Abstract
Asthma is a prevalent chronic disease of the respiratory system and acute asthma exacerbations are among the most common causes of presentation to the emergency department (ED) and admission to hospital particularly in children. Bronchial airways inflammation is the most prominent pathological feature of asthma. Inhaled corticosteroids (ICS), through their anti-inflammatory effects have been the mainstay of treatment of asthma for many years. Systemic and ICS are also used in the treatment of acute asthma exacerbations. Several international asthma management guidelines recommend the use of systemic corticosteroids in the management of moderate to severe acute asthma early upon presentation to the ED. On the other hand, ICS use in the management acute asthma has been studied in different contexts with encouraging results in some and negative in others. This review sheds some light on the role of systemic and ICS in the management of acute asthma and discusses the current evidence behind their different ways of application particularly in relation to new developments in the field.
Keywords: Acute asthma, emergency department, inhaled corticosteroids, systemic corticosteroids
Pathophysiology of Acute Asthma: Brief Overview
Asthma is a chronic respiratory disease that is prevalent worldwide. It is considered as a major cause of morbidity and a main contributor to the high health care expenditure especially in developed countries.[1] There are two major pathological features in asthmatics' airways, inflammation, and hyperresponsiveness. These features are interrelated, but not totally dependent on each other.[2] Airway inflammatory changes include increased airway mucus secretions, airway wall edema, inflammatory cellular infiltrates, epithelial cell damage, smooth muscle hypertrophy, and submucosal fibrosis.[3] The cellular infiltrates are mainly composed of eosinophils, neutrophils, mast cells, lymphocytes, basophils, and macrophages. The ratio of these cells may widely vary between patients indicating asthma heterogeneity.[4] Asthma is classically divided into three main immunopathological phenotypes: Eosinophilic, neutrophilic, and paucigranulocytic. The eosinophilic phenotype is characterized by increased eosinophilic infiltration of the airways. Patients tend to be atopic, have asthma triggered by exposure to allergens and tend to respond well to corticosteroids. The neutrophilic phenotype is characterized by increased neutrophilic infiltration of the airways. Patients tend to have severe, more aggressive, and poorly controlled asthma. They usually do not respond to corticosteroids as well as the eosinophilic type. In the paucigranulocytic phenotype, bronchial neutrophils, and eosinophils are much lower.[4]
Asthmatic patients frequently experience acute exacerbations. These exacerbations are usually triggered by allergens; including pollens, animal dander, dust mites, and mold; viral respiratory tract infections; irritants such as smoke and dust; cold air and exercise. The most common cause of acute asthma exacerbation in both adults and children, but more in children, is viral respiratory tract infections. Viruses may be responsible for up to 80% of wheezing episodes in children and 50-75% of episodes in adults.[5] Many viruses can cause exacerbation of asthma symptoms, the most important and most common is rhinovirus.[6] Respiratory syncycial virus and influenza virus also cause significant proportion of exacerbations. Airway epithelial cells play a major role in the pathology of virally induced asthma exacerbation. In response to infection they secret chemokines like interleukin-8 and CCL-5 that can attract inflammatory cells including neutrophils and lymphocytes that could exacerbate the already existing allergic inflammation.[7] This finding is supported by epidemiologic observations that allergen sensitization and respiratory viral infections can synergize to cause asthma exacerbation.[8] Children who are atopic are more likely to have virally induced wheezing and respiratory distress than nonatopic children.[9] Bacteria like Hemophilus influenze and Moraxella catarrhalis, have been recently shown to be associated with acute wheezing episodes in children.[10] Their role and the role of atypical bacteria as triggers of acute asthma are still controversial.[11]
Current Treatment of Acute Asthma
Acute asthma exacerbations are defined as “episodes of progressive increase in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms.”[12,13] Most recently, an expert group formed by the National Institutes of Health agreed to define acute asthma as “a worsening of asthma requiring the use of systemic corticosteroids to prevent a serious outcome.”[14] Acute exacerbation of asthma symptoms is a common complication of the disease. The frequency in which exacerbations happen vary widely depending on the severity of disease,[15] the degree of control with prophylactic medications,[16] and exposure to triggers. In a multicenter study from the US,[17] the admission rate of all comers to the emergency department (ED) with acute asthma was 23%. On the other hand, a European study showed that only about 7% of all patients with acute asthma exacerbation required hospitalization.[18] We have a similar experience in Saudi Arabia where about 8% of all asthmatics with acute exacerbation are hospitalized, but if we look at only the severe group the rate goes up to 40%.[19] These epidemiological data underscores the importance of effective treatment of asthma exacerbations and their prevention.
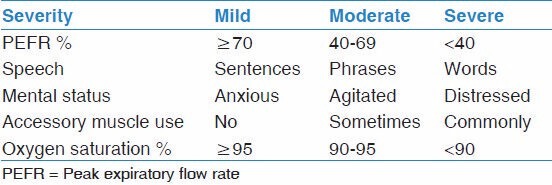
Examination of patients with acute asthma may reveal increased respiratory rate, retractions (accessory respiratory muscle use), wheezing, oxygen desaturation on pulse oximetry and in more severe cases, inability to speak, silent chest, with reduced respiratory lung volumes, cyanosis, and change in mental status. Asthma exacerbations can be classified as mild, moderate, or severe based on the assessment of a group of signs and symptoms as illustrated in Table 1.[20]
Table 1.
General classification of asthma severity
In patients with mild asthma exacerbation, inhaled β2-agonists like albuterol (salbutamol) are usually sufficient in resolving symptoms. The dose can be repeated 3 times every 15-20 min. Levalbuterol, the (R)-enantiomer of albuterol is the effective form of the drug, but clinical trials did not show any advantage of using it over albuterol in terms of efficacy or side-effects.[21] Most patients with mild asthma exacerbation will not require systemic corticosteroids. However, it is recommended that patients who take them regularly or patients who fail initial treatment with albuterol should be given systemic corticosteroids.
Current guidelines recommend that patients with moderate exacerbation should receive three doses of inhaled or nebulized β2-agonist every 15-20 min in the 1st h.[22] Additional doses may be repeated in the next 2-3 h every 30-60 min. All those patients should be treated with systemic corticosteroids at a dose of 2 mg/kg or a maximum dose of 80 mg early in the course of management as it takes at least 4 h to start working.[23] Doses more than 80 mg will not confer any additional benefit. Systemic corticosteroids were found to speed resolution of symptoms, decrease the rate of admission and decrease the rate of relapse if administered for 3-5 days after the acute exacerbation. More detailed discussion about the use of systemic corticosteroids in the treatment of acute asthma can be found below.
Patients with severe asthma exacerbation should obviously be treated more aggressively. High dose inhaled (8-12 puffs) or nebulized β2-agonist should be given every 15-20 min at least in the 1st h, which could be repeated for up to 4 h as required. Data are conflicting whether continuous nebulization using β2-agonist is superior to intermittent nebulization.[22,24] Practically, continuous high dose nebulization could be used for the 1st h and then intermittent nebulization thereafter as required. Ipratropium bromide has been shown to decrease the rate of hospitalization and shorten the stay in the ED in patients with severe or moderate to severe asthma exacerbation in many clinical trials.[25,26,27] Therefore, it is recommended to add it to each treatment of β2-agonist at least in the 1st h of therapy. Its use in patients after admission to the hospital was not shown to make a difference. Systemic corticosteroids should be used as mentioned in patients with moderate exacerbation. Other treatment modalities may be considered like magnesium sulfate and helium oxygen (heliox) therapy in the more severe and nonresponsive patients. Subcutaneous or intravenous (IV) β2-agonists,[28] IV aminophylline,[29] IV montelukast,[30,31] or oral montelukast added to standard therapy in the ED[32] were not shown to be helpful in the treatment of patients with severe asthma exacerbation and therefore are not recommended. Moreover, oral montelukast given to patients post discharge for 5 days was also shown not to be helpful.[33]
β2-agonists can be delivered through a nebulizer or by metered dose inhaler (MDI) with a holding chamber. An MDI dose of 4-8 puffs depending on age is equivalent to a nebulized dose of 2.5-5 mg of albuterol.[34] Nebulizer is preferable in cases of severe symptoms when patients are unable to use the MDI effectively or if other nebulized medications are needed to be mixed with albuterol at the same time or if the patient is requiring oxygen supplementation. Oxygen therapy should be given to maintain saturation ≥90% in adults and ≥95% in pregnant women or children.
Patients who maintain normal oxygen saturation, have no or minimal wheezing on chest auscultation, and have no or mild intercostal retractions can be discharged home after 1 h of assessment on no additional medications in the ED. However, these patients should have a step up in their maintenance medications to prevent relapse. Patients who fail to achieve improvement after 4 h of treatment should be admitted to the hospital for further aggressive therapy.
Introduction and Evolution of Corticosteroids in the Management of Asthma: Historical Background
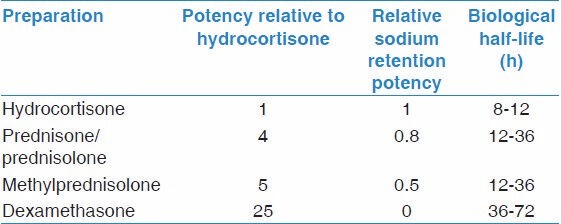
Shortly after the discovery of the structure of adrenal steroid hormones, Hench et al. examined using cortisone to treat arthritis in 1949. The effect was remarkable and that work won the Nobel Prize the next year. It also started a series of trials of corticosteroids in various inflammatory conditions. The first use of corticosteroid to treat acute asthma exacerbation was in 1956.[35] Development of corticosteroids that have less mineralocorticoid activity, like prednisone, and later those that have no mineralocorticoid activity, like dexamethasone, made corticosteroids more attractive therapies to use in asthma. In 1972, Clark showed for the 1st time that inhaled beclomethasone was effective in the management of asthma with less adverse effects than systemic steroids.[36] Numerous reports came afterwards describing the efficacy of oral prednisone and prednisolone , IV methylprednisolone and ICS such as triamcinolone, budesonide, and fluticasone in the management of asthma. These effects are mediated through various genomic and nongenomic mechanisms.[37] Table 2 shows some common systemic corticosteroids and their relative potency.
Table 2.
Common types of systemic corticosteroids and their relative properties
Clinical Evidence of the Effect of Corticosteroids in Acute Asthma
Systemic corticosteroids
Systemic corticosteroids given early in the course of treatment of acute asthma exacerbations in the ED were overall shown to be effective and are recommended by different asthma guidelines like GINA and EPR3. Littenberg and Gluck initially showed that they decrease hospital admission rate.[38] Five subsequent studies had, however, conflicting results. Rodrigo and Rodrigo reviewed all these six studies and concluded that there was no improvement in hospital admission rate or lung function.[39] They, however, reported a trend of improvement in lung function only with medium or high doses systemic corticosteroids. Hence, data in terms of lung function are more encouraging.[40,41] In terms of effect on exacerbation relapse after discharge from the ED, most studies showed less relapse with systemic corticosteroids[35,42] although others did not.[43] One important issue with all these studies is the low number of recruited patients. Almost all had subject number <100 per study and all were performed in adults. On the other hand, Krishnan et al. recently reviewed nine published studies on the use of systemic corticosteroids in acute asthma in adults and concluded “systemic corticosteroids provide clinically meaningful benefits in patients presenting with acute asthma.”[44] In children, more limited data showed benefit of systemic steroids used early in the ED with decreased rate of admission.[45] A Cochrane database review by Rowe et al. showed decrease rate of admission in patients with acute asthma with the use of systemic corticosteroids in adults and children, especially those with severe asthma and those not currently receiving steroids.[46]
There is no significant added benefit from systemic corticosteroids when given at doses above 60-80 mg/day or 2 mg/kg/day in regards to pulmonary function, rate of admission, or length of stay in the hospital. For example, Marquette et al. compared 1 mg/kg/day to 6 mg/kg/day methylprednisolone in 47 adults hospitalized with severe acute asthma and found no benefit of the high dose over the low dose.[47] Manser et al. performed a systematic review of randomized controlled studies of patients with acute severe asthma comparing different doses of corticosteroids with a minimum follow-up of 24 h. They divided the different doses used in the included trials into three groups as an equivalent dose of methylprednisolone over 24 h; low dose (≤80 mg), medium dose (>80 mg and ≤360 mg), and high dose (>360 mg). Nine trials were included with a total patients' number of 344 adults. They found no difference between the different doses.[48]
Studies also showed no difference in the efficacy or onset of action between oral and IV administration. Fifty-two adults with severe acute asthma were treated with either IV hydrocortisone or prednisolone. There was no difference in their peak flow measurements 24 h after admission.[49] Ratto et al. compared four different doses of methylprednisolone; 160 or 320 mg given orally, or 500 or 1000 mg given IV in four divided doses in adults with acute asthma and found no difference in their forced expiratory volume in 1st second (FEV1) measurements or length of hospitalization.[50] In children also, oral prednisolone was found equivalent to IV methylprednisolone in regards to patients' length of hospital stay.[51] In addition, oral treatment was more cost-effective. GINA and the EPR3 guidelines prefer oral administration because it is less invasive except in patients with absorption problems or those who are not able to take orally due to the severity of their respiratory distress or because they are vomiting.
Prescribing a short course of oral corticosteroids following the ED treatment of acute asthma exacerbations was found to reduce the rate of relapse.[52] However, courses longer than 5 days were not found to provide any additional benefit.[53,54] In children, a single dose of dexamethasone 0.6 mg/kg (maximum 18 mg) was found to be equivalent to prednisolone 2 mg/kg/d in two divided doses for 5 days in terms of symptoms resolution.[55] There is also no benefit from using a dose taper over fixed-dose regimen.[44] Due to poor compliance on oral prednisone after discharge from the emergency, intramuscular injection of methylprednisolone was studied as an alternative but was not found superior, plus there was an evidence of injection-site adverse reactions like pain and bruising.[44]
Inhaled corticosteroids
The use of ICS in the treatment of acute asthma was studied in four contexts:
In comparison to placebo,
In comparison to systemic corticosteroids,
As add on therapy to systemic steroids with continuation after discharge from the ED, or
As add on therapy to systemic steroids within the ED stay period only.
In the first context, a systematic review that looked at eight randomized and blinded studies comparing the efficacy of ICS to placebo in acute asthma exacerbation suggested that ICS are superior to placebo especially when given at high doses (>1 mg of budesonide or fluticasone) and to patients with severe exacerbations.[56] It is important to note that those studies were quite heterogeneous in terms of the severity of asthma in recruited patients, the dose and frequency of ICS administered, and in their outcome measures that included clinical symptoms, pulmonary function, oxygen saturation, admission rate, or relapse rate. In addition, a recent study found that preemptive use of high dose fluticasone (750 mcg BID) at the onset of an upper respiratory tract infection in children with recurrent virus induced wheezing and continuing it for 10 days, reduced the use of rescue oral corticosteroids.[57]
When ICSs were compared with systemic corticosteroids in randomized and blinded studies the conclusions were conflicting. Some studies reported superiority of systemic steroids in reducing admission rate,[58] some reported equal efficacy in relation to admission rate as well,[59,60,61] and some reported superiority of ICS.[62,63] A major study compared high dose fluticasone in the ED and for 5 days post discharge to systemic corticosteroids in the same period in patients with mild to moderate asthma found that oral prednisolone lead to faster improvement in FEV1 at 4 h in the ED and less relapse rate at 48 h post discharge.[64] One recent study showed that in patients who were given systemic corticosteroids plus ICS post discharge from the ED, stopping the systemic corticosteroids after 1 week resulted in rebound in the level of patients' exhaled nitric oxide 2 weeks post discharge despite continuing ICS with no effect on the use of rescue medications or on FEV1.[65] GINA guidelines state that “ICS are effective as part of therapy for asthma exacerbations… and can be as effective as oral corticosteroids at preventing relapses,”[13] while the EPR3 guidelines state that “high doses of ICS may be considered in the ED, although current evidence is insufficient to permit conclusions about using ICS rather than oral systemic corticosteroids in the ED.”[12]
Inhaled corticosteroids were also used as add on therapy to systemic corticosteroids in the ED and continued after discharge. In this context, Rowe et al. found a decrease in relapse rate when 1600 mcg/day budesonide for 21 days was added to a 7-day course of 50 mg/day prednisone as compared with placebo.[66] On the other hand, Brenner et al. found no difference in the peak expiratory flow rate (PEFR) between high dose flunisolide used for 24 days added to a 5-day course of prednisone 40 mg/day as compared with placebo.[67] A systematic review of 12 trials concluded no benefit of adding inhaled to systemic corticosteroids in reducing the relapse rate of acute asthma.[68]
There are few randomized and blinded studies examining only the short-term effect of ICS in the ED as add on therapy to systemic corticosteroids plus other standard acute asthma therapy. One study looked at the addition of high dose beclomethasone versus placebo to methylprednisolone in 60 adults and found no difference in FEV1 or symptoms between the two groups.[69] Another study looked at the addition of budesonide nebulizations to methylprednisolone in a population of 26 children with moderate asthma[70] and found no difference in the primary outcome of pulmonary index score, but there was an improvement in the PEFR in the budesonide group compared to placebo. However, the patient number included was very small and PEFR is generally not reliable in young children.[71] The two other randomized and blinded studies that were larger and more rigorous examined the effect of adding 2 mg of budesonide nebulization to prednisone in children with moderate to severe asthma.[72,73] In the study by Sung et al., 44 children with moderate to severe asthma were included. Both groups had no difference in the pulmonary index score. In the other study by Upham et al., 180 children with moderate to severe asthma were included. There was no difference in the asthma score[25] at 2 h after intervention or in the admission rate or time to discharge from the ED between the two groups. Collectively, it was hard to come up with a conclusion from these studies about whether adding ICS to systemic steroids in standard acute asthma therapy will add more benefit or not.[74] In addition, the number of subjects recruited by these studies was very small and would not allow subgroup analysis. Therefore, we recently performed a larger blinded and randomized study to look at this question.[19] We found that there was no added benefit of budesonide nebulization (1500 mcg) in the treatment of moderate to severe acute asthma in 2-12 year old children. However, when we looked at only the subgroup with severe acute asthma, budesonide was able to significantly decrease the admission rate of those patients and to lower their asthma score, suggesting an added value. More large trials specifically targeting patients with severe acute asthma are clearly needed.
Conclusion
Corticosteroids play an important role in the treatment of acute asthma exacerbations in the ED as well as post discharge from the ED. Further research is greatly needed to shed more light on the use of ICS in those patients, their optimal dose and duration, as well as their concomitant use with systemic corticosteroids. In addition, more research is needed on the safety of dispensing oral corticosteroids for home use in case of asthma exacerbation.
Acknowledgment
This review was modified and updated from a chapter entitled “The use of glucocorticoids in the treatment of acute asthma exacerbations” by the same author in “Corticosteroids-new recognition of our familiar friend” edited by Xiaoxiao Qian, 2012. Publisher: INTECH. The author holds exclusive copyright to this chapter. This work was supported by a grant from the Program of Strategic Technologies of the National Plan for Science and Technology and Innovation, Saudi Arabia. Grant number 08-MED520-02.
Footnotes
Source of Support: This work was supported by a grant from the Program of Strategic Technologies of the National Plan for Science and Technology and Innovation, Saudi Arabia. Grant number 08-MED520-02.
Conflict of Interest: None declared.
References
- 1.Subbarao P, Mandhane PJ, Sears MR. Asthma: Epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–90. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–15. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–5. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 4.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–97. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 5.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: Origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–74. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gern JE, Busse WW. Relationship of viral infections to wheezing illnesses and asthma. Nat Rev Immunol. 2002;2:132–8. doi: 10.1038/nri725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: Case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–5. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisgaard H, Hermansen MN, Bønnelykke K, Stokholm J, Baty F, Skytt NL, et al. Association of bacteria and viruses with wheezy episodes in young children: Prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brar T, Nagaraj S, Mohapatra S. Microbes and asthma: The missing cellular and molecular links. Curr Opin Pulm Med. 2012;18:14–22. doi: 10.1097/MCP.0b013e32834dccc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Asthma Education and Prevention Program (2007) Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma Summary Report 2007. J Allergy Clin Immunol. 120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Global Initiative for Asthma (2012) Global strategy for asthma management and prevention. [Last accessed on 2013 Oct 10]. Available: http://www.ginasthma.org/uploads/users/files/GINA_Report_2012.pdf .
- 14.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Jr, Gern J, et al. Asthma outcomes: Exacerbations. J Allergy Clin Immunol. 2012;129:S34–48. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): Findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119:1454–61. doi: 10.1016/j.jaci.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Pollack CV, Jr, Pollack ES, Baren JM, Smith SR, Woodruff PG, Clark S, et al. A prospective multicenter study of patient factors associated with hospital admission from the emergency department among children with acute asthma. Arch Pediatr Adolesc Med. 2002;156:934–40. doi: 10.1001/archpedi.156.9.934. [DOI] [PubMed] [Google Scholar]
- 18.Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: The asthma insights and reality in Europe (AIRE) study. Eur Respir J. 2000;16:802–7. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- 19.Alangari AA, Malhis N, Mubasher M, Al-Ghamedi N, Al-Tannir M, Riaz M, et al. Budesonide nebulization added to systemic prednisolone in the treatment of acute asthma in children: Double-Blind, randomized, controlled trial. Chest. 2014;145:772–8. doi: 10.1378/chest.13-2298. [DOI] [PubMed] [Google Scholar]
- 20.Adams JY, Sutter ME, Albertson TE. The patient with asthma in the emergency department. Clin Rev Allergy Immunol. 2012;43:14–29. doi: 10.1007/s12016-011-8273-z. [DOI] [PubMed] [Google Scholar]
- 21.Kelly HW. Levalbuterol for asthma: A better treatment? Curr Allergy Asthma Rep. 2007;7:310–4. doi: 10.1007/s11882-007-0046-7. [DOI] [PubMed] [Google Scholar]
- 22.Camargo CA, Jr, Spooner CH, Rowe BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev. 2003:CD001115. doi: 10.1002/14651858.CD001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe BH, Edmonds ML, Spooner CH, Diner B, Camargo CA., Jr Corticosteroid therapy for acute asthma. Respir Med. 2004;98:275–84. doi: 10.1016/j.rmed.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo GJ, Rodrigo C. Continuous vs intermittent beta-agonists in the treatment of acute adult asthma: A systematic review with meta-analysis. Chest. 2002;122:160–5. doi: 10.1378/chest.122.1.160. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. N Engl J Med. 1998;339:1030–5. doi: 10.1056/NEJM199810083391503. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax. 2005;60:740–6. doi: 10.1136/thx.2005.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics. 1999;103:748–52. doi: 10.1542/peds.103.4.748. [DOI] [PubMed] [Google Scholar]
- 28.Travers AH, Rowe BH, Barker S, Jones A, Camargo CA., Jr The effectiveness of IV beta-agonists in treating patients with acute asthma in the emergency department: A meta-analysis. Chest. 2002;122:1200–7. doi: 10.1378/chest.122.4.1200. [DOI] [PubMed] [Google Scholar]
- 29.Parameswaran K, Belda J, Rowe BH. Addition of intravenous aminophylline to beta2-agonists in adults with acute asthma. Cochrane Database Syst Rev. 2000:CD002742. doi: 10.1002/14651858.CD002742. [DOI] [PubMed] [Google Scholar]
- 30.Camargo CA, Jr, Gurner DM, Smithline HA, Chapela R, Fabbri LM, Green SA, et al. A randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthma. J Allergy Clin Immunol. 2010;125:374–80. doi: 10.1016/j.jaci.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Morris CR, Becker AB, Piñieiro A, Massaad R, Green SA, Smugar SS, et al. A randomized, placebo-controlled study of intravenous montelukast in children with acute asthma. Ann Allergy Asthma Immunol. 2010;104:161–71. doi: 10.1016/j.anai.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 32.Todi VK, Lodha R, Kabra SK. Effect of addition of single dose of oral montelukast to standard treatment in acute moderate to severe asthma in children between 5 and 15 years of age: A randomised, double-blind, placebo controlled trial. Arch Dis Child. 2010;95:540–3. doi: 10.1136/adc.2009.168567. [DOI] [PubMed] [Google Scholar]
- 33.Schuh S, Willan AR, Stephens D, Dick PT, Coates A. Can montelukast shorten prednisolone therapy in children with mild to moderate acute asthma. A randomized controlled trial? J Pediatr. 2009;155:795–800. doi: 10.1016/j.jpeds.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006:CD000052. doi: 10.1002/14651858.CD000052.pub2. [DOI] [PubMed] [Google Scholar]
- 35.CONTROLLED trial of effects of cortisone acetate in status asthmaticus; report to the Medical Research Council by the subcommittee on clinical trials in asthma. Lancet. 1956;271:803–6. [PubMed] [Google Scholar]
- 36.Clark TJ. Effect of beclomethasone dipropionate delivered by aerosol in patients with asthma. Lancet. 1972;1:1361–4. doi: 10.1016/s0140-6736(72)91094-x. [DOI] [PubMed] [Google Scholar]
- 37.Alangari AA. Genomic and non-genomic actions of glucocorticoids in asthma. Ann Thorac Med. 2010;5:133–9. doi: 10.4103/1817-1737.65040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Littenberg B, Gluck EH. A controlled trial of methylprednisolone in the emergency treatment of acute asthma. N Engl J Med. 1986;314:150–2. doi: 10.1056/NEJM198601163140304. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigo G, Rodrigo C. Corticosteroids in the emergency department therapy of acute adult asthma: An evidence-based evaluation. Chest. 1999;116:285–95. doi: 10.1378/chest.116.2.285. [DOI] [PubMed] [Google Scholar]
- 40.Fanta CH, Rossing TH, McFadden ER., Jr Glucocorticoids in acute asthma. A critical controlled trial. Am J Med. 1983;74:845–51. doi: 10.1016/0002-9343(83)91076-8. [DOI] [PubMed] [Google Scholar]
- 41.Lin RY, Pesola GR, Bakalchuk L, Heyl GT, Dow AM, Tenenbaum C, et al. Rapid improvement of peak flow in asthmatic patients treated with parenteral methylprednisolone in the emergency department: A randomized controlled study. Ann Emerg Med. 1999;33:487–94. doi: 10.1016/s0196-0644(99)70334-3. [DOI] [PubMed] [Google Scholar]
- 42.Schneider SM, Pipher A, Britton HL, Borok Z, Harcup CH. High-dose methylprednisolone as initial therapy in patients with acute bronchospasm. J Asthma. 1988;25:189–93. doi: 10.3109/02770908809071365. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigo C, Rodrigo G. Early administration of hydrocortisone in the emergency room treatment of acute asthma: A controlled clinical trial. Respir Med. 1994;88:755–61. doi: 10.1016/s0954-6111(05)80198-2. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan JA, Davis SQ, Naureckas ET, Gibson P, Rowe BH. An umbrella review: Corticosteroid therapy for adults with acute asthma. Am J Med. 2009;122:977–91. doi: 10.1016/j.amjmed.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–8. [PubMed] [Google Scholar]
- 46.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001:CD002178. doi: 10.1002/14651858.CD002178. [DOI] [PubMed] [Google Scholar]
- 47.Marquette CH, Stach B, Cardot E, Bervar JF, Saulnier F, Lafitte JJ, et al. High-dose and low-dose systemic corticosteroids are equally efficient in acute severe asthma. Eur Respir J. 1995;8:22–7. doi: 10.1183/09031936.95.08010022. [DOI] [PubMed] [Google Scholar]
- 48.Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2001:CD001740. doi: 10.1002/14651858.CD001740. [DOI] [PubMed] [Google Scholar]
- 49.Harrison BD, Stokes TC, Hart GJ, Vaughan DA, Ali NJ, Robinson AA. Need for intravenous hydrocortisone in addition to oral prednisolone in patients admitted to hospital with severe asthma without ventilatory failure. Lancet. 1986;1:181–4. doi: 10.1016/s0140-6736(86)90654-9. [DOI] [PubMed] [Google Scholar]
- 50.Ratto D, Alfaro C, Sipsey J, Glovsky MM, Sharma OP. Are intravenous corticosteroids required in status asthmaticus? JAMA. 1988;260:527–9. [PubMed] [Google Scholar]
- 51.Becker JM, Arora A, Scarfone RJ, Spector ND, Fontana-Penn ME, Gracely E, et al. Oral versus intravenous corticosteroids in children hospitalized with asthma. J Allergy Clin Immunol. 1999;103:586–90. doi: 10.1016/s0091-6749(99)70228-9. [DOI] [PubMed] [Google Scholar]
- 52.Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2001:CD000195. doi: 10.1002/14651858.CD000195. [DOI] [PubMed] [Google Scholar]
- 53.Hasegawa T, Ishihara K, Takakura S, Fujii H, Nishimura T, Okazaki M, et al. Duration of systemic corticosteroids in the treatment of asthma exacerbation; a randomized study. Intern Med. 2000;39:794–7. doi: 10.2169/internalmedicine.39.794. [DOI] [PubMed] [Google Scholar]
- 54.Jones AM, Munavvar M, Vail A, Aldridge RE, Hopkinson L, Rayner C, et al. Prospective, placebo-controlled trial of 5 vs 10 days of oral prednisolone in acute adult asthma. Respir Med. 2002;96:950–4. doi: 10.1053/rmed.2002.1369. [DOI] [PubMed] [Google Scholar]
- 55.Altamimi S, Robertson G, Jastaniah W, Davey A, Dehghani N, Chen R, et al. Single-dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. Pediatr Emerg Care. 2006;22:786–93. doi: 10.1097/01.pec.0000248683.09895.08. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigo GJ. Rapid effects of inhaled corticosteroids in acute asthma: An evidence-based evaluation. Chest. 2006;130:1301–11. doi: 10.1378/chest.130.5.1301. [DOI] [PubMed] [Google Scholar]
- 57.Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–53. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 58.Schuh S, Reisman J, Alshehri M, Dupuis A, Corey M, Arseneault R, et al. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. N Engl J Med. 2000;343:689–94. doi: 10.1056/NEJM200009073431003. [DOI] [PubMed] [Google Scholar]
- 59.Lee-Wong M, Dayrit FM, Kohli AR, Acquah S, Mayo PH. Comparison of high-dose inhaled flunisolide to systemic corticosteroids in severe adult asthma. Chest. 2002;122:1208–13. doi: 10.1378/chest.122.4.1208. [DOI] [PubMed] [Google Scholar]
- 60.Levy ML, Stevenson C, Maslen T. Comparison of short courses of oral prednisolone and fluticasone propionate in the treatment of adults with acute exacerbations of asthma in primary care. Thorax. 1996;51:1087–92. doi: 10.1136/thx.51.11.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scarfone RJ, Loiselle JM, Wiley JF, 2nd, Decker JM, Henretig FM, Joffe MD. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Ann Emerg Med. 1995;26:480–6. doi: 10.1016/s0196-0644(95)70118-4. [DOI] [PubMed] [Google Scholar]
- 62.Devidayal, Singhi S, Kumar L, Jayshree M. Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatr. 1999;88:835–40. doi: 10.1080/08035259950168748. [DOI] [PubMed] [Google Scholar]
- 63.Rodrigo GJ. Comparison of inhaled fluticasone with intravenous hydrocortisone in the treatment of adult acute asthma. Am J Respir Crit Care Med. 2005;171:1231–6. doi: 10.1164/rccm.200410-1415OC. [DOI] [PubMed] [Google Scholar]
- 64.Schuh S, Dick PT, Stephens D, Hartley M, Khaikin S, Rodrigues L, et al. High-dose inhaled fluticasone does not replace oral prednisolone in children with mild to moderate acute asthma. Pediatrics. 2006;118:644–50. doi: 10.1542/peds.2005-2842. [DOI] [PubMed] [Google Scholar]
- 65.Khoo SM, Lim TK. Effects of inhaled versus systemic corticosteroids on exhaled nitric oxide in severe acute asthma. Respir Med. 2009;103:614–20. doi: 10.1016/j.rmed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Rowe BH, Bota GW, Fabris L, Therrien SA, Milner RA, Jacono J. Inhaled budesonide in addition to oral corticosteroids to prevent asthma relapse following discharge from the emergency department: A randomized controlled trial. JAMA. 1999;281:2119–26. doi: 10.1001/jama.281.22.2119. [DOI] [PubMed] [Google Scholar]
- 67.Brenner BE, Chavda KK, Camargo CA., Jr Randomized trial of inhaled flunisolide versus placebo among asthmatic patients discharged from the emergency department. Ann Emerg Med. 2000;36:417–26. doi: 10.1067/mem.2000.110824. [DOI] [PubMed] [Google Scholar]
- 68.Edmonds ML, Milan SJ, Brenner BE, Camargo CA, Jr, Rowe BH. Inhaled steroids for acute asthma following emergency department discharge. Cochrane Database Syst Rev. 2012;12:CD002316. doi: 10.1002/14651858.CD002316.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guttman A, Afilalo M, Colacone A, Kreisman H, Dankoff J. The effects of combined intravenous and inhaled steroids (beclomethasone dipropionate) for the emergency treatment of acute asthma. The Asthma ED Study Group. Acad Emerg Med. 1997;4:100–6. doi: 10.1111/j.1553-2712.1997.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 70.Nuhoglu Y, Atas E, Nuhoglu C, Iscan M, Ozcay S. Acute effect of nebulized budesonide in asthmatic children. J Investig Allergol Clin Immunol. 2005;15:197–200. [PubMed] [Google Scholar]
- 71.Ducharme FM, Davis GM. Measurement of respiratory resistance in the emergency department: Feasibility in young children with acute asthma. Chest. 1997;111:1519–25. doi: 10.1378/chest.111.6.1519. [DOI] [PubMed] [Google Scholar]
- 72.Sung L, Osmond MH, Klassen TP. Randomized, controlled trial of inhaled budesonide as an adjunct to oral prednisone in acute asthma. Acad Emerg Med. 1998;5:209–13. doi: 10.1111/j.1553-2712.1998.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 73.Upham BD, Mollen CJ, Scarfone RJ, Seiden J, Chew A, Zorc JJ. Nebulized budesonide added to standard pediatric emergency department treatment of acute asthma: A randomized, double-blind trial. Acad Emerg Med. 2011;18:665–73. doi: 10.1111/j.1553-2712.2011.01114.x. [DOI] [PubMed] [Google Scholar]
- 74.Edmonds ML, Milan SJ, Camargo CA, Jr, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD002308. doi: 10.1002/14651858.CD002308.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


