Abstract
OBJECTIVE:
The aim was to investigate the effects of two different ventilatory strategies: Pressure-controlled ventilation (PCV) versus volume-controlled ventilation (VCV) in elderly patients with poor pulmonary function during one-lung ventilation (OLV).
PATIENTS AND METHODS:
The patients were enrolled into the study having poor pulmonary function (forced expiratory volume in 1 s <1.5 L) and undergoing radical resection of pulmonary carcinoma requiring at least 2 h of OLV. Patients were respectively allocated to VCV group and PCV group. The intraoperative data, arterial, and mixed venous blood gases were obtained at baseline, 20, 40, 60, 80, 100 and 120 min after OLV and end of surgery. The postoperative data had been recorded and arterial gas measurements were performed at 6, 12 and 24 h after surgery in Intensive Care Unit.
RESULTS:
Comparison of the VCV group and PCV group, PaO2 and P(A-a)O2 were higher and dead space to tidal volume was lower in the PCV group (P < 0.05) after the point of OLV +60, Ppeak was higher in the VCV group (P < 0.05). There were significant advantages in PCV groups with regard to the PaO2 of three points in postoperation, the duration of postoperative ventilation duration, intensive care duration of stay and the days stay in hospital after surgery.
CONCLUSIONS:
The use of PCV compared with VCV during OLV in elderly patients with poor pulmonary function has significant advantages of intraoperative and postoperative oxygenation and it might be a factor, which can beneficial to postoperative recovery.
Keywords: Aged, one-lung ventilation, pressure-controlled ventilation, pulmonary function, volume-controlled ventilation
Introduction
Anesthesia of thoracic surgery conventionally involves one-lung ventilation (OLV) to isolate and protect the lungs and to provide optimum surgical operating conditions. However, nowadays arterial hypoxemia is still a serious complication of OLV,[1] and this complication is easier to take place in elderly patients with poor pulmonary function.[2,3,4] Almost all elderly patients using mechanical ventilation would develop different degree of atelectasis and shunt during anesthesia.[5] Due to degeneration of the respiratory system and decreasing of respiratory function, elderly people have been increased the potential risk of intraoperative hypoxemia.[6,7] Advanced age is a risk factor for the pulmonary complication during thoracic surgery, the changes of pulmonary function with age-related might increase the risk of intraoperative lung injury and postoperative pulmonary complication.[3,4,8] This risk would be increased in radical resection for pulmonary carcinoma, because this kind of surgery is more difficult and need longer operation time, it not only need remove the tumor on the lung, but also need clean the regional lymph nodes conventionally.
Volume-controlled ventilation (VCV) has been considered the conventional method to mechanical ventilation of patients undergoing OLV during thoracic surgery. However, in recent years, Some scholars believe that pressure-controlled ventilation (PCV) might have advantages in decreasing Peak airway pressures and ensuring oxygenation,[9,10,11,12] however the choice of ventilation mode is still controversial, and PCV or VCV which is more suitable for postoperative recovery is still uncertain, especially in elderly patients with poor pulmonary function.
The aim of our study is to investigate, which ventilatory mode is more advantaged between VCV and PCV during OLV undergoing radical resection of pulmonary carcinoma in elderly patients with poor pulmonary function.
Patients and Methods
Study population
The protocol was approved by the Ethics Committee of the authors' hospital, and informed consent was obtained from each patient or from their nearest relatives. No commercial entity providing device or equipment had a role in any aspect of this study. Prior to surgery all patients underwent arterial blood gas and lung spirometry. The patients were enrolled into the study having poor pulmonary function (forced expiratory volume in 1 s [FEV1] <1.5 L) and undergoing radical resection of pulmonary carcinoma requiring at least 2 h of OLV. All patients were American Society of Anesthesiologists physical Status I-III and aged above 65 years. Preoperative exclusion criteria were patient refusal, anticipated inability to perform early postoperative extubation, no signed informed consent form, poor understanding by the patient of the purpose of the study, patient with uncompensated cardiac disease, hepatic or renal disease, previous thoracic surgery, and asthma.
Study protocol
Before anesthetic induction, patients were respectively allocated to VCV group and PCV group with a block randomization scheme produced by computer-generated codes. The ventilation strategy of all the patients used a tidal volume (VT) of 10 ml/kg during two-lung ventilation (TLV) before the operation and reduced to 6 ml/kg during OLV associated with 5 cmH2O positive end-expiratory pressure (PEEP) throughout the operative time, after the operation the VT was used back to 10 ml/kg until extubating the endotracheal tube. The difference is that patients in VCV group were ventilated with VCV and in PCV group the ventilation strategy used PCV during all the mechanical ventilation phase. Inspiration:expiration ratio (I:E) was 1:2 and FIO2 was 100%. EtCO2 was maintained between 35 and 40 mmHg through adjusting the respiratory rate during OLV. The cut-off strategy were unable to control the upper pressure in PCV group <35 cmH2O, unable to perform tracheal intubation in conditions of usual practice, inability to maintain stable mechanical ventilation settings for 30 min, inability to maintain an appropriate EtCO2 and SpO2, abnormal surgical complications, such as large blood vessels burst and unstable state of circulation, such as continuous hypertension or hypotension. The anesthesiologists were not blinded to the groups, but they were not participated in the collection of the data.
Patient treatment
Anesthetic and surgical management were standardized for each patient. After standard monitoring (electrocardiogram, pulse oximetry, and noninvasive blood pressure), an arterial cannula was inserted into the radial artery for invasive arterial pressure monitoring and collecting arterial blood, and a central venous catheter was inserted from the right internal jugular vein to the superior vena cava near the right atrium, used for central venous pressure (CVP) monitoring and collecting mixed venous blood. General anesthesia was induced in all patients with midazolam (0.05 mg/kg), propofol (1.5 mg/kg), fentanyl (4 ug/kg), and rocuronium (1 mg/kg). Anesthesia was maintained with a continuous infusion of remifentanil (0.1-0.2 μg/kg/min), propofol (50-100 μg/kg/min), and supplemental rocuronium discontinuously. No volatile anesthetics were used. The trachea was intubated with a left or right double lumen tube (Mallinckrodt-Endobronchial Tube, Covidien, Made in Ireland) no. 37 for male and no. 35 for female patients. The position of the tube was confirmed by auscultation and fiberoptic bronchoscopy before and after turning the patient to the lateral position. The patients' lungs were ventilated with a Datex-Ohmeda Ventilator (Aestiva/5 7900, Madison, USA). The lumen of the nonventilated side was left open to the air during OLV. During surgery, SpO2 should be kept above 90% at all times. If SpO2 fell below 90%, the following treatments were taken: sucking sputum, adjusting the catheter position, reconfirming by fiberoptic bronchoscopy. After treatment SpO2 can't back to above 90%, TLV was initiated and the patient would be excluded from the study.
Preoperatively standardized fluid replacement had consisted of 10 ml/kg Compound Sodium Chioride Injection (Sichuan Kelun Pharmaceutical Co., Ltd., China), followed by the solution (10 ml/kg/h) perioperatively. If mean arterial pressure was lower than 70 mmHg continuous >5 min, an additional fluid replacement was administered with 10 ml/kg 6% hydroxyethyl starch 130/0.4 (Voluven®; Fresenius Kabi, Germany). If this was not enough, repeated doses of 5 mg ephedrine were administered intravenous.
After surgery, all patients were transferred to Intensive Care Unit?ICU). Extubation was performed when patients met with the following extubation criteria:
Temperature above 36°C,
Normal mean arterial pressure and heart rate (HR),
Arterial oxygen partial pressure (PaO2)/FIO2 ratio >300 mmHg,
Patient' Spontaneous breathing,
Keep awake and adequate cough during suctioning.
Measurements
All measurements were implemented with the patients in the lateral position. Hemodynamic data (HR, systolic arterial pressure, mean arterial pressure, diastolic arterial pressure, CVP) were recorded from the A/S3 (Datex-Engstroem, Helsinki, Finland), Arterial and mixed venous oxygen tension and saturation (PaO2, SaO2, PvO2, SvO2), arterial carbon dioxide tension (PaCO2), arterial pH (PHa), arterial actual bicarbonate (ABCa), arterial base excess (BEa) and arterial hemoglobin (Hba) were analyzed using a model 865 blood gas analyzer (Chiron Diagnostics, Bayer Corp, Tarrytown, NY, USA), ventilatory data (VT, peak inspiratory pressure [Ppeak], plateau inspiratory pressure [Pplateau], mean inspiratory pressure [Pmean]) were recorded at the following points: (1) Baseline: TLV before beginning of OLV; (2) OLV +20 min after OLV; (3) OLV +40 min after OLV; (4) OLV +60 min after OLV; (5) OLV +80 min after OLV; (6) OLV +100 min after OLV; (7) OLV +120 min after OLV; (8) end of surgery: 20 min after reestablishing TLV. Blood gases were processed within 5 min after extraction, and values were corrected for body temperature. Derived values were calculated from standard formulae.[13]
After the patients were transferred to ICU, the data of postoperative ventilation duration, days in ICU, hospitalization duration, postoperative complications had been recorded. Arterial gas measurements were performed and the ratios PaO2/FIO2 were recorded at 6, 12 and 24 h after surgery in ICU.
Statistical analysis
Data were analyzed using the SPSS 17.0 package (SPSS Inc., Chicago, IL) and were expressed as the mean and standard deviation. The normality of the distribution of quantitative variables was analyzed using the Kolmogorov-Smirnov test. A power analysis with a pilot study revealed a sample size of 24 patients per group would be enough to achieve a type-I error of 5% and type-II error of 20%. Paired t-test was used to compare the measurements in different study periods. Differences in categorical variables between groups were analyzed by using Chi-square test or Fisher's exact test when appropriate. P < 0.05 were accepted as statistically significant.
Results
The study was performed from September 2011 to December 2013. A total of 106 patients were assessed for study eligibility and 86 patients were enrolled into the study [Figure 1]. The patients were randomly allocated to two experimental groups: VCV (n = 43) and PCV (n = 43). One patient in the VCV group had an unexpectedly difficult intubation followed by serious hypoxemia. Two patients in VCV group and four patients in PCV group did not meet the prescribed time of OLV during surgery. SpO2 of three patients in VCV group and two patients in PCV group could not keep above 90% during OLV and TLV was initiated to solve it. The left atrium of one patient in the PCV group was injured during surgery and that required increased the fluids and blood transfusion during surgery. These patients were excluded from the study according to the study protocol. There was no significant difference between the two groups in demographic characteristics, preoperative lung function and data on the surgical procedure [Tables 1 and 2].
Figure 1.
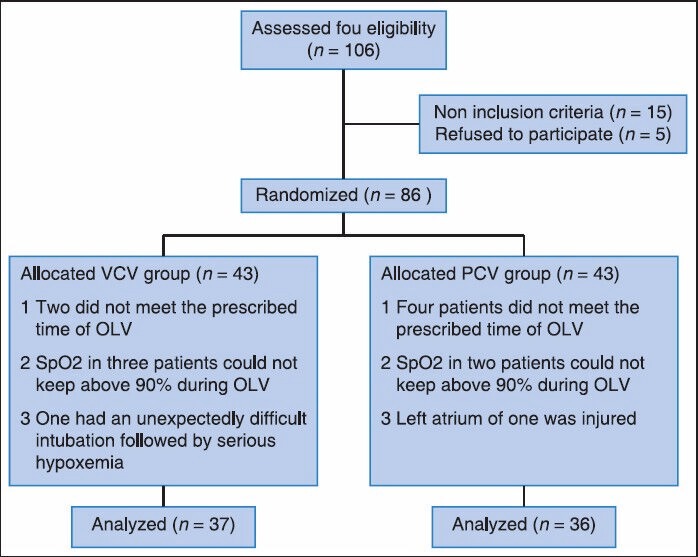
Flow diagram of subjects
Table 1.
Characteristics of the patients at study inclusion
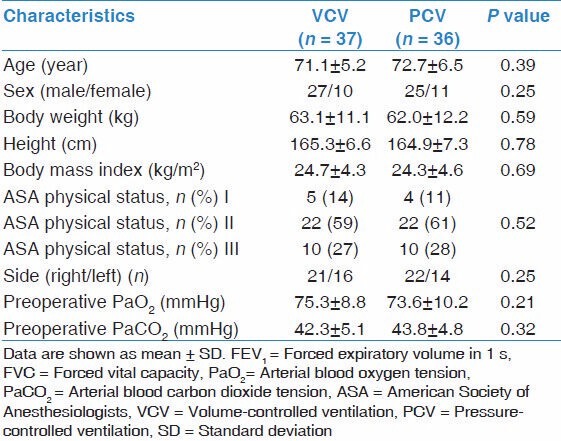
Table 2.
Intraoperative data of the patients
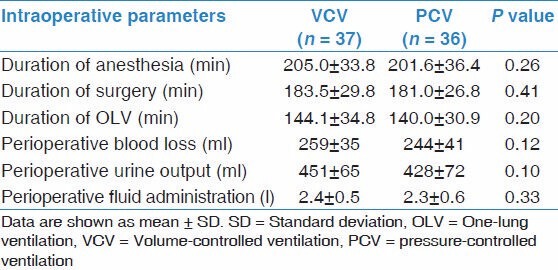
The study data of both two groups obtained during OLV are listed in Table 3. As expected, the beginning of OLV with either VCV or PCV group had a significant increase in Ppeak, Pmean and Pplateau (P < 0.05) compared with the initial TLV, and the PaO2 during OLV with both two groups was significantly lower compared with TLV (P < 0.01). Comparison of the VCV group and PCV group, PaO2 and P(A-a)O2 were higher and dead space to VT was lower in the PCV group (P < 0.05) after the point of OLV +60, Ppeak was higher in the VCV group (P < 0.05), and there was no significant difference in Pplateau or Pmean during OLV.
Table 3.
Intraoperative variables of the patients
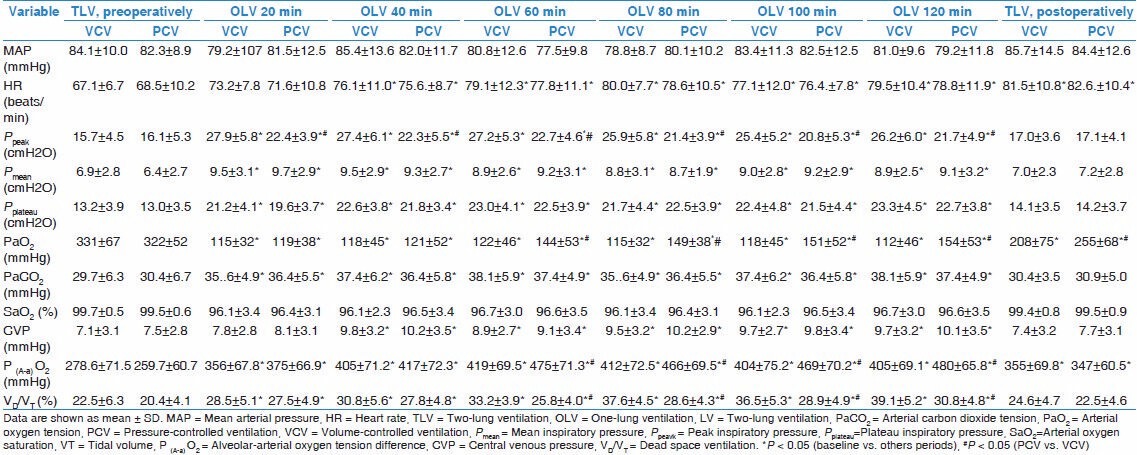
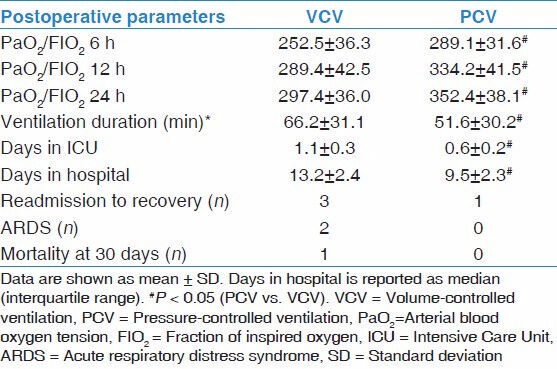
There were significant differences between the two groups with regard to the PaO2 of three points in postoperation, the duration of postoperative ventilation duration, the days stay in ICU and the days stay in hospital after surgery [Table 4]. Compared with the VCV group, PCV group had obvious advantages in postoperation. Five patients in the VCV group and two patients in the PCV group appeared deferring of extubation after surgery, and other patients in the study were extubated uneventfully in ICU. Three patients in VCV group and one patient in PCV group needed to be readmitted to the ICU because of respiratory failure. Two patients in VCV group had acute respiratory distress syndrome that was diagnosed after surgery and there into one patient died in hospital because of multiple organ dysfunction syndrome.
Table 4.
Postoperative data of the patient
Discussion
The advantages of PCV on intraoperative and postoperative arterial oxygenation have recently shown conflicting outcomes during OLV. Our results showed that compared with VCV, the PCV during OLV undergoing radical resection of pulmonary carcinoma in elderly patients with poor pulmonary function (FEV1 <1.5 L) had a statistically significant decrease in Ppeak, it could improve intraoperative and postoperative arterial oxygenation in patients, and have obviously advantages in postoperative recovery.
Our results were consistent with some studies comparing VCV with PCV, which reported that PCV could provide advantage over VCV for as much as improving oxygenation. Tuğrul et al.[10] showed that, PCV improved arterial oxygenation during OLV, especially in patients with a lower forced vital capacity (VC). This improvement might be explained by the flow profile with PCV, decreasing in pulmonary shunt fraction and lower airway pressures than with VCV. Sentürk et al.[11] showed that PCV with a PEEP of 4 cmH2O was associated with an improvement in oxygenation compared with VCV and zero PEEP. In contrast, some studies had found that PCV offered no advantage over VCV for improving oxygenation. Unzueta et al.[14] have reported that the use of PCV during OLV did not lead to improve oxygenation during OLV compared with VCV for patients with good preoperative pulmonary function. Pardos et al.[15] showed that compared with VCV, the use of PCV did not affect arterial oxygenation during OLV or early postoperative oxygenation.
According to our study results, we think the PCV during OLV have some advantages in elderly patients with poor pulmonary function (FEV1 <1.5 L) and the reasons are as follows:
The first of all, we had chosen the elderly patients with poor pulmonary function in our study. The lung tissues undergo changes with age that lead to an increase in alveolar size. The changes can lower the alveolar surface tension, which cause a reduction in maximum achievable flow in the airways. Muscle performance diminishes with age and the chest wall becomes stiffer, which lead to an increased residual volume.[6] These changes impact on lung function; static and dynamic measurements fall. VC is generally decreased almost 25% or 40%, FEV1 clearly drops at a rate depending on age and the FEV1 /FVC ratio drops progressively with age.[7] PCV has the lower peak airway pressure that might reduce the degree of barotraumas during mechanical ventilation.[16,17] PCV ventilator mode generates appropriate flow to maintain the set aspiratory pressure, and it usually can make peak airway pressures lower, improve the static and dynamic lung compliance, and decelerate flow waveform.[18] Due to these characteristics of the elderly patients, PCV shows its advantages.
Second, our ventilation strategy was small VT (6 ml/kg) during OLV associated with 5 cmH2O PEEP throughout the operative time; therefore, the airway pressure is relatively lower during OLV in both two groups. VCV uses a constant inspired flow, which has been gained as the VT delivered through producing a progressive increase of airway pressure to the peak inspiratory pressure. However, in contrast, the PCV ventilation model has advantage that it can improve the homogeneous distribution of inspired gas, gain better oxygenation and decrease the risk of lung injury.
Third, in our study, PCV ventilation strategy was used throughout the whole process of ventilation in PCV group, including the mechanical ventilation in postoperative phase. We found that the PaO2/FIO2 ratio in PCV group had a significant advantage in arterial oxygenation than VCV group in early postoperative recovery phase. The PaO2/FIO2 ratio is an universal accepted parameter that can be used to compare the oxygenation among patients in the recovery period after thoracic surgery, and it is often measured in early postoperative phase because lung injury often appears very early after lung resection surgery.[19,20] Although, there is no obvious difference of postoperative complications in two groups, but we found that compared with VCV group, the patients in PCV group had a significant decrease in the length of postoperative ventilation duration, the days stay in ICU and in hospital.
In addition, all the patients in our study had been undergone radical resection of pulmonary carcinoma. This kind of surgery need a longer operating time. Furthermore, during OLV in lateral decubitus, the compression atelectasis of dependent lung regions, the loss of elastic recoil after thoracotomy, and mediastinal surgical manipulations can markedly reduce the aerated lung capacity, impair ventilation distribution, and worsen ventilation/perfusion mismatch. Therefore, PCV might exert its advantages in a longer time in the process of mechanical ventilation.
Several limitations of this study should be mentioned. First, it was a single-center study with a small group of patients, and limiting the generalizability of the conclusions. Second, the results cannot extrapolate how severe degree of the preoperative pulmonary function is safe during OLV. Third, the different difficulty in operation and scope of surgical resection may produce some effects on the results of the study.
Conclusion
The use of PCV compared with VCV during OLV undergoing radical resection of pulmonary carcinoma in elderly patients with poor pulmonary function (FEV1 <1.5 L) have significant advantages of intraoperative and postoperative oxygenation and it might be a factor, which can beneficial to postoperative recovery.
Acknowledgments
The following grants and foundations supported this work: Research grant No. 81060008 from the National Natural Science Foundation of China and Research grant No. 200203 from the Bureau of Health, Guangxi, and Research grant No. 2010GXNSFB013087 from Natural Science Foundation of Guangxi Province of China.
Footnotes
Source of Support: The following grants and foundations supported this work: Research grant no. 81060008 from the National Natural Science Foundation of China and Research grant no. 200203 from the Bureau of Health, Guangxi, and Research grant no. 2010GXNSFB013087 from Natural Science Foundation of Guangxi Province of China.
Conflict of Interest: None declared.
References
- 1.Motsch J, Wiedemann K, Roggenbach J. Airway management for one-lung ventilation. Anaesthesist. 2005;54:601–22. doi: 10.1007/s00101-005-0866-6. [DOI] [PubMed] [Google Scholar]
- 2.Torchio R, Guglielmo M, Giardino R, Ardissone F, Ciacco C, Gulotta C, et al. Exercise ventilatory inefficiency and mortality in patients with chronic obstructive pulmonary disease undergoing surgery for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2010;38:14–9. doi: 10.1016/j.ejcts.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Ma LY, Liao ZF, Wang GJ. Risk factors for post-surgical pulmonary complications in patients after esophagectomy for cancer: A multivariate logistic analysis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23:625–8. [PubMed] [Google Scholar]
- 4.Cheng YD, Duan CJ, Dong S, Zhang H, Zhang SK, Wang SQ, et al. Clinical controlled comparison between lobectomy and segmental resection for patients over 70 years of age with clinical stage I non-small cell lung cancer. Eur J Surg Oncol. 2012;38:1149–55. doi: 10.1016/j.ejso.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Gunnarsson L, Tokics L, Gustavsson H, Hedenstierna G. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br J Anaesth. 1991;66:423–32. doi: 10.1093/bja/66.4.423. [DOI] [PubMed] [Google Scholar]
- 6.Miller MR. Structural and physiological age-associated changes in aging lungs. Semin Respir Crit Care Med. 2010;31:521–7. doi: 10.1055/s-0030-1265893. [DOI] [PubMed] [Google Scholar]
- 7.Ruivo S, Viana P, Martins C, Baeta C. Effects of aging on lung function. A comparison of lung function in healthy adults and the elderly. Rev Port Pneumol. 2009;15:629–53. [PubMed] [Google Scholar]
- 8.Sprung J, Gajic O, Warner DO. Review article: Age related alterations in respiratory function-Anesthetic considerations. Can J Anaesth. 2006;53:1244–57. doi: 10.1007/BF03021586. [DOI] [PubMed] [Google Scholar]
- 9.Lohser J. Evidence-based management of one-lung ventilation. Anesthesiol Clin. 2008;26:241–72, v. doi: 10.1016/j.anclin.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Tuğrul M, Camci E, Karadeniz H, Sentürk M, Pembeci K, Akpir K. Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. Br J Anaesth. 1997;79:306–10. doi: 10.1093/bja/79.3.306. [DOI] [PubMed] [Google Scholar]
- 11.Sentürk NM, Dilek A, Camci E, Sentürk E, Orhan M, Tuğrul M, et al. Effects of positive end-expiratory pressure on ventilatory and oxygenation parameters during pressure-controlled one-lung ventilation. J Cardiothorac Vasc Anesth. 2005;19:71–5. doi: 10.1053/j.jvca.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Heimberg C, Winterhalter M, Strüber M, Piepenbrock S, Bund M. Pressure-controlled versus volume-controlled one-lung ventilation for MIDCAB. Thorac Cardiovasc Surg. 2006;54:516–20. doi: 10.1055/s-2006-924413. [DOI] [PubMed] [Google Scholar]
- 13.Daoud EG. Airway pressure release ventilation. Ann Thorac Med. 2007;2:176–9. doi: 10.4103/1817-1737.36556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unzueta MC, Casas JI, Moral MV. Pressure-controlled versus volume-controlled ventilation during one-lung ventilation for thoracic surgery. Anesth Analg. 2007;104:1029–33. doi: 10.1213/01.ane.0000260313.63893.2f. [DOI] [PubMed] [Google Scholar]
- 15.Pardos PC, Garutti I, Piñeiro P, Olmedilla L, de la Gala F. Effects of ventilatory mode during one-lung ventilation on intraoperative and postoperative arterial oxygenation in thoracic surgery. J Cardiothorac Vasc Anesth. 2009;23:770–4. doi: 10.1053/j.jvca.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Prella M, Feihl F, Domenighetti G. Effects of short-term pressure-controlled ventilation on gas exchange, airway pressures, and gas distribution in patients with acute lung injury/ARDS: Comparison with volume-controlled ventilation. Chest. 2002;122:1382–8. doi: 10.1378/chest.122.4.1382. [DOI] [PubMed] [Google Scholar]
- 17.Esteban A, Alía I, Gordo F, de Pablo R, Suarez J, González G, et al. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest. 2000;117:1690–6. doi: 10.1378/chest.117.6.1690. [DOI] [PubMed] [Google Scholar]
- 18.Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28:406–13. doi: 10.1007/s00134-001-1178-1. [DOI] [PubMed] [Google Scholar]
- 19.Baudouin SV. Lung injury after thoracotomy. Br J Anaesth. 2003;91:132–42. doi: 10.1093/bja/aeg083. [DOI] [PubMed] [Google Scholar]
- 20.Cinnella G, Grasso S, Natale C, Sollitto F, Cacciapaglia M, Angiolillo M, et al. Physiological effects of a lung-recruiting strategy applied during one-lung ventilation. Acta Anaesthesiol Scand. 2008;52:766–75. doi: 10.1111/j.1399-6576.2008.01652.x. [DOI] [PubMed] [Google Scholar]


