Abstract
Background:
The efficacy of steroid administration in the prevention of postextubation complications in critical care remains controversial. The purpose of this study was to determine whether administration of nebulized budesonide in critically ill patients reduces the occurrence of postextubation airway complications.
Materials and Methods:
This was a prospective, randomized, double-blind, placebo-controlled study. We prospectively followed up 70 adult patients in the intensive care unit who were intubated for more than 48 h. Patients received either nebulized budesonide (1 mg every 12 h; n = 35) or placebo (normal saline; n = 35) until 48 h after extubation. Then, the postextubation complications were assessed and recorded within 48 h of extubation.
Results:
The incidence of respiratory distress was lower in budesonide group (8.6% vs. 31.4%, P = 0.017). Reintubation with mechanical support was necessary in 8.6% (3.35) of patients in the budesonide group and 31.4% (11.35) of patients in the placebo group (P = 0.017).
Conclusion:
Nebulized budesonide after extubation is effective in reducing the incidence of reintubation and respiratory distress in adult patients.
Keywords: Budesonide, extubation complication, intensive care unit, nebulized
INTRODUCTION
To facilitate mechanical ventilation in the operating room and intensive care unit, endotracheal intubation is needed; but in case of prolonged intubation, this procedure may be associated with the potential development of glottic or subglottic edema due to mucosal damage, resulting in stridor shortly after extubation.[1]
Postextubation laryngeal edema may necessitate medical intervention and reintubation, which may result in increased morbidity [e.g., prolonged length of intensive care unit (ICU) stay, additional costs] and mortality.[2]
It is reported that the prevalence of postextubation stridor ranges between 3.5% and 22% in intubated patients and between 1% and 17% in critically ill patients, therefore upper airway potency may be assessed indirectly in patients at risk to develop postextubation edema by test. The reduced percent cuff leak (PCL) can predict the occurrence of laryngotracheal edema in high-risk patients.[1,2,3]
Laryngeal edema is more common after endotracheal intubation for more than 36 h. Other factors which are correlated with these complications include older age, female gender, low Glasgow Coma Scale (GCS) score, and excessive endotracheal tube (ETT) size.[4]
Any intervention that increases the chances of successful extubation is therefore of great interest. Previous studies reported conflicting results regarding the effectiveness of prophylactic steroid therapy on laryngeal edema, and steroid after extubation. Almost in all of these studies, patients received corticosteroid prior to extubation. However, the timing and methods of administration were different.[5,6]
Aerosolized budesonide, when used in the treatment of croup, was found to reduce edema without any side effects. Budesonide is a steroid used for the treatment of asthma and non-infectious rhinitis (including hay fever and other allergies), and for the treatment and prevention of nasal polyposis.[7,8]
The present study was conducted to evaluate the effect of administration of nebulized budesonide after extubation. The specific objectives of our study were to determine whether multiple doses of nebulized budesonide are effective in reducing or preventing postextubation edema.
MATERIALS AND METHODS
After obtaining institutional ethics committee approval and taking written informed consent from patients or their relatives prior to entrance in this trial, 70 adult (18-65 years) intubated ICU patients between 1 April 2010 and 1 November 2011, who met the inclusion criteria, were included in this randomized, double-blind, prospective study.
Patients who were intubated for more than 48 h after surgery, and met the weaning criteria defined as respiratory rate (RR) <30 breaths/min, negative tidal volume >5 ml/kg ideal body weight, and shallow index (RR/tidal volume) <105 breaths/min/l were included.
The exclusion criteria included any history of corticosteroid therapy in the previous week, nasal or throat disease/surgery, pulmonary airway disease, and gastrointestinal bleeding.
Cuff leak test
One of the ways to do cuff leak test to anticipate postextubation stridor is to measure the PCL. To measure it, we recorded the difference in exhaled tidal volume before and after the endotracheal tube's cuff deflation. Divide this number by the exhaled tidal volume before cuff deflation. This gives the PCL. Patients with a cuff leak of less than 10% are at risk for shorter or reintubation. Patients who met the weaning criteria were therefore included in this trial. Following extubation, all patients received humidified oxygen by nasal prongs or facemask with an oxygen flow of 6 l/min. Patients also randomly received either nebulized budesonide (1 mg/4ml every 12 h for 48 h) [(Group I (B); n = 35] or normal saline at an equivalent volume (placebo) (4 ml every 12 h for 48 h) [Group II (S); n = 35] via nebulizer face mask with an oxygen flow of 6 l/min in 20 min.
A physician who was not involved in the patient care prepared the study drug and the identical-looking placebo, and the other physician administered the treatment (i.e., budesonide or placebo). Both physicians were blinded.
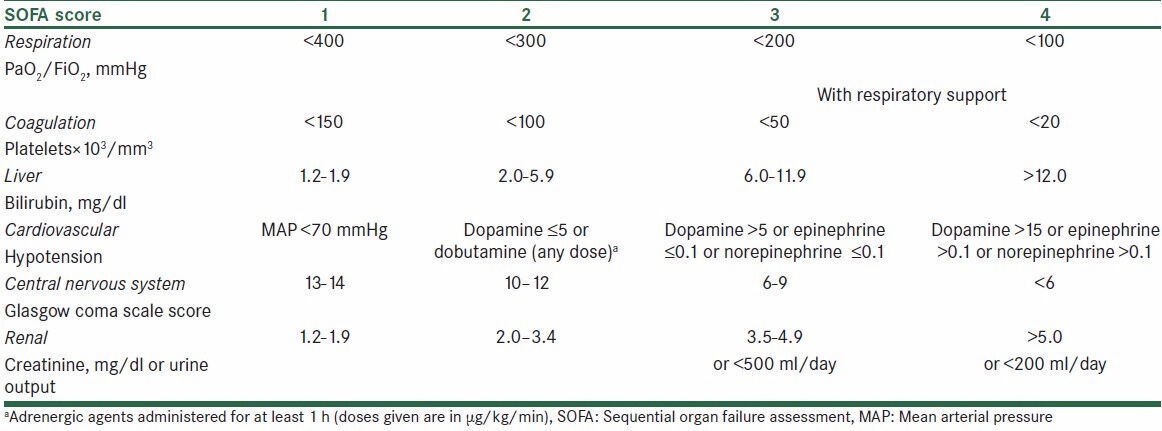
RR, heart rate (HR), blood pressure (BP), and oxygen saturation (SPO2) were recorded for each patient immediately before aerosol administration (time 0), at 30 and 60 min, and at 2, 4, 8, 12, 24, 36, and 48 h. The presence of respiratory distress stridor (heard with the aid of stethoscope), the need to reintubation, and the sequential organ failure assessment (SOFA) score were recorded within 48 h of extubation. SOFA score is used to evaluate patient's status during ICU admission [Table 1].
Table 1.
The SOFA score
To calculate PCL before extubation, patients were mechanically ventilated in the volume-assisted control mode with a tidal volume of 8 ml/kg of ideal body weight, an RR of 12 breaths/minute, and a zero positive end expiratory pressure (PEEP). This selected inspiratory tidal volume displayed as an expiratory tidal volume which was recorded.
The balloon cuff was deflated, and the expiratory tidal volume was recorded over the six subsequent respiratory cycles, and the average of the lowest three values was used for subsequent analyses. The PCL was determined as the percentage of volume that leaked.
Respiratory distress is defined as: (a) respiratory acidosis: An arterial pH of less than 7.35 with a partial pressure of arterial carbon dioxide of more than 45 mm Hg, (b) clinical signs suggestive of respiratory muscle fatigue or increased respiratory effort (i.e., use of accessory muscles, intercostals’ retraction, or paradoxical motion of the abdomen), (c) an RR of more than 25 breaths/min for two consecutive hours, and (d) hypoxemia: An arterial oxygen saturation of less than 90% or a PaO2 of less than 80 mmHg with an FiO2 of more than 50%.
Patients were reintubated with mechanical ventilation support if they met at least one of the following criteria: (a) a pH of less than 7.3 with a pressure of carbon dioxide increase of more than 15 mmHg, (b) a change in mental status rendering the patient unable to tolerate non-invasive respiratory support, (c) a decrease in the oxygen saturation to less than 85% despite the use of a high FiO2 (a PaO2 of less than 50 mmHg with an FiO2 more than 70%), (d) lack of improvement in signs of respiratory muscle fatigue, (e) hypotension with a systolic blood pressure (SBP) of less than 80 mmHg more than 30 min despite adequate volume challenge, (f) a diastolic blood pressure drop of more than 20 mmHg, or (g) copious secretions that could not be cleared adequately or that were associated with acidosis, hypoxemia, or change in mental status (somnolence, agitation, or diaphoresis).
Statistical analysis
The results are expressed as mean ± standard deviation (SD) for continuous variables and frequencies (%) for categorical variables. Statistical analyses were conducted using the SPSS software package (version 18.0). Univariate analyses between the two groups were conducted using Student's t-test for continuous variables and Pearson Chi-square test for categorical variables. For all tests, P value of less than 0.05 was considered statistically significant.
RESULTS
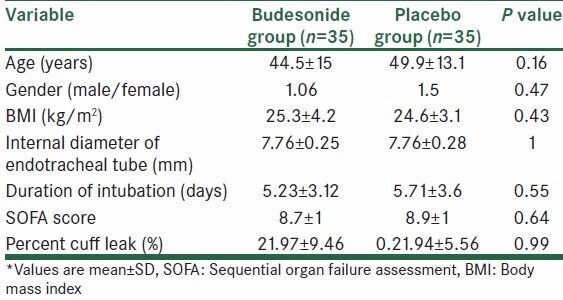
Seventy patients met the inclusion criteria. These patients were randomly assigned to the budesonide group (n = 35) or the placebo group (n = 35). The budesonide and placebo groups did not significantly differ in the demographic characteristics including age, gender, body mass index (BMI), duration of intubation, diameter of ETT, SOFA score, and PCL [Table 2].
Table 2.
Demographic data of patients*
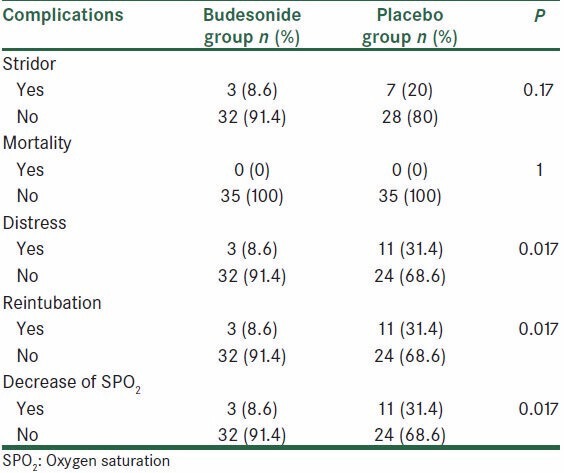
The trends in RR, HR, BP, SPO2, pH, PaCO2, and HCO3 in both groups were not significantly different when assessed over time. The incidence of postextubation stridor was 8.6% (3.35) in the budesonide group and 20% (7.35) in the placebo group. However, the difference in the postextubation stridor rate was not statistically significant between the budesonide and the placebo groups (P = 0.17).
There was statistically significant difference in respiratory distress and reintubation rate between the two groups. The incidence of respiratory distress was lower in the budesonide group (8.6% vs. 31.4%, P = 0.017). Reintubation with mechanical support was necessary in 8.6% (3.35) of patients in the budesonide group and 31.4% (11.35) of patients in the placebo group (P = 0.017). There was no mortality during the period of our study [Table 3].
Table 3.
Frequency distribution of postextubation complications in the two groups
DISCUSSION
The aim of this study was to determine the effect of nebulized budesonide in preventing postextubation complications in critically ill patients. Postoperative complications such as sore throat and hoarseness are the common complaints in patients receiving tracheal intubation. Although not major complications and usually self-limiting, they affect the satisfaction of patients to a significant extent. Steroids, known for their anti-inflammatory function, are widely used in clinic. The inhaled corticosteroids (ICSs), in particular, are widely used for patients at risk of airway diseases, as they can be directly delivered to the airways without introducing a systemic exposure. Previous studies have shown that ICSs are capable of decreasing the incidence and severity of postoperative complications such as sore throat, cough, and hoarseness caused by tracheal intubation.[1,2]
Endotracheal intubation is a useful approach to manage critically ill patients who may or may not require mechanical ventilation; however, this intervention may induce laryngeal mucosa trauma as a result of mucosa abrasion and necrosis secondary to compression, which leads to postextubation stridor. Histological findings of postextubation stridor are characterized by mucosal inflammation, mucosal ulceration, and edema. Corticosteroids have been used in infants and children to prevent or reduce postextubation stridor by suppressing mucosal inflammation, inhibition of leukocyte migration, maintenance of cell membrane integrity, attenuation of lysosome release, and reduction of fibroblast proliferation and tissue swelling.[2,3,8,11] There are few data in adult patients, compared with pediatric patients, with respect to the use of corticosteroids.
In our study, there was no significant difference between the two groups in demographic characteristics. Thus, the above groups are comparable and probably there is not any confounding effect between the two groups. Also, according to results of this study, no statistically significant difference was found between two groups in RR, HR, BP, SPO2, pH, PaCO2, and HCO3. As the results, nebulized budesonide had no harmful effects on hemodynamic of patients. Also, respiratory distress and reintubation rate in the intervention group were lower than in the placebo group (8.6% vs. 31.4%). In addition, reintubation with mechanical support was necessary in 8.6% of patients in the budesonide group, but in 31.4% of patients in the placebo group. Some other studies showed that the use of nebulized budesonide can lead to decrease in postextubation complications. A study done by Chao-Hsienlee et al. showed that prevalence of Chao-Hsienlee stridor was 6%-37%,[7,8] but in our study, the prevalence of stridor was 8.6% in patients with nebulized budesonide. According to Sinha et al.'s study, use of nebulized L-epinephrine (1000 μg) can decrease postextubation complications.[8,9,10] It is believed that the postoperative symptoms are the result of mucosal injury-induced inflammation. Under normal circumstances, the inflammation of mucosa (mucositis) is considered as an immune response to airway instrumentation (i.e., laryngoscopy and suctioning) or irritating foreign objects (i.e., endotracheal tube, laryngeal mask airway (LMA), or oral airway). In the studies performed by Bellissant and Malhotra, use of corticosteroids was found to decrease the airway complications.[10,11] After the potential role of inflammation in the postoperative squeal of airways had been recognized, the use of inhaled and topical corticosteroids was described. It is well known that ICSs’ inhalation is a direct method of treating airway diseases [i.e., asthma, chronic obstructive pulmonary disease (COPD), and acute pharyngitis] due to their high efficacy in controlling and preventing symptoms with reduced systemic side effects, compared to the therapy with other corticosteroids. The risk-benefit ratio of ICSs is determined by multiple factors. Beneficial properties include low oral bioavailability, high systemic clearance, and sufficient receptor-binding affinity. Since ICSs’ inhalation possesses these beneficial properties, it has been considered a more secure treatment. In conclusion, our study indicated that administration of nebulized budesonide before tracheal extubation could significantly prevent and reduce the occurrence of throat complaints in ICU patients.
ACKNOWLEDGEMENT
The authors wish to sincerely thank the support of all the colleagues in intensive care unit staff of Alzahra University Hospital affiliated to Isfahan University of Medical Sciences in Isfahan, Iran. Furthermore, our special thanks to the patients, who wholeheartedly and actively assisted us to carry out this research. No conflict of interest existed. This prospective randomized observational study was approved by the Ethics Committee of our university, (Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran) and all patients gave written, informed consent.
Footnotes
Source of Support: Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Epstein SK. Corticosteroids prevent of post-extubation upper airway obstruction. Crit Care. 2007;11:156. doi: 10.1186/cc5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng KC, Hou CC, Huang HC, Lin SC, Zhang H. Intravenous injection methylprednisolone reduces the incidence of post-extubation stridor in ICU. Crit Care Med. 2006;34:1345–50. doi: 10.1097/01.CCM.0000214678.92134.BD. [DOI] [PubMed] [Google Scholar]
- 3.Meade MO, Guyatt GH, Cook DJ, Sinuff T, Butler R. Trial of corticosteroids to prevent of post-extubation airway complications. Chest. 2001;120(6 Suppl):464S–8. doi: 10.1378/chest.120.6_suppl.464s. [DOI] [PubMed] [Google Scholar]
- 4.Wang CL, Tsai YH, Huang CC, Wu YK, Ye MZ, Chou HM, et al. The role of cuff leak test in predicting the effect of corticosteroid treatment on post-extubation stridor. Chang Gung Med J. 2007;30:53–61. [PubMed] [Google Scholar]
- 5.Wittekamp BH, van Mook WN, Tjan DH, Zwaveling JH, Bergmans DC. Post-extubation laryngeal edema and extubation failure. Crit Care. 2009;13:233. doi: 10.1186/cc8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandhu RS, Pasquale MD, Miller K, Wasser TE. Measurement of endotracheal tube cuff leak to predict postextubatoin strior and need for reintubation. J Am Coll Surg. 2000;190:682–7. doi: 10.1016/s1072-7515(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Peng MJ, Wu CL. Dexametas one to prevent post-extubation airway obstraction in adults. Crit Care. 2007;11:R72. doi: 10.1186/cc5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha A, Jayashree M, Singhi S. Aerosolized L-epinephrine vs budesonide for post-extubation stridor. Indian Pediatr. 2010;47:317–22. doi: 10.1007/s13312-010-0060-z. [DOI] [PubMed] [Google Scholar]
- 9.Young D, Watkinson P. Preventing post-extubation airway complication in adults. BMJ. 2008;337:a1565. doi: 10.1136/bmj.a1565. [DOI] [PubMed] [Google Scholar]
- 10.François B, Bellissant E, Gissot V, Desachy A, Normand S, Boulain T, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: A randomised double-blind trial. Lancet. 2007;369:1083–9. doi: 10.1016/S0140-6736(07)60526-1. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra SA, Curgoo AN, Shora AK, Farooqi MS, Qazi BA, Dar SQ. Role of dexamethasone in prevention of post extubation upper airway complications in paediatric patients in an intensive care unit. Internet J Anesthesiol. 2009;19:1. [Google Scholar]


