Abstract
Objectives:
The aim of this study was to evaluate the efficacy and safety of combinations of lercanidipine (L) and enalapril (E) at different doses on office and home blood pressure (BP) in patients with Stage 2 hypertension.
Study design:
This was a randomized, double-blind, placebo-controlled, factorial study conducted in 100 centres from seven countries. Patients with office DBP 100–109 mmHg and home DBP at least 85 mmHg at the end of a 2-week placebo run-in period were randomized to a 10-week treatment with placebo, L (10 or 20 mg), E (10 or 20 mg) or the four L-E combinations. The efficacy parameters were office DBP at trough (primary), SBP at trough and home SBP and DBP. Office BP was measured at each visit in both the sitting and the standing position, while home BP was measured twice in the morning and twice in the evening for at least 3 days before treatment and at study end. Safety parameters included adverse events, laboratory tests and 12-lead ECG.
Results:
A total of 1039 patients were randomized (48% men, mean age 54 years, mean BMI 30 kg/m2, 40% obese patients). Baseline BP was similar in all groups and lower for home than for office values (149/95 and 159/103 mmHg, respectively). A marked placebo effect was observed on office but not on home BP. Combination therapy was superior to placebo at all doses for both office and home BP. The greatest effect was observed in the L20/E20 group, in which the SBP/DBP fall amounted to −19.2/−15.2 and −13.2/−7.5 mmHg for sitting office and home BP, respectively. Similar reductions were observed on standing office BP. The L20/E20 combination was associated with less cough, palpitations and leg oedema than monotherapies, with no increased rate of dizziness or hypotension.
Conclusion:
In Stage 2 hypertension, a fixed-dose combination of L and E ensures a control of both office and out-of-office BP, with a favourable tolerability profile.
Keywords: combination therapy, enalapril, essential hypertension (Stage 2), factorial study design, home blood pressure, lercanidipine
INTRODUCTION
All guidelines on hypertension regard monotherapy as unable to effectively reduce blood pressure (BP) in the majority of patients with a BP elevation and recommend use of combinations of two or more drugs in order to increase the rate by which BP is controlled in the hypertensive population [1–3]. They also favour initiation of antihypertensive treatment with two rather than one drug in order to guarantee a more rapid BP control and earlier protective effect [4], and combination of two agents into a single tablet, because simplification of treatment has a favourable effect on adherence to the prescribed regimen [5,6].
Several drug combinations are indicated by guidelines as suitable for preferential use [1]. Among them, the combination of a dihydropiridine calcium antagonist and an angiotensin-converting enzyme (ACE) inhibitor is of particular importance because randomized trials have shown that this combination may lower effectively the elevated BP [7,8], exert a superior cardiovascular protective effect than the diuretic/beta-blocker and ACE inhibitor/calcium antagonist combinations [9,10] and reduce the incidence of the most common calcium antagonist related side effect, that is ankle oedema [11].
A fixed-dose combination between the calcium antagonist lercanidipine (L) and the ACE inhibitor enalapril (E) is of clinical interest, as both enalapril and lercanidipine are among the most used drugs in their class. Furthermore, E has proved to exert clearcut protective effects in both hypertension and heart failure [12–15], while L has been shown to be a long-acting drug [16], with a smooth antihypertensive effect, a favourable tolerability profile and a protective influence on organs damaged by hypertension [17,18]. Compared with other calcium antagonists, the L-E combination has also been found to be associated with no chronic reflex activation of the sympathetic nervous [19], thereby avoiding the adverse effects that sympathetic hyperactivity may have on organ structure and function as well as on cardiovascular risk [20].
The L-E combination has already been shown to be effective in hypertensive patients unresponsive to monotherapies [21,22]. However, the efficacy and safety of the two drugs when administered at their full therapeutic doses (20 mg/day) has not yet been established by comparisons with monotherapy, combinations of the two drugs at lower doses or placebo. Aim of the present study was to provide this information by a large-scale, randomized, placebo-controlled study on moderate hypertensive patients. The antihypertensive effect was assessed not only by office but also by home BP changes because home BP has been found to have an important prognostic value [23–25] and is more and more frequently used by physicians to check the effect of the prescribed treatment regimen in an environment closer to daily life than the physician's office.
MATERIALS AND METHODS
Study population
The study population consisted of male and female hypertensive patients aged 18 to 75 years, who were untreated, intolerant to the prescribed drugs or not well controlled by the existing antihypertensive therapy. Patients were recruited if their trough office BP values (24 ± 2 h after dosing) were between 100 and 109 mmHg for DBP and less than 180 mmHg for SBP after 2 weeks of single-blind placebo treatment, and average home DBP was at least 85 mmHg in the week before randomization. This was done to exclude individuals with isolated office hypertension.
Other key exclusion criteria included secondary or severe hypertension (patients with a DBP ≥110 mmHg or an SBP ≥180 mmHg), history or presence of cardiovascular disease (transient ischemic attacks, stroke, hypertensive encephalopathy, angina pectoris, myocardial infarction, myocardial revascularization procedures, heart failure), haemodynamically significant valve disease, clinically significant ventricular or supraventricular arrhythmias, heart rate greater than 100 beats/min, severe renal or hepatic insufficiency or diabetes on drug treatment. Women who were pregnant, breastfeeding, planning a pregnancy or having a childbearing potential without using an effective method of contraception were also excluded from the study and so were those with a history of intolerance to dihydropiridine calcium antagonists or ACE inhibitors.
Study design
This was an international, multicentre, randomized, placebo-controlled, parallel group study conducted on a total of 100 sites in France, Germany, Italy, Poland, Russia, Spain and Ukraine. The study consisted of a 2-week single-blind placebo run-in period, during which all previous antihypertensive medications (if any) were discontinued, followed by a 10-week double-blind active treatment period. The eligible patients were randomly allocated to one of nine treatment groups: placebo, monotherapy with 10 or 20 mg daily of L, monotherapy with 10 or 20 mg daily of E, or L/E combination at the following daily doses: L10/E10, L10/E 20, L20/10 mg and L20/E20. One capsule containing one or two tablets of L 10 mg and one tablet of E10 or 20 mg were used and all drugs were given in the morning, usually around 0800 h. Randomization was accomplished by assigning each patient a unique individual identification number (composed of the study site number and a screening number) via an interactive voice response system. For safety reasons, individuals assigned to be treated with L20, E20, L10/E20, L20/E10 or L20/E20 mg received a lower dose in the first 2 weeks of treatment before being titrated to the final dosage. Furthermore, individuals with a DBP at least 110 mmHg or an SBP at least 180 mmHg taken at office were removed from the study at any time. Clinic visits were scheduled 2 weeks after the initiation of the single-blind placebo period, at the end of the placebo period and at 2, 4, 6 and 10 weeks after randomization to treatment. Adverse events and concomitant medications were checked at each visit. Laboratory tests, a physical examination and an electrocardiography were performed at screening, before randomization to treatment, at study end or at the time of an early study interruption. Adherence to treatment was assessed at each visit by pill counting.
The first patient was enrolled in March 2010, and the last patient completed the study in March 2011. The study was conducted in agreement with the principles of the Declaration of Helsinki through its recent version (59th General Assembly, Seoul 2008) and with the Good Clinical Practice guidelines (CPMP/ICH/135/1995, Directive 2001/20/EC for Europe), and the local regulations for clinical trials. The study protocol and the informed consent procedure were reviewed and approved by the Ethics Committees of the study sites. All patients provided written informed consent before any study-specific procedure was implemented.
Office blood pressure
Office BP was measured at all study visits with a validated semi-automatic device (Omron 705 IT; Omron Matsusaka Co. Ltd. Japan) using a cuff of appropriate size. The device provided printouts of BP values together with the time and date of the measurements. At the initial visit, BP was checked in both arms and the arm with the higher value was used for all subsequent measurements. Individuals with a between-arm BP difference more than 20 mmHg for systolic or more than 10 mmHg for diastolic were excluded from the study. Three measurements were made with the patient in the sitting position since at least 5 min and the average of the three values was used as the reference value for the visit. Two additional measurements were obtained immediately upon and 2 min after standing. For each BP value, the device also provided heart rate values in beats/minute.
Home blood pressure
Home BP was measured with Microlife WatchBP Home (Widnau, Switzerland), a device that has been validated according to the International Study Protocols of the European Society of Hypertension [26]. Patients received oral and written instructions to obtain duplicate measurements between 0600 and 0900 h and between 1800 and 2100 h on each day of the week preceding randomization to treatment and of the week prior to the last in-treatment visit. The device automatically excluded the first day measurements and provided the mean BP values of the remaining period together with the number from which the mean was calculated. As mentioned above, the home BP values obtained in the week prior to randomization were used as a criterion for patient inclusion in the study.
Study endpoints
The primary efficacy variable was the change from prerandomization values in office trough (24 ± 2 h after dosing) sitting DBP after the 10 weeks of treatment. The main secondary efficacy variable was the corresponding change in office trough SBP. Other secondary efficacy variables were the effect of the 10 weeks of treatment on the rate of BP control (<140/90 mmHg), the responder rate (office SBP and DBP reductions, respectively, >20 and >10 mmHg), the home BP changes from the prerandomization week and the changes in standing office SBP and DBP.
Statistical analysis and sample size calculation
Enrolment of 100 patients in each group (900 total) was required to provide 90% power to detect a difference in mean change from baseline of 3 mmHg (considered as a clinically relevant difference) between monotherapy, combination therapy and placebo at a 0.05 two-sided significance level, assuming a common standard deviation of 6.5 mmHg. Considering approximately a 10% withdrawal rate recruitment of a total of 990 patients was planned.
The study population was analysed on an intention-to-treat (ITT) basis, which included all randomized individuals receiving at least one dose of the study drug who had at least one measurement of office sitting BP within 48 h after taking the study drug(s). The last observation carried forward (LOCF) approach was used. The analysis was performed by using an analysis of covariance (ANCOVA) with treatment and centres as main effects and baseline value as a covariate. The effect of treatment on BP was adjusted for baseline BP values. Medical history and adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) coding dictionary Version 13.0. Prior and concomitant medications were coded by the WHO DRUG Q1-2010 dictionary. All analyses and summaries were conducted by using SAS System Version 9.2 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Study population
A total of 1638 individuals were screened. One thousand and thirty-nine individuals successfully completed the placebo run-in period and were randomly assigned to the double-blind study drug phase (Fig. 1). The primary reason for discontinuation before randomization was failure to satisfy all inclusion/exclusion criteria.
FIGURE 1.
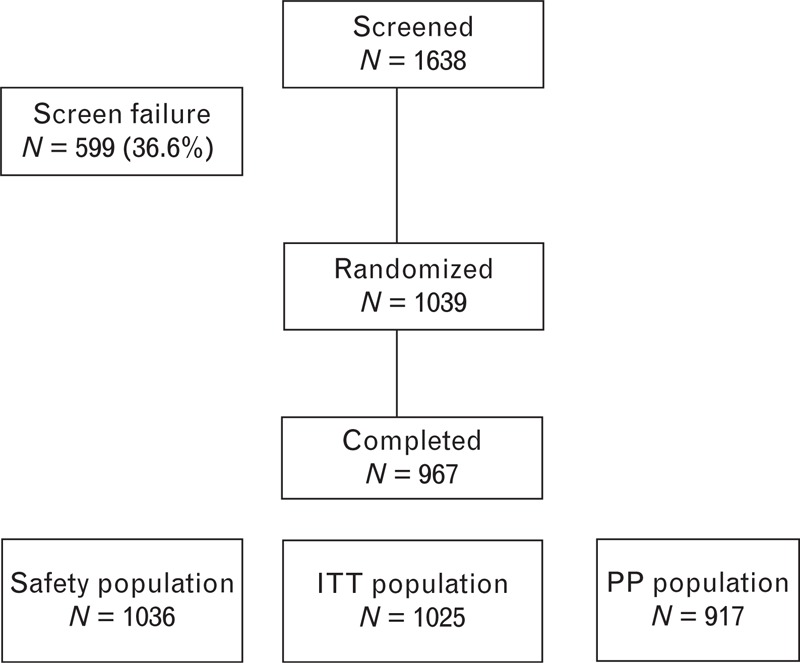
Flow chart of the patients screened and found to be eligible for the study. ITT, intention-to-treat; PP, per protocol.
As summarized in Table 1, among the 1025 individuals included in the ITT population, 491 were men (47.9%) and 99.4% were white. Mean age was 53.9 years, and the percentage of individuals aged 65 years or more 13.3%; the mean BMI was 29.9 kg/m2 and the proportion of obese individuals (BMI ≥ 30 kg/m2) about 40%. There were no statistically significant differences in demographic or other baseline characteristics between treatment groups. In the group as a whole, the mean sitting office DBP and SBP were 103.1 and 159.3 mmHg, respectively, again with no significant between-group differences.
TABLE 1.
Baseline demographic and clinical characteristics of the intention-to-treat population
| Placebo N = 111 | E10 N = 118 | L10 N = 116 | E20 N = 111 | L20 N = 109 | L10 + E10 N = 116 | L10 + E20 N = 118 | L20 + E10 N = 110 | L20 + E20 N = 116 | Overall N = 1025 | |
| Males (%) | 50.5 | 47.5 | 44.8 | 48.6 | 44.0 | 49.1 | 52.5 | 48.2 | 45.7 | 47.9 |
| Age (years) | 54.7 ± 9.8 | 54.8 ± 9.9 | 53.6 ± 9.8 | 52.1 ± 9.4 | 55.4 ± 9.7 | 54.0 ± 10.2 | 53.0 ± 10.1 | 51.9 ± 10.2 | 55.8 ± 10.1 | 53.9 ± 9.10 |
| White (%) | 99.1 | 99.2 | 99.1 | 99.1 | 100 | 100 | 99.2 | 100 | 99.1 | 99.4 |
| BMI (kg/m2) | 29.89 ± 4.9 | 29.34 ± 5.2 | 30.60 ± 4.9 | 30.12 ± 5.2 | 29.73 ± 4.8 | 30.38 ± 4.8 | 29.72 ± 4.8 | 29.86 ± 4.7 | 29.11 ± 5.3 | 29.86 ± 4.10 |
| Office SBP (mmHg) | 160.5 ± 10.5 | 158.8 ± 10.3 | 159.5 ± 10.4 | 159.9 ± 9.7 | 159.2 ± 10.7 | 159.1 ± 10.2 | 159.4 ± 10.5 | 158.6 ± 10.4 | 159.0 ± 10.7 | 159.3 ± 10.4 |
| Office DBP (mmHg) | 103.2 ± 2.4 | 103.2 ± 2.2 | 102.9 ± 2.5 | 103.1 ± 2.6 | 103.3 ± 2.8 | 103.0 ± 2.6 | 102.9 ± 3.4 | 102.8 ± 3.1 | 103.1 ± 3.10 | 103.1 ± 2.9 |
| Office HR (b/min) | 77.6 ± 10.8 | 77.3 ± 11.0 | 77.8 ± 9.3 | 77.9 ± 10.6 | 78.5 ± 9.9 | 79.4 ± 9.5 | 78.7 ± 11.7 | 78.4 ± 11.3 | 77.1 ± 9.9 | 78.1 ± 10.5 |
Data are shown as % or as mean ± standard deviation (SD). E, enalapril; HR, heart rate; ITT, intention to treat; L, lercanidipine.
Home BP measurements in the week before randomization to treatment were available in 969 patients of the ITT population. As expected, home values (148.9 mmHg SBP and 94.8 mmHg DBP, respectively) were noticeably lower than office BP values, but clearly greater than the values defined as the upper limit of home BP normality, that is 135/85 mmHg [1,24] (Table 2).
TABLE 2.
Baseline home DBP and SBP – intention-to-treat population
| Placebo N = 105 | E10 mg N = 109 | L10 mg N = 110 | E20 mg N = 104 | L20 mg N = 103 | L10 + E10 mg N = 114 | L10 + E20 mg N = 113 | L20 + E10 mg N = 102 | L20 + E20 mg N = 109 | Overall N = 969 | |
| Home SBP (mmHg) | 148.0 ± 12.4 | 147.7 ± 12.3 | 150.8 ± 12.1 | 147.2 ± 12.3 | 149.8 ± 12.3 | 150.0 ± 11.5 | 150.1 ± 11.3 | 148.5 ± 12.0 | 148.0 ± 11.9 | 148.9 ± 12.0 |
| Home DBP (mmHg) | 93.5 ± 5.6 | 93.6 ± 6.0 | 95.8 ± 5.8 | 94.9 ± 7.1 | 94.4 ± 5.6 | 95.5 ± 5.7 | 95.7 ± 6.3 | 95.6 ± 6.5 | 94.4 ± 6.1 | 94.8 ± 6.1 |
| Home HR (beats/min) | 75.2 ± 9.8 | 74.4 ± 8.9 | 76.0 ± 8.9 | 77.1 ± 9.5 | 76.2 ± 8.7 | 76.4 ± 10.2 | 75.9 ± 9.6 | 75.9 ± 9.3 | 75.8 ± 9.0 | 75.9 ± 9.3 |
Data are shown as % or as mean ± standard deviation (SD). E, enalapril; HR, heart rate; ITT, intention to treat; L, lercanidipine..
Effects of treatment on office blood pressure
Table 3 summarizes that the 10-week treatment was associated with a significant reduction of office DBP in all groups. Compared with prerandomization values, the reductions were significantly greater with all L/E combinations (L10/E10, L10/E20, L20/E10 and L20/E20) than with placebo (P < 0.001, P = 0.003, P < 0.001 and P < 0.001, respectively), whereas the differences between combination treatments and monotherapies were less consistent. The largest BP-lowering effect was observed in the L20/E20 group in which DBP decreased by 15.2 mmHg in comparison with individuals treated with E20 alone (−11.3 mmHg, P = 0.004) and L20 alone (−13.0 mmHg, P = 0.092). Similar results were obtained for office SBP that decreased by −19.2 mmHg in the L20/E20 group compared with −13.0 mmHg in the L20 group (P = 0.002) and −15.3 mmHg in the E20 group (P = 0.005). In the various treatment groups, BP reductions were similar after immediate assumption of standing position (Table 4) and 2 min later (data not shown).
TABLE 3.
Adjusted office and home blood pressure changes at end of treatment in the intention-to-treat population
| Placebo (N = 111) | L10 mg (N = 116) | L20 mg (N = 109) | E10 mg (N = 118) | E20 mg (N = 111) | L10 + E10 (N = 116) | L10 + E20 (N = 118) | L20 + E10 (N = 110) | L20 + E20 (N = 116) | |
| Office BP (N = 1025) | |||||||||
| SBP (mmHg) | −9.6 | −11.0 | −13.0 | −14.7** | −15.3** | −15.8** | −16.2** | −17.1* | −19.2* |
| DBP (mmHg) | −8.8 | −10.4 | −13.0** | −13.8* | −11.3 | −14.2* | −12.8** | −14.0* | −15.2* |
| Placebo (N = 89) | L10 mg (N = 96) | L20 mg (N = 87) | E10 mg (N = 96) | E20 mg (N = 91) | L10 + E10 (N = 102) | L10 + E20 (N = 103) | L20 + E10 (N = 91) | L20 + E20 (N = 99) | |
| Home BP (N = 854) | |||||||||
| SBP (mmHg) | −2.4 | −8.8* | −7.7** | −9.1* | −9.2* | −11.2* | −10.6* | −9.6* | −13.2* |
| DBP (mmHg) | −1.5 | −4.6** | −5.5** | −6.2* | −5.3** | −6.4* | −6.5* | −6.8* | −7.5* |
Data refer to the differences between last on-treatment visit and baseline, adjusted for baseline values. Means ± SD are shown. P values refer to between-group differences.
*P < 0.001 versus placebo.
**P < 0.05 versus placebo.
TABLE 4.
Difference between office immediate standing and sitting blood pressure at end of treatment in the safety population (N = 1036)
| Placebo (N = 113) | L10 mg (N = 117) | L20 mg (N = 113) | E10 mg (N = 119) | E20 mg (N = 111) | L10 + E10 (N = 117) | L10 + E20 (N = 118) | L20 + E10 (N = 112) | L20 + E20 (N = 116) | |
| SBP (mmHg) | 1.0 (9.8) | −2.9 (7.7) | −0.5 (10.0) | −0.5 (9.6) | 0.3 (8.8) | −0.5 (9.7) | −2.0 (10.7) | 0.5 (9.9) | −1.1 (9.1) |
| DBP (mmHg) | 2.4 (6.7) | 2.8 (5.9) | 3.3 (6.3) | 3.5 (5.8) | 2.7 (6.3) | 4.1 (7.1) | 3.4 (7.0) | 3.5 (7.1) | 2.5 (5.7) |
As shown in Fig. 2, the responder and normalization rates of BP values were achieved by a significantly greater percentage of individuals treated with combination therapy than with placebo and individuals treated with L20/E20 had the highest responder rate and frequency of normalization. In patients receiving the L20/E20 combination, the absolute office BP values were lower than in the placebo and monotherapy groups at all in-treatment visits (Fig. 3).
FIGURE 2.
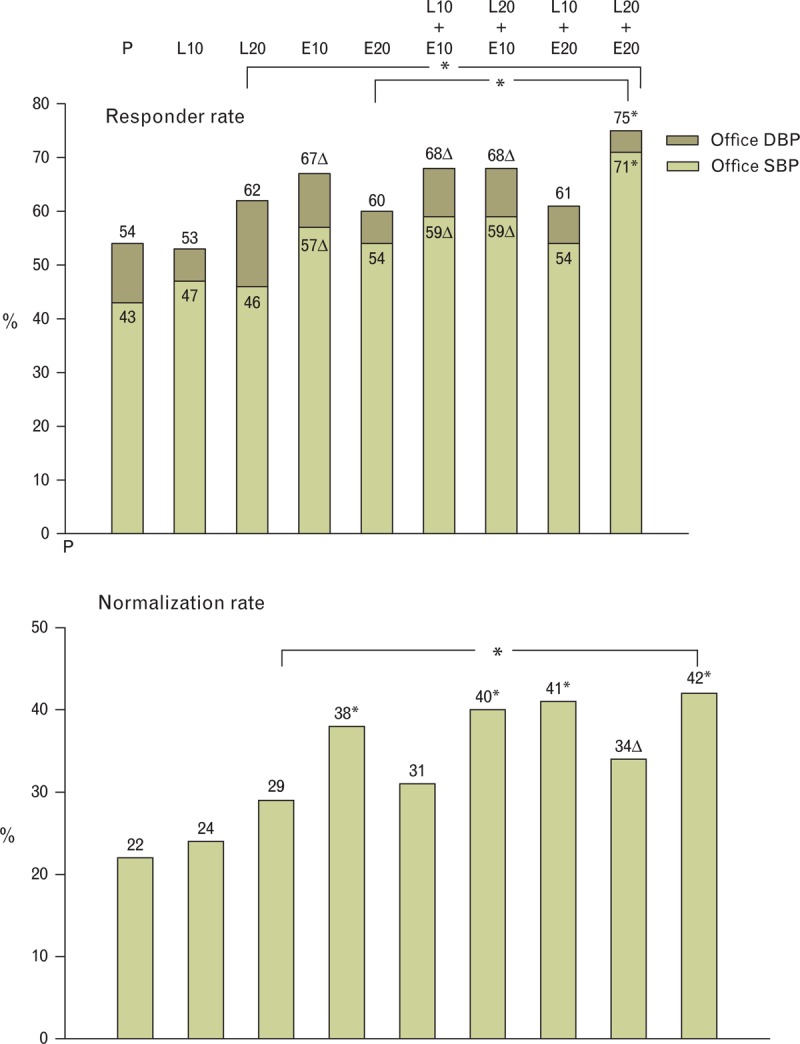
Responder (upper panel) and normalization rate (lower panel) in the study groups. Responder rate was defined as a SBP and DBP reduction respectively greater than 20 and 10 mmHg. Normalization was defined as a BP reduction to <140/90 mmHg. E, enalapril; L, lercanidipine; P, placebo. ∗ and Δ above histograms refer to significant difference (P < 0.0001 and P < 0.005, respectively) versus placebo.
FIGURE 3.
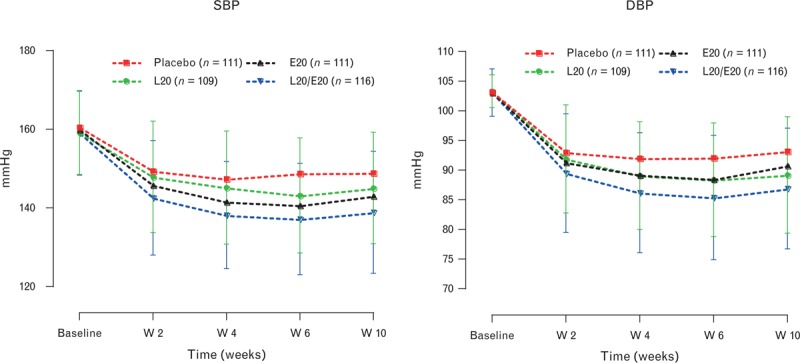
Office SBP and DBP values at baseline and throughout the treatment period in differently treated groups. Explanations as in Figure 2.
Effects of treatment on home blood pressure
Data on the effect of treatment on home BP were available in 854 randomized individuals (ITT population). As summarized in Table 3, the placebo effect was much smaller for home than for office BP. For all active treatments, including the monotherapies, the BP-lowering effect was significantly superior to placebo. The greatest effect was exhibited by the L20/E20 treatment group in which the SBP reductions was significantly and markedly different both versus the L20 and the E20 group (−13.2 versus −7.7 mmHg, P = 0.002, with L20 and −9.2 mmHg, P = 0.021, with E20, respectively). In patients receiving the combination therapy, the absolute BP values were lower than those observed in patients receiving placebo or monotherapies (Fig. 4).
FIGURE 4.
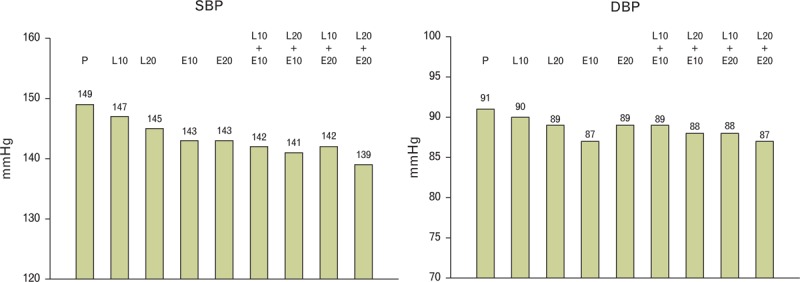
Absolute home true SBP and DBP values during treatment in differently treated groups. Explanations as in Figure 2.
Safety, compliance and tolerability
Out of 1039 randomized patients, 72 (6.9%) discontinued the study, the primary reason being lack of efficacy (23 individuals, 2.2%), mostly occurring in the placebo and monotherapy groups, followed by adverse events (19 individuals, 1.8%), withdrawal of consent (17 individuals, 1.6%) and protocol violation (10 individuals, 1.0%). Two individuals (0.2%) were lost to follow-up and one discontinued for family reasons. Adherence to study medications for the overall treatment period was 99.3 ± 3.5%, similarly for all treatment groups.
As shown in Table 5, in the safety population, 240 individuals (23.2%) reported a total of 348 adverse events, 8.3% of which were considered possibly, probably or definitely related to the study drugs. Eighteen individuals (1.7%) had adverse events that led to withdrawal from the study. There was no difference among the treatment groups in the number of patients exhibiting adverse events or withdrawing from the study because of adverse events.
TABLE 5.
Adverse events during the double-blind treatment period (safety population)a
| Adverse events | Placebo N = 113 | E10 mg N = 119 | L10 mg N = 117 | E20 mg N = 111 | L20 mg N = 113 | L10 + E10 mg N = 117 | L10 + E20 mg N = 118 | L20 + E10 mg N = 112 | L20 + E20 mg N = 116 | Overall N = 1036 |
| Any TEAE | ||||||||||
| No. of events | 36 | 34 | 39 | 43 | 39 | 30 | 50 | 35 | 42 | 348 |
| n (%) | 27 (23.9) | 23 (19.3) | 26 (22.2) | 30 (27.0) | 29 (25.7) | 20 (17.1) | 30 (25.4) | 26 (23.2) | 29 (25.7) | 240 (23.2) |
| Treatment-related adverse eventsb | ||||||||||
| No of events | 8 | 11 | 13 | 14 | 13 | 14 | 16 | 14 | 12 | 115 |
| n (%) | 6 (5.3) | 9 (7.6) | 7 (6.0) | 12 (10.8) | 10 (8.8) | 10 (8.5) | 12 (10.2) | 10 (8.9) | 10 (8.6) | 86 (8.3) |
| Adverse events leading to withdrawal | ||||||||||
| No of events | 2 | 3 | 2 | 2 | 2 | 3 | 1 | 4 | 2 | 21 |
| n (%) | 2 (1.8) | 2 (1.7) | 2 (1.7) | 2 (1.8) | 2 (1.8) | 2 (1.7) | 1 (0.8) | 3 (2.7) | 2 (1.7) | 18 (1.7) |
aAdverse events with onset or increased severity anytime after the date of first dose of double-blind study drug and up to 30 days after the last dose.
bConsidered definitely, probably or possibly related to study drug.
The most frequently occurring treatment-related adverse events were cough, tachycardia and headache. Cough occurred mainly with E20 alone (four individuals; 3.6%) or in combination with L10 (six patients, 5.1%), but was reported by only two individuals (1.7%) in the L20/E20 mg group. The percentage of patients who reported tachycardia or palpitations was highest in the L20 mg group (seven individuals; 6.2%); however, combination therapy with L20/E20 mg reduced this side effect to one individual only (0.9%). The percentage of patients who reported headache ranged from 0.0 to 2.6% in the various groups. Only one patient (0.9%) reported headache in the L20/E20 group. The incidence of peripheral oedema was low in all groups (0.8–2.7%) and no case was reported in the L20/E20 group. The incidence of dizziness or hypotension was not increased by combination therapy (data not shown).
There were five not treatment-related cardiovascular and noncardiovascular events during the study. Two cases (ischemic stroke and worsening of hypertension) occurred in placebo group, one case (acute leukemia) occurred in the L10/E20 mg group and two cases (myocardial infarction and anaphylactic shock) occurred in the L20/E20 mg treatment group.
There were no clinically meaningful changes in any of the laboratory parameters, heart rate, difference between standing and sitting BP or ECGs in any of the treatment groups.
DISCUSSION
The population of the present study was characterized by moderate hypertension, the BP elevation clearly extending also to home BP values. In this population, the administration of L plus E at the daily dose of 20 mg each lowered office DBP significantly more than placebo and the combination components in monotherapy. This was associated with a similarly greater SBP reduction, a larger number (70% approximately) of responders to the drugs administration and a more common achievement of the BP value (<140/90 mmHg) recommended by guidelines as the target to pursue in middle age and elderly hypertensive individuals [1]. It can thus be concluded that the combination of 20 mg of E and 20 mg of L is an effective therapeutic measure in the large number of patients who exhibit a moderate BP increase. It should additionally be emphasized that a large number of our patients were overweight (mean BMI 29.9 kg/m2) or even frankly obese. This is clinically relevant because obesity is a condition frequently associated with hypertension [27] in which BP reduction and control are particularly difficult [1] and a combination of two or more drugs needs to be frequently considered.
The following additional results deserve to be discussed. First, the conclusion that the L20/E20 combination is therapeutically effective is confirmed by the home BP data provided by the study. In line with current knowledge [1,28,29], baseline home BP was lower than office BP, which made the overall BP-lowering effect of treatment less pronounced. Once again, however, the BP reduction obtained with the combination of 20 mg of either E and L was the maximal one observed, the difference being significant compared with all monotherapies, including those in whom only 20 mg of L or 20 mg of E was used.
Second, compared with placebo, all active treatments were well tolerated, and a small number of side effects was also seen for the L20/E20 combination. Indeed, the L20/E20 combination was associated with less cough and leg oedema compared with the combination components in monotherapy. Furthermore, compared with either monotherapy or the L/E combinations at lower doses, the L20/E20 combination did not increase palpitation, dizziness and heart rate and did not show any greater BP-lowering effect on standing than on sitting BP, this being the case either for BP values taken immediately and for those taken after a more prolonged exposure to orthostatic stress. Taken together, these observations imply that the combination of L and E at the highest dose has a favourable tolerability profile and that its greater BP-lowering effect does not translate into a risk of hypotensive episodes nor lead to reflex sympathetic activation.
Third, office BP was reduced throughout the treatment period with combination therapy, including the measurements made 2 weeks after randomization. As mentioned by the recent European guidelines [1], this may represent a further advantage for treatment because at least in hypertensive patients with a more pronounced cardiovascular risk profile, the goal BP should be achieved more promptly to ensure cardiovascular protection also against the possible occurrence of early cardiovascular morbid or fatal events [3]. Furthermore, recent evidence show that early achievement of BP control, such as when combination treatment is used as first step, may reduce discontinuation of the prescribed drug regimen during the chronic treatment phase [26,30]. This also translates into cardiovascular protection because treatment discontinuation is associated with a greater rate of hospitalization for cardiovascular disease [31–33].
Two final points are worthy a mention. One, in the present study, patients exhibited an unusually large placebo effect, which is probably responsible for the fact that, except for the L/E combinations, the office BP reductions exhibited by monotherapies, although by no means quantitatively marginal, were not invariably significantly more pronounced than those seen in the placebo group. However, as known from previous studies [23,26,29,33], the placebo effect was much less pronounced with home BP measurements, which made the difference of home BP effects by active treatments and placebo more consistent. This speaks in favour of a more frequent use of home BP in antihypertensive treatment studies. Two, our study has several elements of strength, including the high number of patients recruited, the double-blind design and the high adherence to treatment in the recruited population. Perhaps the most important element of strength, however, is the high number of patients in whom BP was also measured at home over several days either before or during treatment. This allows to conclude that the effectiveness of the L/E combination applies to daily life BP.
In conclusion, in this large population of adult overweight and even frankly obese patients with moderate hypertension combination therapy with L and E proved to be more effective than placebo in reducing both office BP at trough and BP values taken at home. The greatest effect was observed with L20/E20 that lowered BP significantly more than placebo and L or E monotherapies even when home BP values were considered. Compared with monotherapies, the L20/E20 combination also showed a favourable tolerability profile, that is less cough, tachycardia/palpitations and leg oedema with no increase in dizziness or hypotension. Home BP proved to be a useful tool to evaluate the antihypertensive effect of medications because unlike office BP, it was largely devoid of the confounding effect of placebo-dependant BP fall.
ACKNOWLEDGEMENTS
Conflicts of interest
G.M. has received personal fees as speaker/chairman or consultant from Boehringer Ing., Daiichi Sankyo, Medtronic, Menarini, Novartis, Recordati, Sanofi, Servier, Takeda in the past 3 years. X.G. received honoraria as speaker or consultant from Novartis, Daiichi-Sankyo, Bayer, Menarini in the past 3 years. A.C. has received personal fees from the main drug companies in the cardiovascular area. Y.S. has received personal fees from Recordati. H.H., D.P.-M., I.C. and P.P. have no conflicts of interest to declare.
Reviewer's Summary Evaluation
Reviewer 1
The effect of enalapril and lercanidipine combination at high dose has not been examined with respect to other combinations of the same compounds at lower dose, monotherapy with these compounds or placebo. In this paper the authors confirmed that enalapril and lercanidipine combination at high dose is superior than other combinations and its effects is also seen in home blood pressure. This combination has also been proved to be safe and well tolerated.
Reviewer 2
The study provides significant information for everyday clinical practice and has several strengths: first, the double-blind, randomized, placebo-controlled study design which renders available data of high quality; then, the large number of participants; and the use of home blood pressure monitoring, which accounts for white-coat hypertension and of equal importance attenuates the placebo effect. The inclusion of Caucasian patients alone and the under-representation of elderly patients consist the limitations of the study.
Footnotes
Abbreviations: ANCOVA, analysis of covariance; BP, blood pressure; ITT, intention to treat; LOCF, last observation carried forward; MedDRA, Medical Dictionary for Regulatory Activities
Trial Registration: ClinicalTrials.gov Identifier: NCT01093807.
REFERENCES
- 1.Mancia G, Fagard R, Krzysztof N, Narkiewicz K, Redon J, Zanchetti A, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 2.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 Expert Consensus Document on Hypertension in the Elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2011; 123:2434–2506. [DOI] [PubMed] [Google Scholar]
- 3.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009; 122:290–300. [DOI] [PubMed] [Google Scholar]
- 4.Weber MA, Julius S, Kjeldsen SE, Brunner HR, Ekman S, Hansson L, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet 2004; 363:2049–2051. [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007; 120:713–719. [DOI] [PubMed] [Google Scholar]
- 6.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23:1296–1310. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR. Anglo-Scandinavian Cardiac Outcomes Trial Investigators Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial: blood pressure lowering arm and the relative influence of antihypertensive medication. Diabetes Care 2008; 31:982–988. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence. Hypertension. Clinical management of clinical hypertension in adults. NICE Clinical Guideline 127. August 2011. London, UK: National Clinical Guideline Centre, Royal College of Physicians. [Google Scholar]
- 9.Dahlöf B, Sever P, Poulter NR, Wedel H, Beevers D, Caulfield M, et al. for the ASCOT investigators et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomized controlled trial. Lancet 2005; 366:895–906. [DOI] [PubMed] [Google Scholar]
- 10.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, et al. for the ACCOMPLISH trial investigators Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 11.Lund-Johansen P, Stranden E, Helberg S, Wessel-Aas T, Risberg K, Ronnevik PK, et al. Quantification of leg oedema in postmenopausal hypertensive patients treated with lercanidipine or amlodipine. J Hypertens 2003; 21:1003–1010. [DOI] [PubMed] [Google Scholar]
- 12.Schrier RW, Estacio RO, Jeffers B. Appropriate blood pressure control in NIDDM (ABCD) trial. Diabetologia 1996; 39:1646–1654. [DOI] [PubMed] [Google Scholar]
- 13.Wing LM, Reid CM, Ryan P, et al. for the Second Australian National Blood Pressure Study Group. A comparison of outcomes with angiotensin-converting–enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 2003; 348:583–592. [DOI] [PubMed] [Google Scholar]
- 14.The SOLVD Investigators∗ Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302. [DOI] [PubMed] [Google Scholar]
- 15.The Consensus Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987; 316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 16.Meredith PA. Lercanidipine: a novel lipophilic dihydropyridine calcium antagonist with long duration of action and high vascular selectivity. Exp Opin Invest Drugs 1999; 8:1043–1062. [DOI] [PubMed] [Google Scholar]
- 17.Dalla Vestra M, Pozza G, Mosca A, Grazioli V, Lapolla A, Fioretto P, Crepaldi G. Effect of lercanidipine compared with ramipril on albumin excretion rate in hypertensive type 2 diabetic patients with microalbuminuria: DIAL study. Diab Nutr Mtab 2004; 17:259–266. [PubMed] [Google Scholar]
- 18.Grassi G, Quarti-Trevano F, Scopelliti F, Seravalle G, Cuspidi C, Mancia G. Effect of long term lercanidipine or hydrochlorotiazide administration on hypertension-related vascular structural changes. Blood Pressure 2006; 15:268–274. [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, Seravalle G, Turri C, Bolla G, Mancia G. Short-versus long-term effects of different dihydropyridines on sympathetic and baroreflex function in hypertension. Hypertension 2003; 41:558–562. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Grassi G. The central sympathetic nervous system in hypertension. Handb Clin Neurol 2013; 117:329–335. [DOI] [PubMed] [Google Scholar]
- 21.Hair PI, Scott LJ, Perry CM. Fixed dose-combination Lercanidipine/Enalapril. Drugs 2007; 67:95–106. [DOI] [PubMed] [Google Scholar]
- 22.Chatzikyrkou C, Haller H, Menne J. Efficacy and safety of fixed-dose Lercanidipine-Enalapril for the treatment of hypertension. Clin Med Ther 2009; 1:63–76. [Google Scholar]
- 23.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 2008; 26:1505–1530. [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, Zanchetti A, Agabiti-Rosei E, Benemio G, De Cesaris R, Fogari R, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation 1997; 95:1464–1470. [DOI] [PubMed] [Google Scholar]
- 25.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population. Follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 26.Stergiou GS, Jaenecke B, Giovas PP, Chang A, Chung-Yueh Y, Tan TM. A tool for reliable self-home blood pressure monitoring designed according to the European Society of Hypertension recommendations: the Microlife WatchBP Home monitor. Blood Press Monit 2007; 12:127–131. [DOI] [PubMed] [Google Scholar]
- 27.Bombelli M, Facchetti R, Fodri D, Brambilla G, Sega R, Grassi G, Mancia G. Impact of body mass index and waist circumpherence on the cardiovascular risk and all-cause death in a general population: data from the PAMELA study. Nutr Metab Cardiovasc Dis 2013; 23:650–656. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G, Sega R, Grassi G, Cesana G, Zanchetti A. Defining ambulatory and home blood pressure normality: further considerations based on data from the PAMELA study. J Hypertens 2001; 19:995–999. [DOI] [PubMed] [Google Scholar]
- 29.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European Society of Hypertension guidelines for blood pressure monitoring. J Hum Hypertens 2010; 24:779–785. [DOI] [PubMed] [Google Scholar]
- 30.Corrao G, Parodi A, Zambon A, Heiman F, Filippi A, Cricelli C, et al. Reduced discontinuation of antihypertensive treatment by two-drug combination as first step. Evidence from daily life practice. J Hypertens 2010; 28:1584–1590. [DOI] [PubMed] [Google Scholar]
- 31.Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, Mancia G. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens 2011; 29:610–618. [DOI] [PubMed] [Google Scholar]
- 32.Sokol MD, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005; 43:521–530. [DOI] [PubMed] [Google Scholar]
- 33.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006; 333:15. [DOI] [PMC free article] [PubMed] [Google Scholar]


