoulos S.; Nguyen T.; Dwyer G.; Edwards D. S.; Liu S.; Bartis J.; Ellars C.; Onthank D.; Yalamanchili P.; Heminway S.; Robinson S.; Lazewatsky J.; Barrett J. (2006) Structure-activity relationships of 111In- and 99mTc-labeled quinolin-4-one peptidomimetics as ligands for the vitronectin receptor: potential tumor imaging agents. Bioconjugate Chem. 17, 1294–1313. [DOI] [PubMed] [Google Scholar]
- Shi J.; Wang L.; Kim Y. S.; Zhai S.; Liu Z.; Chen X.; Liu S. (2008) Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic arginine-glycine-aspartic (RGD) dimers with triglycine linkers. J. Med. Chem. 51, 7980–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Shi J.; Kim Y. S.; Zhai S.; Jia B.; Zhao H.; Liu Z.; Wang F.; Chen X.; Liu S. (2009) Improving tumor-targeting capability and pharmacokinetics of 99mTc-labeled cyclic RGD dimers with PEG4 linkers. Mol. Pharmaceutics 6, 231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Wang L.; Kim Y. S.; Zhai S.; Jia B.; Wang F.; Liu S. (2009) 99mTcO (MAG2-3G3-dimer): a new integrin αvβ3-targeted SPECT radiotracer with high tumor uptake and favorable pharmacokinetics. Eur. J. Nucl. Med. Mol. Imaging 36, 1874–1884. [DOI] [PubMed] [Google Scholar]
- Shi J.; Kim Y. S.; Zhai S.; Liu Z.; Chen X.; Liu S. (2009) Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjugate Chem. 20, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S.; Shi J.; Kim Y. S.; Zhou Y.; Jia B.; Wang F.; Liu S. (2011) Evaluation of 111In-labeled cyclic RGD peptides: tetrameric not tetravalent. Bioconjugate Chem. 21, 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Kim Y. S.; Chakraborty S.; Jia B.; Wang F.; Liu S. (2009) 2-Mercaptoacetylglycylglycyl (MAG2) as a bifunctional chelator for 99mTc-labeling of cyclic RGD dimers: effect of technetium chelate on tumor uptake and pharmacokinetics. Bioconjugate Chem. 20, 1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Zhou Y.; Chakraborty S.; Kim Y. S.; Jia B.; Wang F.; Liu S. (2011) Evaluation of 111In-labeled cyclic RGD peptides: effects of peptide and linker multiplicity on their tumor uptake, excretion kinetics and metabolic stability. Theranostics 1, 322–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Kim Y. S.; Chakraborty S.; Zhou Y.; Wang F.; Liu S. (2011) Impact of bifunctional chelators on biological properties of 111In-labeled cyclic peptide RGD dimers. Amino Acids 41, 1059–1070. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Kim Y. S.; Chakraborty S.; Shi J.; Gao H.; Liu S. (2011) 99mTc-labeled cyclic RGD peptides for noninvasive monitoring of tumor integrin αvβ3 expression. Mol. Imaging 10, 386–397. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Kim Y. S.; Lu X.; Liu S. (2012) Evaluation of 99mTc-labeled cyclic RGD dimers: impact of cyclic RGD peptides and 99mTc chelates on biological properties. Bioconjugate Chem. 23, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.; Czerwinski A.; Zhou Y.; Shao G.; Valenzuela F.; Sowiński P.; Chauhan S.; Pennington M.; Liu S. (2013) 99mTc-Galacto-RGD2: a novel 99mTc-labeled cyclic RGD peptide dimer useful for tumor imaging. Mol. Pharmacol. 10, 3304–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubner R.; Bruchertseifer F.; Bock M.; Kessler H.; Schwaiger M.; Wester H. J. (2004) Synthesis and biological evaluation of a 99mTc-labeled cyclic RGD peptide for imaging the αvβ3 expression. Nuklearmedizin 43, 26–32. [DOI] [PubMed] [Google Scholar]
- Ma Q.; Ji B.; Jia B.; Gao S.; Ji T.; Wang X.; Han Z.; Zhao G. (2011) Differential diagnosis of solitary pulmonary nodules using 99mTc-3P4-RGD2 scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 38, 2145–2152. [DOI] [PubMed] [Google Scholar]
- Zhu Z.; Miao W.; Li Q.; Dai H.; Ma Q.; Wang F.; Yang A.; Jia B.; Jing X.; Liu S.; Shi J.; Liu Z.; Zhao Z.; Wang F.; Li F. (2012) 99mTc-3PRGD2 for integrin receptor imaging of lung cancer: a multicenter study. J. Nucl. Med. 53, 716–722. [DOI] [PubMed] [Google Scholar]
- Zhao D.; Jin X.; Li F.; Liang J.; Lin Y. (2012) Integrin αvβ3 imaging of radioactive iodine–refractory thyroid cancer using 99mTc-3PRGD2. J. Nucl. Med. 53, 1872–1877. [DOI] [PubMed] [Google Scholar]
- Carter D. C.; Ho J. X. (1994) Structure of serum albumin. Adv. Protein Chem. 45, 153–203. [DOI] [PubMed] [Google Scholar]
- Sugio S.; Kashima A.; Mochizuki S.; Noda M.; Kobayashi K. (1999) Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 12, 439–446. [DOI] [PubMed] [Google Scholar]
- He X. M.; Carter D. C. (1992) Atomic structure and chemistry of human serum albumin. Nature 358, 209–215. [DOI] [PubMed] [Google Scholar]
- Moriggi L.; Yaseen M. A.; Helm L.; Caravan P. (2012) Serum albumin targeted, pH-dependent magnetic resonance relaxation agents. Chem.—Eur. J. 18, 3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros E.; Caravan P. (2013) Structure-relaxivity relationships of serum albumin targeted MRI probes based on a single amino acid Gd complex. J. Med. Chem. 56, 1782–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre V. C.; Allen M. J.; Caravan P. (2014) Contrast agents for MRI: 30+ years and where are we going?. J. Biol. Inorg. Chem. 19, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. D.; Sworin M.; Williams N.; Rajopadhye M.; Damphousse P. R.; Glowacka D.; Poirier M. J.; Yu K. (1999) Synthesis of stable hydrazones of a hydrazinonicotinyl-modified peptide for the preparation of 99mTc-labeled radiopharmaceuticals. Bioconjugate Chem. 10, 808–814. [DOI] [PubMed] [Google Scholar]
- Shao G.; Zhou Y.; Wang F.; Liu S. (2013) Monitoring glioma growth and tumor necrosis with the U-SPECT-II/CT scanner by targeting integrin αvβ3. Mol. Imaging 12, 39–48. [PubMed] [Google Scholar]
- Zhou Y.; Shao G.; Liu S. (2012) Monitoring breast tumor lung metastasis by U-SPECT-II/CT with an integrin αvβ3-targeted radiotracer 99mTc-3P-RGD2. Theranostics 2, 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.; Zheng Y.; Shao G.; Zhou Y.; Liu S. (2013) Integrin αvβ3–targeted radiotracer 99mTc-3P-RGD2 useful for noninvasive monitoring of breast tumor response to antiangiogenic linifanib therapy but not anti-integrin αvβ3 RGD2 therapy. Theranostics 3, 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.; Zhou Y.; Voorbach M. J.; Shao G.; Zhang Y.; Fox J. B.; Albert D. H.; Luo Y.; Liu S.; Mudd S. R. (2013) Monitoring tumor response to linifanib therapy with SPECT/CT using the integrin αvβ3-targeted radiotracer 99mTc-3P-RGD2. J. Pharmacol. Exp. Ther. 346, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]