Abstract
AIM:
The purpose of this study was to assess the contribution of 18F-fluorodeoxyglucose (FDG) Positron Emission Tomography (PET)/Computed Tomography (CT) in detection and staging of pulmonary carcinoid tumors.
METHODS:
A total of 22 patients with pulmonary carcinoid tumors (14 typical, 8 atypical) were reviewed in this retrospective study. PET/CT images of all patients were evaluated for primary tumor as well as metastatic regional lymph nodes, bone and other distant metastases. PET/CT positivity of primary tumors was determined by visual interpretation. Tumor size, SUVmax and Hounsfield Unit (HU) values of the tumors were used to test for differences between tumor groups (typical carcinoids and atypical carcinoids).
RESULTS:
SUVmax of carcinoids ranged from 1.24 to 11.1 (mean, 5.0; median, 2.67). The mean largest diameter of primary tumors was 2.7 ± 1.3 cm, ranging from 1 to 5.5 cm. The overall sensitivity of FDG PET/CT for detection of pulmonary carcinoid tumors was 81.8%. Tumor size, SUVmax and Hounsfield Unit (HU) values of the atypical carcinoids were higher than those for typical carcinoids. However, the results were not statistically meaningful (P > 0.05). The sensitivity and specificity of FDG PET/CT in the detection of mediastinal and hilar lymph nodes metastases were 25% and 83% respectively. One patient had bone metastasis.
CONCLUSION:
Although FDG PET/CT can be a useful tool for the detection of pulmonary carcinoid tumors and distant metastasis, it cannot discriminate typical carcinoids from atypical ones and absence of an FDG avid lesion cannot exclude pulmonary carcinoid tumors. Moreover, PET/CT is not a reliable tool in the staging of mediastinal and hilar lymph nodes especially for those patients with typical carcinoids.
Keywords: FDG PET/CT, pulmonary carcinoid tumor, synchronous pulmonary carcinoids
Pulmonary carcinoids are rare malignant tumors, comprising 1-5% of all primary lung cancers.[1] These tumors were categorized as low-grade typical carcinoids or intermediate-grade atypical carcinoids.[2,3] While about 10-20% of pulmonary carcinoids are atypical carcinoids; the remaining 80-90% are typical carcinoids.[1,4]
Anatomical imaging methods such as chest radiography and computed tomography (CT) are essential in the diagnosis of pulmonary carcinoid tumors. Pulmonary carcinoid tumors usually appear as a smooth, spherical, ovoid or slightly lobulated nodule having calcification or ossification in approximately 30% of cases on computed tomography (CT) scans. Approximately 80% of them are located in the central airways and accompanying evidence of bronchial obstruction such as atelectasis or obstructive pneumonitis can be seen frequently.[4]
Although 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) have been widely used in the staging, restaging, follow-up and response of many malignancies, the use of PET/CT scan for detection and management of pulmonary carcinoids is controversial.[5] In this study, we aimed to assess the contribution of FDG PET/CT in detection and staging of pulmonary carcinoids.
Methods
Patients
We retrospectively evaluated data from 22 patients (14 males and 8 females; mean age, 46 ± 14 years; age range, 21-68 years) with histopathologically proven pulmonary carcinoid tumors diagnosed between January 2010 and December 2012. Primary tumors underwent complete resection. Systematic lymph node dissection (LND) or sampling was performed during surgery. Pulmonary carcinoids were classified as atypical or typical tumors according to the criteria of the World Health Organization/International Association for the study of Lung Cancer classification scheme on histopathological evaluation.[6] The demographic data including age, gender and histological findings were recorded for all patients. This study was approved by the ethics committee.
FDG PET/CT imaging
PET/CT imaging was performed forty-five to sixty minutes after intravenous injection of 259-518 MBq (7-14 mCi) of FDG with a Siemens Biograph 6 HI-REZ integrated PET/CT scanner (Siemens Medical Solutions, Knoxville, TN, USA). All patients fasted for at least 6 hours before PET/CT imaging without water restriction. The blood glucose levels of patients were confirmed to be less than 180 mg/dL before FDG injection. PET/CT data was acquired from the top of skull to the upper thigh with the arms up position. The maximum standardized uptake value (SUVmax) corrected for body weight was computed by standard methods from the activity in the most intense voxel in the three-dimensional tumor region from the transaxial whole body images on attenuation-corrected PET/CT images. Iodinated intravenous (IV) CT contrast material was used in only four of 22 patients.
FDG PET/CT analysis
All PET/CT images were retrospectively evaluated by two experienced nuclear medicine physicians and one radiologist. All primary tumors were reviewed for CT features such as tumor location, existence of tumor calcification, existence of findings of endobronchial obstruction (atelectasis or consolidation). PET/CT positivity was determined using visual interpretation according to the following criteria: a) FDG uptake of primary tumor was compared with that of mediastinum in patients without endobronchial obstruction, b) in the presence of obstruction, FDG activity of tumor was compared with that of atelectasis or consolidation. Differentiation of primary tumor from atelectasis/consolidation or mediastinum was categorized as “easy” or “difficult”. Easily differentiated tumors were considered as PET positive. PET/CT images of all patients were also evaluated for metastatic mediastinal and hilar lymph nodes, bone and other distant metastases. Lymph nodes were considered as positive if there was a FDG uptake higher than the surrounding mediastinal blood pool.
Quantitative assessment
In addition to visual assessment, SUVmax of all primary tumors, longest tumor size and SUVmax of each mediastinal and hilar nodes were recorded. Non-contrast mean attenuation measurements (in Hounsfield Unit, HU) for 18 patients obtained from the CT images of PET/CT were assessed. HU values of primary tumors were not measured in the remaining four of 22 patients who received IV iodinated contrast material on CT imaging.
Statistical analysis
The Mann-Whitney U test was used to test for differences between tumor sizes, SUVmax and HU values and tumor groups (typical carcinoids and atypical carcinoids). The Spearman's rank correlation test was used to test the hypothesis that quantifiable data (SUVmax, HU, primary tumor size) of the patients with typical carcinoids and atypical carcinoids differ from each other. All quantitative values are given as mean ± standard deviation (SD). A statistically significant difference was defined as a P-value < 0.05.
Results
Clinical and histopathological data
Complete resection was done for all of 22 patients. Twenty one of the 22 patients had thoracotomy for primary tumor resection within one month after PET/CT imaging. Lobectomy was performed for one patient after treatment with bronchoscopic cryotherapy and six cycles of chemotherapy. According to pathological examination, the tumors were typical carcinoids in 14 (63.64%) and atypical carcinoids in 8 patients (36.36%). There were synchronous multiple pulmonary carcinoid tumors in two patients (one typical, one atypical). There were multiple tumorlets in one patient who had typical carcinoid tumor in the right lung accompanying bronchioloalveolar cell carcinoma (BCA) in the left lung. Of the eight patients with atypical carcinoid tumors, one showed oncocytic changes in the tumor specimens.
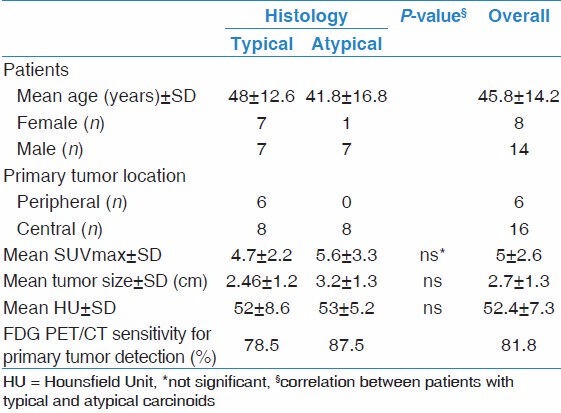
While 16 of 22 carcinoids were located in the central bronchial system, 6 of 22 carcinoids were located in peripheral bronchial system. All of the six peripheral tumors were typical carcinoids. All of the eight patients with atypical carcinoids had the primary tumor located in the central bronchial system [Table 1].
Table 1.
Patient characteristics, pathological data and measurements of PET/CT findings
Sixteen of 22 patients (72.7%) had postsurgical follow-up at our institution. Mean observation time was 13 months (range, 1-25 months). Other than one progressive patient with multiple bone and liver metastasis detected by PET/CT, none of the patients died or showed progression during the follow-up period.
FDG PET/CT findings
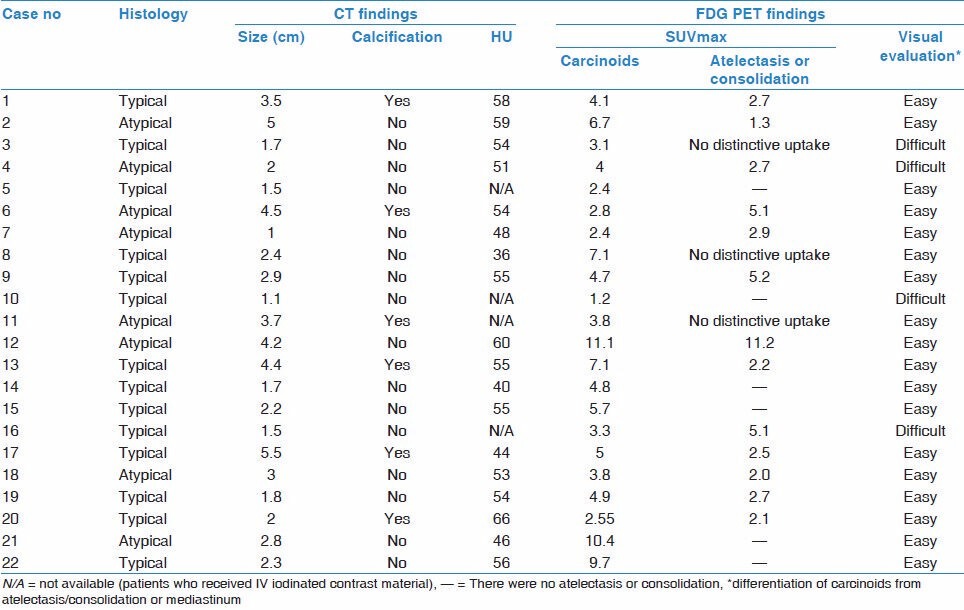
The FDG PET/CT findings of 22 patients with pulmonary carcinoids are given in Table 2. SUVmax of carcinoids ranged from 1.24 to 11.1 (mean, 5.0; median, 2.67). The mean largest size of primary tumors was 2.7 ± 1.3 cm, ranging from 1 to 5.5 cm. There was a marked correlation between the SUVmax values and tumor sizes (P = 0.010). There was accompanying obstructive pneumonia or atelectasis distal to endobronchial tumor in 16 of 22 patients. Activity of primary tumors were not easily discriminated from atelectasis or consolidation (n = 2) or mediastinal blood pool (n = 2) for four patients (three typical, one atypical carcinoids). These four patients were interpreted as false negatives. The remaining 18 of 22 (81.8%) pulmonary carcinoids that had distinctive activity from adjacent lung tissue or mediastinum were interpreted as true positives.
Table 2.
PET/CT findings of pulmonary carcinoid tumors
The overall sensitivity of FDG PET/CT for detection of pulmonary carcinoid tumors was 81.8%. Although tumor size, SUVmax and HU values of the atypical carcinoids were higher than typical carcinoids, the results were not statistically meaningful for each comparison (P > 0.05) [Table 1].
The PET/CT showed distant metastasis in only one patient with bone metastases on the sternum.
Assessment of lymph nodes
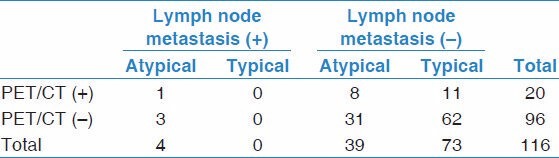
Of the 22 patients, 20 also had hilar and mediastinal lymphadenectomy on surgery. Systematic LN dissection was performed in 19 patients. A hundred and fourteen mediastinal and hilar stations were dissected in these patients. Lymph nodes were completely removed from these stations. The right lower paratracheal nodes (level 4R) and subcarinal nodes (level 7) were sampled in a patient with a right-sided tumor. A total 116 lymph node stations were evaluated hystopathologically. Metastases were diagnosed histopathologically in four of 116 stations in four individual patients. Histologic and imaging results are shown in Table 3. Twenty of 116 lymph nodes stations were found to be FDG-avid on PET/CT. However, only one of 20 FDG positive lymph nodes was proven to be metastatic on histopathological examination. The remaining 19 FDG false positive lymph nodes were revealed as reactive lymphoid proliferation and/or anthracosis (n = 18) or silicosis (n = 1) on histopathological examination. The SUVmax value of positive lymph node stations were ranging between 1.95 and 4.37, mean ± SD: 3.04 ± 0.69. SUV max of the metastatic lymph node was 2.46. All of the patients with lymph node metastases had atypical carcinoid tumors. None of the patients with typical carcinoid tumors had metastatic lymph nodes on histopathological examination. The sensitivity, specificity, positive and negative predictive values, false-positive and false-negative rates of PET/CT in detecting mediastinal and hilar lymph node metastases on a per-nodal station basis were 25, 83, 5, 96.8, 16.9 and 75% respectively.
Table 3.
Results of FDG-PET/CT and histopathological findings of lymph nodes in 20 patients who underwent lymph node dissection during surgery
Discussion
The present study revealed that the detection rate of FDG PET/CT imaging for pulmonary carcinoid tumors was 81.8%. Erasmus et al.[7] reported that FDG PET had a low sensitivity (14.2%) for detecting pulmonary carcinoid tumors in seven patients. However, several other reports that included a larger number of patients and large-size tumors indicate that FDG PET or PET/CT have a higher sensitivity similar to our results.[8,9,10,11] There were some limitations of FDG PET/CT for evaluating pulmonary carcinoid tumors: (a) low FDG affinity of especially small carcinoid tumors; (b) FDG uptake of these endobronchial tumors may be obscure from intense FDG uptake of distal obstructive atelectasis. Kayani et al.[12] reported the PET/CT results of 18 patients with pulmonary neuroendocrine tumors comparing the performance of 68Ga-DOTATATE, a novel selective somatostatin receptor 2 PET ligand, and FDG in the detection of pulmonary neuroendocrine tumors. They showed that FDG uptake was more intense than the 68Ga-DOTATATE uptake in a collapsed lung distal to endobronchial carcinoid tumor. However, in 14 (87.5%) of 16 carcinoids with accompanying distal atelectasis or consolidation, the activity of the tumor could be successfully separated from these paranchimal lesions in our study [Figure 1].
Figure 1.
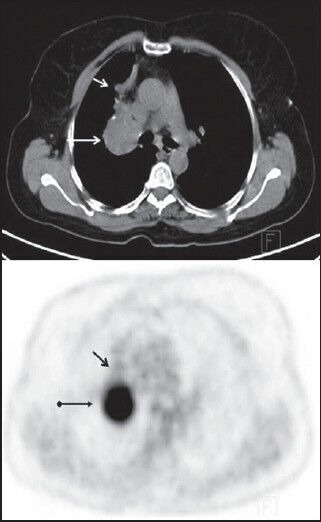
Typical carcinoid tumor (Case no. 13). Axial PET/CT image shows high FDG uptake in the central lobulated mass with punctate calcifications (long arrow). There was atelectasis with low FDG uptake at the right upper lobe (short arrow)
Some authors noticed that histologic type and lymph-node involvement were the most important prognostic factors for pulmonary carcinoid tumors.[13] Reactive hilar or mediastinal lymph node hyperplasia may be seen due to recurrent pneumonia on patients with carcinoid tumors.[14] Confirming this data, there were a lot of anthracosis and reactive lymphoid hyperplasia in lymph nodes in the present study. Due to high ratio of false negativity and false positivity in the staging of mediastinal lymph nodes in the pulmonary carcinod tumors, LN dissection should be preferred in all patients.
It was shown that there was a positive correlation between the tumor size and SUVmax in lung cancer.[15] Similarly, our study revealed that SUVmax of the primary carcinoid tumor has a positive correlation with the tumor diameter for 22 patients. It was reported that FDG uptake correlates well with Glut-1 expression.[16] Ozbudak et al.[17] revealed that Glut-1 was observed in 7% (3/46) of typical carcinoids and 21% (6/29) of the atypical carcinoids. It is expected that there is higher FDG uptake in atypical carcinoids than typical carcinoids. Daniels et al.,[9] reported that atypical carcinoids were more likely to be PET true positive than were typical carcinoids (80% versus 72.7%, respectively). Similar to these reports, although there is no statistically significant difference, mean SUVmax, tumor size and CT density were higher in atypical carcinoids than typical carcinoids. It seems that differentiation of atypical carcinoids from typical ones according to PET/CT findings is almost impossible. Future studies with a larger group of patients may yield more satisfactory results.
Synchronous multiple pulmonary carcinoids are extremely unusual cases.[18,19] There were two patients with synchronous multiple pulmonary carcinoid tumors in our study group. The incidence of multiple pulmonary carcinoid tumors may be greater than expected. Also there were multiple tumorlets, pulmonary carcinoid and bronchioloalveolar carcinoma in the same patient. Tumorlets are nodular aggregates of neuroendocrine cells with a diameter of less than 0.5 cm. The morphology of tumorlets is similar to that of carcinoid tumors. Carcinoid tumors may be seen in patients with multiple tumorlets.[20] In the presence of multiple pulmonary nodules, especially those with low FDG affinity, synchronous pulmonary carcinoids and/or tumorlets should be considered in the differential diagnosis.
Conclusion
Although FDG PET/CT can be a useful tool for the detection of pulmonary carcinoid tumors and distant metastasis, it cannot discriminate typical carcinoids from atypical ones and absence of an FDG avid lesion cannot exclude pulmonary carcinoid tumors. Moreover, PET/CT is not a reliable tool in the staging of mediastinal and hilar lymph nodes, especially for those patients with typical carcinoids.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M, et al. Pulmonary carcinoid: Presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from literature. Chest. 2001;119:1647–51. doi: 10.1378/chest.119.6.1647. [DOI] [PubMed] [Google Scholar]
- 2.Arrigoni MG, Woolner LB, Bernatz PE. Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg. 1972;64:413–21. [PubMed] [Google Scholar]
- 3.Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–44. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Neuroendocrine tumors of the lung: Clinical, pathologic, and imaging findings. Radiographics. 2006;26:41–57. doi: 10.1148/rg.261055057. [DOI] [PubMed] [Google Scholar]
- 5.Bertino EM, Confer PD, Colonna JE, Ross P, Otterson GA. Pulmonary neuroendocrine/carcinoid tumors: A review article. Cancer. 2009;115:4434–41. doi: 10.1002/cncr.24498. [DOI] [PubMed] [Google Scholar]
- 6.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–7. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Erasmus JJ, McAdams HP, Patz EF, Jr, Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol. 1998;170:1369–73. doi: 10.2214/ajr.170.5.9574618. [DOI] [PubMed] [Google Scholar]
- 8.Krüger S, Buck AK, Blumstein NM, Pauls S, Schelzig H, Kropf C, et al. Use of integrated FDG PET/CT imaging in pulmonary carcinoid tumours. J Intern Med. 2006;260:545–50. doi: 10.1111/j.1365-2796.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 9.Daniels CE, Lowe VJ, Aubry MC, Allen MS, Jett JR. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest. 2007;131:255–60. doi: 10.1378/chest.06-0711. [DOI] [PubMed] [Google Scholar]
- 10.Wartski M, Alberini JL, Leroy-Ladurie F, De Montpreville V, Nguyen C, Corone C, et al. Typical and atypical bronchopulmonary carcinoid tumors on FDG PET/CT imaging. Clin Nucl Med. 2004;29:752–3. doi: 10.1097/00003072-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Moore W, Freiberg E, Bishawi M, Halbreiner MS, Matthews R, Baram D, et al. FDG-PET imaging in patients with pulmonary carcinoid tumor. Clin Nucl Med. 2013;38:501–5. doi: 10.1097/RLU.0b013e318279f0f5. [DOI] [PubMed] [Google Scholar]
- 12.Kayani I, Conry BG, Groves AM, Win T, Dickson J, Caplin M, et al. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med. 2009;50:1927–32. doi: 10.2967/jnumed.109.066639. [DOI] [PubMed] [Google Scholar]
- 13.Aydin E, Yazici U, Gulgosteren M, Agackiran Y, Kaya S, Gulhan E, et al. Long-term outcomes and prognostic factors of patients with surgically treated pulmonary carcinoid: Our institutional experience with 104 patients. Eur J Cardiothorac Surg. 2011;39:549–54. doi: 10.1016/j.ejcts.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Webb WR, Gamsu G, Bimgerg FA. CT appearance of bronchial carcinoid with recurrent pneumonia and hyperplastic hilar lymphadenopathy. J Comput Assist Tomogr. 1983;7:707–9. doi: 10.1097/00004728-198308000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Sahiner I, Atasever T, Akdemir UO, Ozturk C, Memis L. Relationship between primary lesion metabolic parameters and clinical stage in lung cancer. Rev Esp Med Nucl Imagen Mol. 2013;32:357–63. doi: 10.1016/j.remn.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y, et al. [18F] FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia. 2005;7:369–79. doi: 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozbudak IH, Shilo K, Tavora F, Rassaei N, Chu WS, Fukuoka J, et al. Glucose transporter-1 in pulmonary neuroendocrine carcinomas: Expression and survival analysis. Mod Pathol. 2009;22:633–8. doi: 10.1038/modpathol.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazici U, Gulhan E, Agackiran Y, Tastepe I, Yaran P. Synchronous bilateral multiple typical pulmonary carcinoid tumors. Ann Thorac Surg. 2010;89:1278–80. doi: 10.1016/j.athoracsur.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Choi YD, Kim BJ, Oh IJ, Song SY, Nam JH, et al. Multiple peripheral typical carcinoid tumors of the lung: Associated with sclerosing hemangiomas. Diagn Pathol. 2013;8:97. doi: 10.1186/1746-1596-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aubry MC, Thomas CF, Jr, Jett JR, Swensen SJ, Myers JL. Significance of multiple carcinoid tumors and tumorlets in surgical lung specimens: Analysis of 28 patients. Chest. 2007;131:1635–43. doi: 10.1378/chest.06-2788. [DOI] [PubMed] [Google Scholar]


