Abstract
BACKGROUND:
Several studies showed that the implementation of the Institute for Healthcare Improvement (IHI) ventilator bundle alone or with other preventive measures are associated with reducing Ventilator-Associated Pneumonia (VAP) rates. However, the association with ventilator utilization was rarely examined and the findings were conflicting. The objectives were to validate the bundle association with VAP rate in a traditionally high VAP environment and to examine its association with ventilator utilization.
MATERIALS AND METHODS:
The study was conducted at the adult medical-surgical intensive care unit (ICU) at King Abdulaziz Medical City, Saudi Arabia, between 2010 and 2013. VAP data were collected by a prospective targeted surveillance as per Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network (NHSN) methodology while bundle data were collected by a cross-sectional design as per IHI methodology.
RESULTS:
Ventilator bundle compliance significantly increased from 90% in 2010 to 97% in 2013 (P for trend < 0.001). On the other hand, VAP rate decreased from 3.6 (per 1000 ventilator days) in 2010 to 1.0 in 2013 (P for trend = 0.054) and ventilator utilization ratio decreased from 0.73 in 2010 to 0.59 in 2013 (P for trend < 0.001). There were negative significant correlations between the trends of ventilator bundle compliance and VAP rate (cross-correlation coefficients −0.63 to 0.07) and ventilator utilization (cross-correlation coefficients −0.18 to −0.63).
CONCLUSION:
More than 70% improvement of VAP rates and approximately 20% improvement of ventilator utilization were observed during IHI ventilator bundle implementation among adult critical patients in a tertiary care center in Saudi Arabia. Replicating the current finding in multicenter randomized trials is required before establishing any causal link.
Keywords: Bundle, health-care-associated infection, Institute for Healthcare Improvement, quality improvement, ventilator-associated pneumonia, ventilator utilization
Ventilator-Associated Pneumonia (VAP) is a significant source of morbidity and mortality among critically ill patients.[1] Additionally, it is associated with a considerable increase in resource utilization and health care costs.[2] Therefore, VAP prevention at intensive care unit (ICU) is recognized as an important patient-safety initiative and health care quality indicator.[3] A number of organizations published several strategies to reduce the incidence of health-care-associated pneumonia. These include the American Thoracic Society and Infectious Disease Society of America (ATS/IDSA),[4] the Centers for Disease Control and Prevention (CDC),[5] and the European Task Force.[6] However, the implementation of these multiple guidelines is difficult and often inconsistent due to lack of strategy.[7,8] Moreover, several randomized controlled studies and recent meta-analyses showed that although several preventive measures can reduce the rate of VAP, only few of them are able to reduce patient mortality, ventilation duration, or length of stay.[9]
The Institute for Healthcare Improvement (IHI) introduced the concept of preventive bundles, including ventilator bundle, to facilitate the implementation of evidence-based preventive strategies for health-care-associated infections.[10] The original IHI ventilator bundle consisted of four elements that need to be implemented collectively and reliably; elevation of the head of the bed to 30-45 degrees, daily sedation hold, deep vein thrombosis (DVT) prophylaxis, and gastric ulcer prophylaxis.[10] Oral care was further added in 2010 as the fifth component of the ventilator bundle.[11] Several studies have shown that implementation of the components of IHI bundle alone or with other preventive measures are associated with reducing VAP rates.[12,13,14] However, a relatively fewer number of studies focused on examining the association between the ventilator bundle compliance and ventilator utilization with conflicting results.[14,15,16]
The objectives of the current study were to validate the association between the ventilator bundle compliance and VAP rate in a traditionally high VAP environment[17] and to examine the association between ventilator bundle compliance and ventilator utilization among adult patients at a medical-surgical ICU.
Materials and Methods
Setting and population
The current study was conducted at adult ICU of King Abdulaziz Medical City (KAMC) in Riyadh, Saudi Arabia. KAMC is an approximately 900-bed tertiary care facility that provides health care services to about 600,000 Saudi National Guard soldiers, employees, and their families. The care provided ranges from primary and preventive care to tertiary care. The adult ICU at KAMC is a 21-bed closed medical-surgical-trauma unit covered by onsite board-certified intensivists 24 hours per day, 7 days a week and admits approximately 900 patients per year. The nurse to patient ratio is 1:1. The study population was adult patients (>18 years) who were admitted to the adult ICU irrespective of admission diagnosis (excluding burns) and required ventilation during the study period.
Study design
Two methodological designs were adopted between June 2010 and December 2013; VAP data were collected by a prospective surveillance as per the National Healthcare Safety Network (NHSN) definitions and methodology.[18] Bundle data were collected by a cross-sectional design as per IHI methodology. All ventilated patients were followed for VAP development, whereas samples of ventilated patients were checked for bundle compliance. VAP surveillance and examination (not implementation) of bundle compliance were intermittent as per targeted risk-directed infection control surveillance plan. All required ethical approvals as per the KAMC regulations were obtained.
VAP definitions
VAP was diagnosed using the Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network (NHSN) 2009 definition.[18] Accordingly, VAP was defined as a pneumonia identified using a combination of radiologic, clinical, and laboratory criteria in a patient who was intubated and ventilated at the time of or within 48 hours before the onset of the infection. According to the type of clinical and laboratory findings, patients were further divided into:
Clinical pneumonia,
Pneumonia with specific laboratory findings, or
Pneumonia in immune-compromised patients.
Ventilator bundle
The following four IHI bundle components were checked throughout the study duration:
Elevation of the head of the bed to between 30-45 degrees,
Daily sedation interruption and daily assessment of readiness to extubate,
Peptic ulcer disease prophylaxis, and
Deep venous thrombosis prophylaxis.[10]
Starting late 2011, daily oral care (using 0.12% oral chlorhexidine as mouth rinse 3-4 times per day) was added as the fifth component of ventilator bundle.[11]
Metric definitions
The VAP rate was expressed as cases of VAP per 1000 ventilator days. Ventilator days were defined as the sum of daily counts of patients with ventilator. Patient days were defined as the sum of daily counts of all patients admitted to the adult medical-surgical Intensive Care Unit (ICU). Ventilator utilization was expressed as ratio of ventilator days to patient days. Ventilator bundle compliance was expressed as percentage and was defined as the daily number of ventilated patients with all components of ventilator bundle compliant (unless contraindicated) in relation to the total number of ventilated patients reviewed.
Statistical analysis
Demographic and clinical data were presented using frequencies and percentages for categorical data and mean and standard deviation (SD) for continuous data. The difference between two VAP rates was tested using Z-test. The differences between two bundle compliance percentages or ventilator utilization ratios were tested using chi-square test. The significance of decreasing or increasing trends of VAP rate, ventilator bundle compliance, and ventilator utilization were examined using Mantel-Haenszel chi-square test of trend. Association between trends of ventilator bundle compliance with VAP rate and ventilator utilization were examined using cross-correlation function. All P-values were two-tailed. P-value < 0.05 was considered as significant. Statistical Package for the Social Sciences (SPSS) software (release 20.0, SPSS Inc., Chicago, U S) and Programs for Epidemiologist for Windows (WINPEPI) software (version 11.1)[19] were used for statistical analyses.
Results
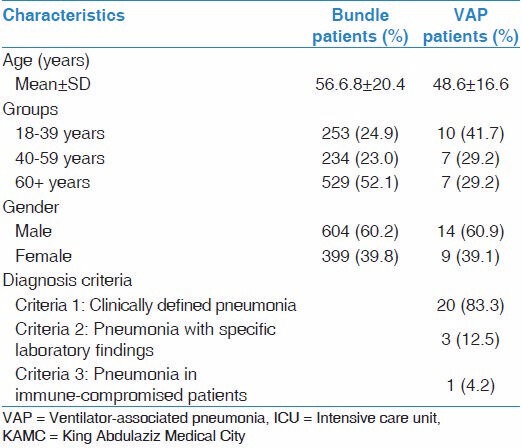
Table 1 showed the demographic and clinical characteristics of the studied population. Mean age of ventilated patients was 56.68 ± 20.4 years while the mean age of patients who developed VAP was 48.6 ± 16.6 years. Approximately 52% of ventilated patients and 29% of patients who developed VAP were ≥ 60 years old. Males represented 60% of ventilated patients and 61% of patients who developed VAP. Approximately 83.3% of ventilated patients who developed VAP were diagnosed based on clinical criteria while fewer cases were diagnosed based on specific laboratory findings (12.5%) and pneumonia in immuno-compromised patients (4.2%).
Table 1.
Demographic and clinical characteristics of ventilated patients admitted to adult medical-surgical ICU of KAMC (Riyadh) between 2010 and 2013
During the study period, 24 VAP events were diagnosed during a total of 9,099 ventilator days and 14,521 patient days [Table 2]. During the same period, a total 2,521 out of 2,722 patient days examined for ventilator bundle were compliant. Quarterly frequency of VAP diagnosis ranged between 0-6 in the last year to the first year of the study.. Quarterly frequency of ventilator days ranged from 400 to 1,382 ventilator days. Quarterly patient days ranged from 589 to 1,845 patient days. Quarterly examined days for ventilator bundle compliance ranged from 124 to 396 days with about 107-379 days of compliance.
Table 2.
Patient days, ventilator days, VAP events, bundle compliance among patients admitted to adult medical-surgical ICU of KAMC (Riyadh) between 2010 and 2013
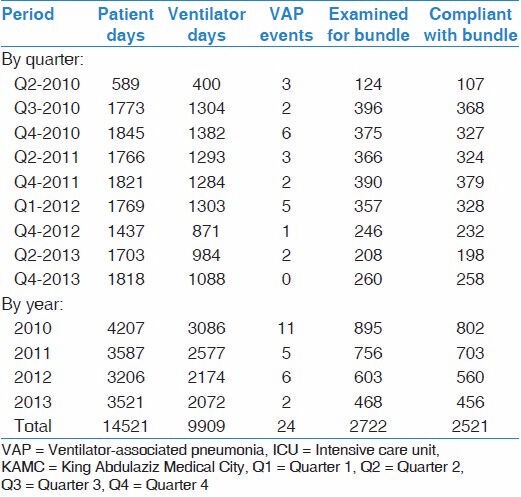
Figure 1 showed the trends of ventilator bundle compliance with VAP rate and ventilator utilization. There was a significant increasing trend of ventilator bundle compliance throughout the study period (Mantel-Haenszel chi-square test of trend was 29.75, P < 0.001). Simultaneous with this increasing bundle compliance trend, there were a marginally significant decreasing trend of VAP rate (Mantel-Haenszel chi-square test of trend was 3.71, P = 0.054) and a significant decreasing trend of ventilator utilization (Mantel-Haenszel chi-square test of trend was 190.81, P < 0.001). Comparing yearly metrics [Figure 2], ventilator bundle compliance significantly increased from 90% in 2010 to 97% in 2013 (P-value of chi-square <0.001). On the other hand, VAP rate decreased from 3.6 (per 1000 ventilator days) in 2010 to 1.0 in 2013 (P-value of Z-test = 0.068) and ventilator utilization ratio decreased from 0.73 in 2010 to 0.59 in 2013 (P-value of chi-square test <0.001).
Figure 1.
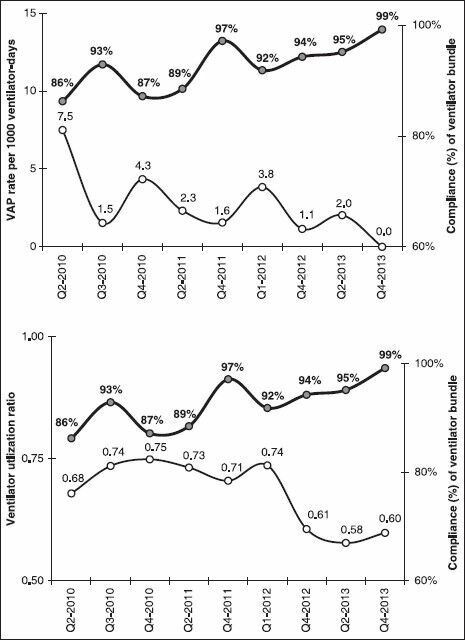
Trends of ventilator bundle compliance with VAP rate (above) and ventilator utilization (below) among admitted patients at medical-surgical ICU of KAMC (Riyadh) between 2010 and 2013 VAP = Ventilator-associated pneumonia, ICU = Intensive care unit, KAMC = King Abdulaziz Medical City
Figure 2.
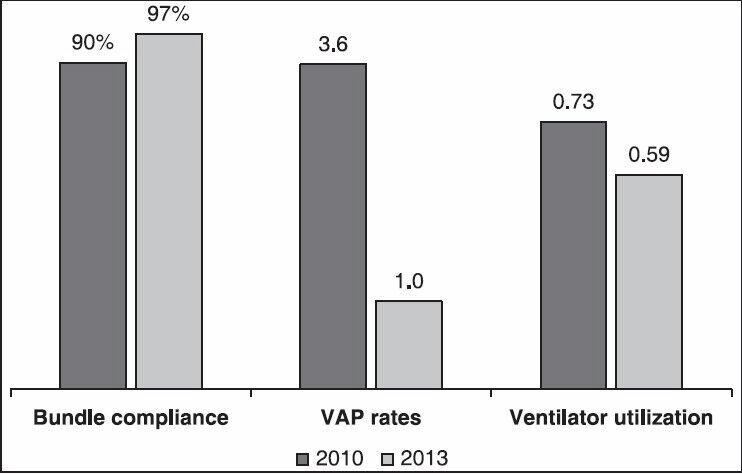
Comparisons of ventilator bundle compliance, VAP rate, and ventilator utilization between 2010 and 2013VAP = Ventilator-associated pneumonia
Figure 3 showed the correlations of trends of ventilator bundle compliance with VAP rate and ventilator utilization. There was a significant negative correlation between ventilator bundle compliance and the VAP rate as indicated by negative cross-correlation coefficients. Cross-correlation coefficients ranged between −0.63 (standard error, 0.20) to 0.07 (standard error, 0.21). Both zero and negative lags (in months) were significant (crossing the lower confidence limit), indicating that the decrease in VAP rate occured at the same time or 1 month after the ventilator bundle compliance increase. Additionally, there was a significant negative correlation between ventilator bundle compliance and ventilator utilization. Cross-correlation coefficients ranged between −0.18 to −0.63 (standard error, 0.21 for each). Both zero and positive lags (in months) were significant, indicating that the decrease in ventilator utilization happened at the same time or within 2 months before the increase in ventilator bundle compliance.
Figure 3.

Cross-correlation coefficient of ventilator bundle compliance with VAP rate (above) and ventilator utilization (below) among admitted patients at medicalsurgical ICU of KAMC (Riyadh) between 2010 and 2013 VAP = Ventilator-associated pneumonia, KAMC = King Abdulaziz Medical City
Discussion
We are reporting approximately 73% improvement in VAP rates and approximately 20% improvement in ventilator utilization during a 4-year continuous IHI ventilator bundle implementation among adult patients at a medical-surgical ICU. The current VAP rate decline was similar to findings from several reports, which examined data from different hospitals in both developed and developing countries.[12,13,14,20] For example, in a recent review of eight studies (between 2004 and 2009) using IHI ventilator bundle alone or as a part of a multifaceted VAP prevention strategy at ICU settings, VAP rate declined by 38% to 85% after bundle implementation.[12] Additionally, implementation of multiple preventive measures including the components of IHI bundle in 44 ICUs in 13 developing countries was associated with 56% reduction in VAP rate.[20] The ventilator bundle is believed to improve the outcome of ICU patients with VAP by setting priority, standardizing patient care, promoting adherence, and enhancing reliability and accountability.[21,22] On the other hand, some reports failed to show decline in VAP rate after implementation of IHI ventilator bundle.[23]
In the current study, we were able to achieve zero VAP rate towards the end of the study; however, the sustainability of this zero rate needs further surveillance. A number of hospitals have reported a zero VAP rate after aggressive bundle implementation.[15,24,25] Achieving and sustaining zero rates or rates very close to zero seem possible but probably need continuous monitoring and persistent adherence to preventive bundle with more than 95% compliance rates.[25] On the other hand, there is a growing skepticism about the possibility of achieving and sustaining zero rates. A number of multifaceted intervention programs failed to achieve zero VAP rate despite aggressive use of preventive bundles.[26,27] This could be at least partially explained by non-modifiable host factors.[26] Moreover, regulations that mandate public reporting of VAP rates may have encouraged conservative interpretation of subjective signs of VAP definition which may result in artifactual lower rates.[28]
Although a significant correlation was found, the current decline of VAP rates and ventilator utilization with bundle implementation cannot prove any causal link for many reasons; the finding was derived from ecological analysis of prospectively collected data, the design does not account for many ongoing preventive and infection control measures such as hand hygiene, aspiration of subglottic secretions, VAP surveillance feedback, Acinetobacter surveillance,[17] and finally only a sample of the ventilated patients checked for bundle compliance. Nevertheless, we believe that the magnitude of the observed VAP and ventilator utilization decline suggest a strong association that can be elicited only in a randomized design. In absence of opposing evidence, the current finding is a guarantee to continue ventilator bundle as a pivotal patient-safety initiative.
Although ventilator utilization is a reflection of ventilation duration, a major focus of preventive bundles, which is more objective to measure than VAP,[9] ventilator utilization was rarely examined as a primary outcome for ventilator bundle. We found a significant correlation between the increase in ventilator bundle compliance and the decline of ventilator utilization. Similarly, a number of studies showed that IHI bundle was associated with a significant decline in ventilator utilization.[12,14] However, several studies that showed a decline in VAP rates with different VAP preventive strategies failed to detect such a reduction in ventilation duration.[15,16,29] Interestingly, the decline in ventilator utilization in this study happened at or before the increase in ventilator bundle compliance. This may be indicative that at least part of the decline in ventilator utilization may not be explained by the ventilator bundle compliance and, in such a case, both should be regarded as independent good-care outcome.
Similar to other institutions, starting implementation and surveillance of preventive bundle in KAMC (Riyadh) was not without challenges.[30,31] Physician's skeptism about the available evidence, the nurse worry about extra work associated with bundle documentation and extra risk associated with sedation vacation, ICU staff worry from being watched and audited, and the need for teamwork organization. The dynamic cooperation between infection control and ICU staff, appointment of a resident to oversee bundle implementation, daily multidisciplinary rounds, periodic educational and training sessions, administration encouragement, and no blame policy were some of the measures taken to facilitate bundle implementation.
Our study has multiple strengths; the large number of ventilator days examined, the long duration of the study, and the ascertainment of VAP events by infectious disease consultant. However, we acknowledge some limitations; the single-center experience may limit generalizability; the design does not allow causal link, the ecologic analysis of aggregate data does not allow patient-based outcome inference which may result in ecological bias, and finally, as mentioned before, inability to account for other ongoing routine preventive and infection control measures during the study.
In conclusion, more than 70% improvement of VAP rates and approximately 20% improvement of ventilator utilization were observed during IHI ventilator bundle implementation among adult critical patients in a tertiary care center in Saudi Arabia. In consideration of data limitation in the current and previous studies, replicating the current finding in multicenter randomized trials is required before establishing any causal link between bundle implementation and hard outcomes in ventilated patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. VAP Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–21. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 3.Uckay I, Ahmed QA, Sax H, Pittet D. Ventilator-associated pneumonia as a quality indicator for patient safety? Clin Infect Dis. 2008;46:557–63. doi: 10.1086/526534. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society, Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 5.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R CDC. Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care-associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1–36. [PubMed] [Google Scholar]
- 6.Torres A, Carlet J. Ventilator-associated pneumonia. European Task Force on ventilator-associated pneumonia. Eur Respir J. 2001;17:1034–45. doi: 10.1183/09031936.01.17510340. [DOI] [PubMed] [Google Scholar]
- 7.Cason CL, Tyner T, Saunders S, Broome L. Centers for Disease Control and Prevention. Nurses' implementation of guidelines for ventilator-associated pneumonia from the Centers for Disease Control and Prevention. Am J Crit Care. 2007;16:28–36. [PubMed] [Google Scholar]
- 8.Sinuff T, Muscedere J, Cook D, Dodek P, Heyland D. Canadian Critical Care Trials Group. Ventilator-associated pneumonia: Improving outcomes through guideline implementation. J Crit Care. 2008;23:118–25. doi: 10.1016/j.jcrc.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Bouadma L, Wolff M, Lucet JC. Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis. 2012;25:395–404. doi: 10.1097/QCO.0b013e328355a835. [DOI] [PubMed] [Google Scholar]
- 10.Evans B. Best-practice protocols: VAP prevention. Nurs Manage. 2005;36:10, 12, 14 passim. doi: 10.1097/00006247-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 11.How-to Guide: Prevent Ventilator-Associated Pneumonia. Cambridge, MA: Institute for Healthcare Improvement; 2012. [Last accessed on 2012 Oct]. Available from: http://www.ihi.org/knowledge/Pages/Tools/HowtoGuidePreventVAP.aspx . [Google Scholar]
- 12.Lawrence P, Fulbrook P. The ventilator care bundle and its impact on ventilator-associated pneumonia: A review of the evidence. Nurs Crit Care. 2011;16:222–34. doi: 10.1111/j.1478-5153.2010.00430.x. [DOI] [PubMed] [Google Scholar]
- 13.O' Keefe-McCarthy S, Santiago C, Lau G. Ventilator-associated pneumonia bundled strategies: An evidence-based practice. Worldviews Evid Based Nurs. 2008;5:193–204. doi: 10.1111/j.1741-6787.2008.00140.x. [DOI] [PubMed] [Google Scholar]
- 14.Lim KP, Kuo SW, Ko WJ, Sheng WH, Chang YY, Hong MC, et al. Efficacy of ventilator-associated pneumonia care bundle for prevention of ventilator-associated pneumonia in the surgical intensive care units of a medical center. J Microbiol Immunol Infect. 2013 doi: 10.1016/j.jmii.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Caserta RA, Marra AR, Durao MS, Silva CV, Pavao dos Santos OF, Neves HS, et al. A program for sustained improvement in preventing ventilator associated pneumonia in an intensive care setting. BMC Infect Dis. 2012;12:234. doi: 10.1186/1471-2334-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25:174 e11–8. doi: 10.1016/j.jcrc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Al-Dorzi HM, El-Saed A, Rishu AH, Balkhy HH, Memish ZA, Arabi YM. The results of a 6-year epidemiologic surveillance for ventilator-associated pneumonia at a tertiary care intensive care unit in Saudi Arabia. Am J Infect Control. 2012;40:794–9. doi: 10.1016/j.ajic.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Division of Healthcare Quality Promotion, National Center for Infectious Diseases. Atlanta: Georgia; 2009. Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual. Patient Safety Component Protocol. [Google Scholar]
- 19.Abramson JH. WINPEPI (PEPI-for-Windows): Computer programs for epidemiologists. Epidemiol Perspect Innov. 2004;1:6. doi: 10.1186/1742-5573-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthal VD, Rodrigues C, Alvarez-Moreno C, Madani N, Mitrev Z, Ye G, et al. INICC members. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in adult intensive care units from 14 developing countries of four continents: Findings of the International Nosocomial Infection Control Consortium. Crit Care Med. 2012;40:3121–8. doi: 10.1097/CCM.0b013e3182657916. [DOI] [PubMed] [Google Scholar]
- 21.Sedwick MB, Lance-Smith M, Reeder SJ, Nardi J. Using evidence-based practice to prevent ventilator-associated pneumonia. Crit Care Nurse. 2012;32:41–51. doi: 10.4037/ccn2012964. [DOI] [PubMed] [Google Scholar]
- 22.Marwick C, Davey P. Care bundles: The holy grail of infectious risk management in hospital? Curr Opin Infect Dis. 2009;22:364–9. doi: 10.1097/QCO.0b013e32832e0736. [DOI] [PubMed] [Google Scholar]
- 23.Bloos F, Muller S, Harz A, Gugel M, Geil D, Egerland K, et al. Effects of staff training on the care of mechanically ventilated patients: A prospective cohort study. Br J Anaesth. 2009;103:232–7. doi: 10.1093/bja/aep114. [DOI] [PubMed] [Google Scholar]
- 24.Berriel-Cass D, Adkins FW, Jones P, Fakih MG. Eliminating nosocomial infections at Ascension Health. Jt Comm J Qual Patient Saf. 2006;32:612–20. doi: 10.1016/s1553-7250(06)32079-x. [DOI] [PubMed] [Google Scholar]
- 25.Marra AR, Cal RG, Silva CV, Caserta RA, Paes AT, Moura DF, Jr, et al. Successful prevention of ventilator-associated pneumonia in an intensive care setting. Am J Infect Control. 2009;37:619–25. doi: 10.1016/j.ajic.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Apisarnthanarak A, Warren DK, Fraser VJ. The long-term outcome of a multifaceted intervention to reduce ventilator-associated pneumonia: Can zero really be achieved? Am J Infect Control. 2011;39:613–4. doi: 10.1016/j.ajic.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Bouadma L, Deslandes E, Lolom I, Le Corre B, Mourvillier B, Regnier B, et al. Long-term impact of a multifaceted prevention program on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis. 2010;51:1115–22. doi: 10.1086/656737. [DOI] [PubMed] [Google Scholar]
- 28.Klompas M. Is a ventilator-associated pneumonia rate of zero really possible? Curr Opin Infect Dis. 2012;25:176–82. doi: 10.1097/QCO.0b013e3283502437. [DOI] [PubMed] [Google Scholar]
- 29.Morris AC, Hay AW, Swann DG, Everingham K, McCulloch C, McNulty J, et al. Reducing ventilator-associated pneumonia in intensive care: Impact of implementing a care bundle. Crit Care Med. 2011;39:2218–24. doi: 10.1097/CCM.0b013e3182227d52. [DOI] [PubMed] [Google Scholar]
- 30.Dawson D, Endacott R. Implementing quality initiatives using a bundled approach. Intensive Crit Care Nurs. 2011;27:117–20. doi: 10.1016/j.iccn.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Berenholtz S, Pronovost PJ. Barriers to translating evidence into practice. Curr Opin Crit Care. 2003;9:321–5. doi: 10.1097/00075198-200308000-00012. [DOI] [PubMed] [Google Scholar]


