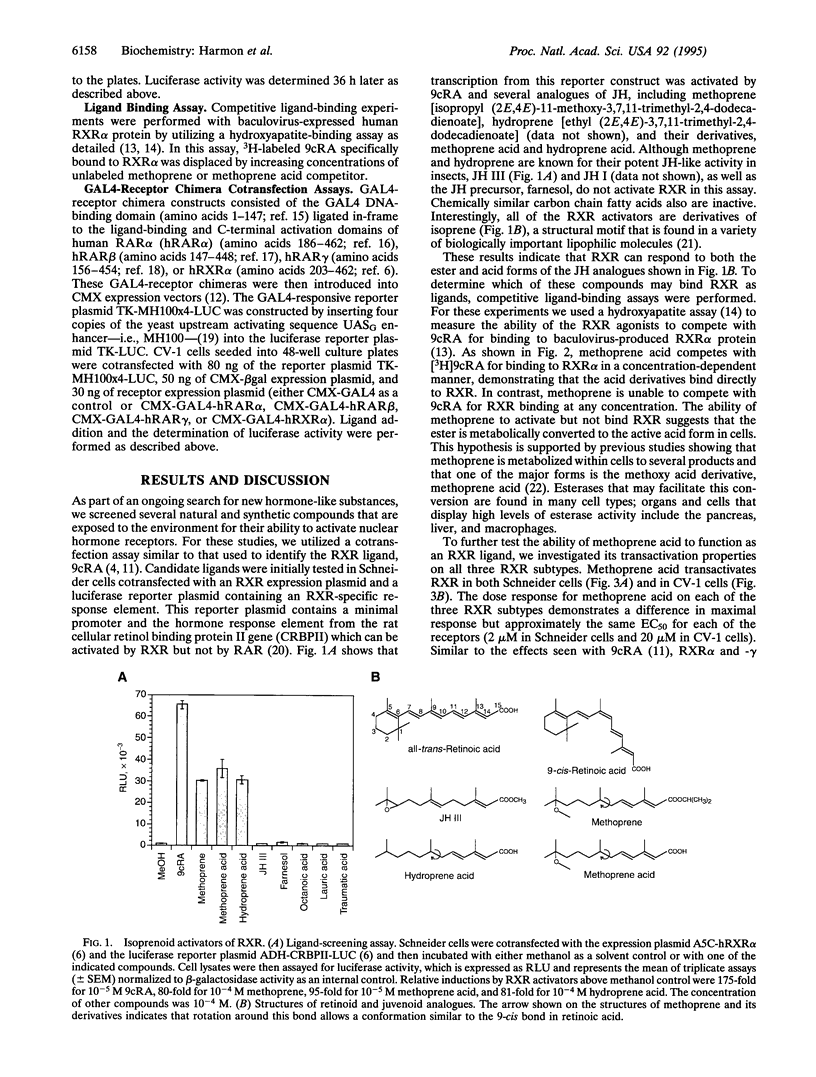
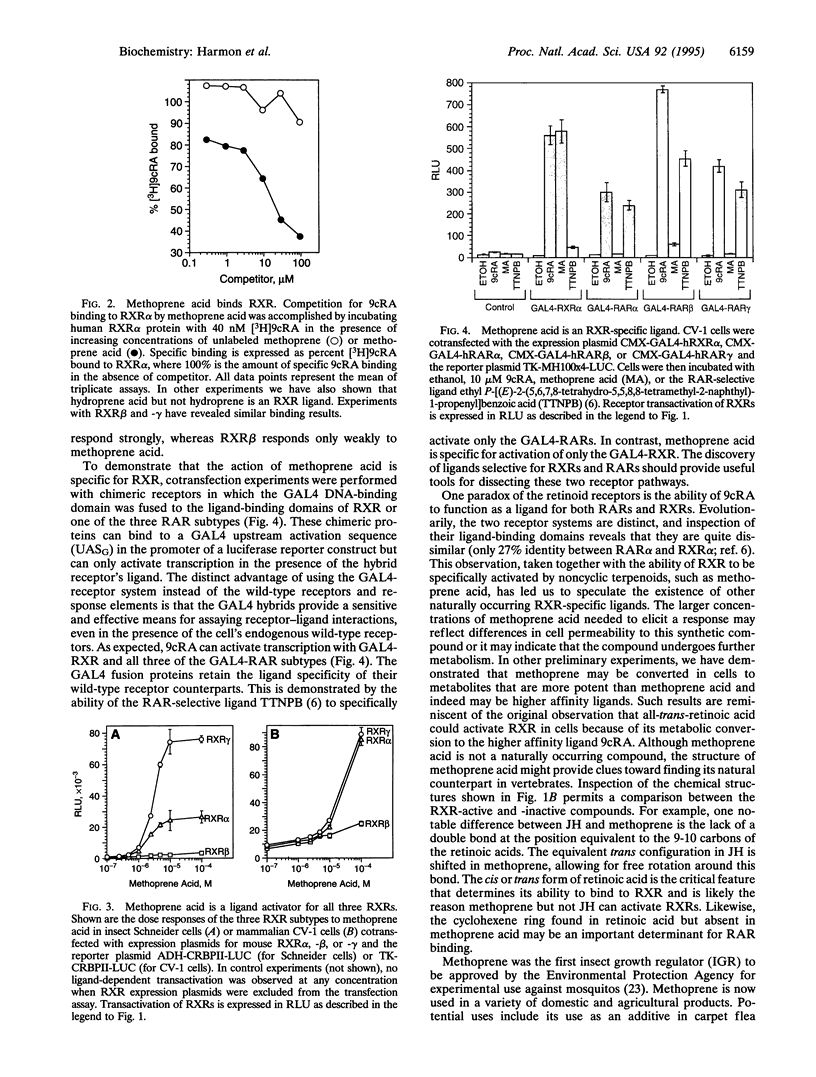
Abstract
We report that methoprene and its derivatives can stimulate gene transcription in vertebrates by acting through the retinoic acid-responsive transcription factors, the retinoid X receptors (RXRs). Methoprene is an insect growth regulator in domestic and agricultural use as a pesticide. At least one metabolite of methoprene, methoprene acid, directly binds to RXR and is a transcriptional activator in both insect and mammalian cells. Unlike the endogenous RXR ligand, 9-cis-retinoic acid, this activity is RXR-specific; the methoprene derivatives do not activate the retinoic acid receptor pathway. Methoprene is a juvenile hormone analog that acts to retain juvenile characteristics during insect growth, preventing metamorphosis into an adult, and it has been shown to have ovicidal properties in some insects. Thus, a pesticide that mimics the action of juvenile hormone in insects can also activate a mammalian retinoid-responsive pathway. This finding provides a basis through which the potential bioactivity of substances exposed to the environment may be reexamined and points the way for discovery of new receptor ligands in both insects and vertebrates.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehm M. F., Zhang L., Badea B. A., White S. K., Mais D. E., Berger E., Suto C. M., Goldman M. E., Heyman R. A. Synthesis and structure-activity relationships of novel retinoid X receptor-selective retinoids. J Med Chem. 1994 Sep 2;37(18):2930–2941. doi: 10.1021/jm00044a014. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Fiorella P. D., Napoli J. L. Expression of cellular retinoic acid binding protein (CRABP) in Escherichia coli. Characterization and evidence that holo-CRABP is a substrate in retinoic acid metabolism. J Biol Chem. 1991 Sep 5;266(25):16572–16579. [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Umesono K., Mangelsdorf D. J., Aburatani H., Stanger B. Z., Shibasaki Y., Imawari M., Evans R. M., Takaku F. A functional retinoic acid receptor encoded by the gene on human chromosome 12. Mol Endocrinol. 1990 Jun;4(6):837–844. doi: 10.1210/mend-4-6-837. [DOI] [PubMed] [Google Scholar]
- Kang T., Martins T., Sadowski I. Wild type GAL4 binds cooperatively to the GAL1-10 UASG in vitro. J Biol Chem. 1993 May 5;268(13):9629–9635. [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Oro A. E., McKeown M., Evans R. M. Relationship between the product of the Drosophila ultraspiracle locus and the vertebrate retinoid X receptor. Nature. 1990 Sep 20;347(6290):298–301. doi: 10.1038/347298a0. [DOI] [PubMed] [Google Scholar]
- Prestwich G. D., Touhara K., Riddiford L. M., Hammock B. D. Larva lights: a decade of photoaffinity labeling with juvenile hormone analogues. Insect Biochem Mol Biol. 1994 Sep;24(8):747–761. doi: 10.1016/0965-1748(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Quistad G. B., Staiger L. E., Schooley D. A. Environmental degradation of the insect growth regulator methoprene (isopropyl (2E,4E)-11-Methoxy-3,7,11-trimethyl-2,4-dodecadienoate). I. Metabolism by alfalfa and rice. J Agric Food Chem. 1974 Jul-Aug;22(4):582–589. doi: 10.1021/jf60194a022. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989 Sep 25;17(18):7539–7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal G. B. Insect growth regulators with juvenile hormone activity. Annu Rev Entomol. 1975;20:417–460. doi: 10.1146/annurev.en.20.010175.002221. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth B., Hennen S., Krishnakumaran A., Ting P., Hoffman N. Teratogenic evaluation of terpenoid derivatives. Life Sci. 1974 Nov 1;15(9):1649–1655. doi: 10.1016/0024-3205(74)90331-2. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Norman A. W. An hydroxylapatite batch assay for the quantitation of 1alpha,25-dihydroxyvitamin D3-receptor complexes. Anal Biochem. 1979 Jan 15;92(2):314–323. doi: 10.1016/0003-2697(79)90664-x. [DOI] [PubMed] [Google Scholar]
- Wright J. E. Environmental and toxicological aspects of insect growth regulators. Environ Health Perspect. 1976 Apr;14:127–132. doi: 10.1289/ehp.7614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T. P., Segraves W. A., Oro A. E., McKeown M., Evans R. M. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell. 1992 Oct 2;71(1):63–72. doi: 10.1016/0092-8674(92)90266-f. [DOI] [PubMed] [Google Scholar]