Abstract
Neutrophil firm adhesion to endothelial cells plays a critical role in inflammation in both health and disease. The process of neutrophil firm adhesion involves many different adhesion molecules including members of the β2 integrin family and their counter-receptors of the ICAM family. Recently, naturally occurring genetic variants in both β2 integrins and ICAMs are reported to be associated with autoimmune disease. Thus, the quantitative adhesive capacity of neutrophils from individuals with varying allelic forms of these adhesion molecules is important to study in relation to mechanisms underlying development of autoimmunity. Adhesion studies in flow chamber systems can create an environment with fluid shear stress similar to that observed in the blood vessel environment in vivo. Here, we present a method using a flow chamber assay system to study the quantitative adhesive properties of human peripheral blood neutrophils to human umbilical vein endothelial cell (HUVEC) and to purified ligand substrates. With this method, the neutrophil adhesive capacities from donors with different allelic variants in adhesion receptors can be assessed and compared. This method can also be modified to assess adhesion of other primary cell types or cell lines.
Keywords: Immunology, Issue 89, neutrophil adhesion, flow chamber, human umbilical vein endothelial cell (HUVEC), purified ligand
Introduction
Genetic variants in both β2 integrins and in ICAM ligands are now recognized to be associated with the development of autoimmune disease1,2. The determination of the functional consequences of these variants in cells derived from individuals with these variants is necessary for our understanding of how these variants contribute to autoimmune disease pathogenesis. Such functional studies allow for the determination of the mechanisms by which naturally occurring genetic variants shape the immune response in both health and disease. In the specific example of SLE, we now know that variants in ITGAM (CD11b) and its ligand, ICAM-1, strongly associate with development of disease1,2. Because neutrophils are critical in inflammatory responses, the quantitative study of neutrophil adhesion may provide mechanistic insights into how genetic variants in ITGAM/ICAM alter inflammation.
Neutrophil firm adhesion to endothelial cells (EC) is a highly regulated process and plays an essential role in inflammatory responses3,4. The firm adhesion of neutrophil follows initial neutrophil rolling and capture on the EC and ultimately can result in transmigration in vivo. These processes involve many different kinds of adhesion molecules, including ICAM-1, ICAM-2, P-Selectin, E-Selectin on the endothelial cells and β2 integrins on the neutrophil5-9. Thus, careful quantification of neutrophil adhesion from donors with different allelic variants of adhesion molecules will be important to understand the functional and pathological consequences of these genetic variants.
Experimental use of a flow chamber can create an in vitro environment with fluid shear stress similar to that observed in the blood vessel environment in vivo10-12. Indeed, a flow chamber assay coupled with human umbilical vein endothelial cell (HUVEC) can mimic the in vivo environment of a blood vessel. Using this method, one can study the overall cellular adhesive properties towards endothelial cell. In addition, the highly controlled environment of the flow chamber also allows assessment of cell binding to purified adhesion ligands such as ICAM-1 to facilitate the study of specific receptor-ligand interactions.
We present here a method utilizing a flow chamber adhesion assay system to study the adhesive properties of human peripheral blood neutrophils to HUVEC and to purified ligand substrates. Using this method with cells from donors expressing different adhesion molecule allelic variants allows us to assess how these genetic variants can alter human neutrophil firm adhesion.
Protocol
All donors recruited for this study gave written informed consent and the study was approved by the University of Alabama at Birmingham Institutional Review Board.
1. HUVEC Initial Culture and Subculture
Culture human umbilical vein endothelial cells (HUVEC) in vitro in complete growth medium that is comprised of endothelial cell basal medium (see List of Materials) supplemented with endothelial cell growth factors. Note: The growth factors used in this study include: 5 ng/ml human recombinant Epidermal Growth Factor (hEGF), 1.0 mg/ml hydrocortisone, 50 mg/ml Gentamicin and 50 ng/ml amphotericin-B (GA-1000), 2% Fetal Bovine Serum (FBS), 0.5 ng/ml Vascular Endothelial Growth Factor (VEGF), 10 ng/ml human Fibroblast Growth Factor-Basic with heparin (hFGF-B), 20 ng/ml human recombinant Insulin-like Growth Factor (R3-IGF-1), 1 µg/ml ascorbic acid, and 22.5 µg/ml heparin. The complete medium is prepared initially at 37 °C and can then be stored at 4 °C for use within 1 month of preparation.
To prepare a flask for cells, complete growth medium is added to a 75 cm2 tissue culture flask (1 ml/5 cm2) and then the flask is allowed to equilibrate to 37 °C/5% CO2 in a humidified incubator for at least 30 min. Human HUVEC will be seeded directly into this equilibrated culture flask at a density of 2,500-5,000 cells/cm2.
While the media is equilibrating, quickly thaw the stock HUVEC cryovial in a 37 °C water bath. Disperse the cells in the original storage vial by vortexing and then add them directly to the culture flask containing the pre-equilibrated HUVEC complete growth medium to achieve a density of 2,500-5,000 cells/cm2. Gently rock the flask to evenly distribute the cells and then return the flask to the incubator. These cells now represent the 1st passage.
Medium should be changed every two days until the cells are 70-80% confluent.
The HUVECs can now be harvested from the culture flask with Trypsin/EDTA. The culture media is aspirated from the culture flasks followed by a PBS rinse to remove any residual protein and calcium from the cells. A 0.25% Trypsin-EDTA solution is added and within 2-6 min cell detachment should be evident as assessed by light microscopy.
When 90% of the cells are rounded up off the plate, stop the trypsinization by adding an equal volume of 2x trypsin inhibitor. To facilitate the harvest of cells, add another equal amount of complete growth medium. Transfer the detached cells to a sterile 15 ml centrifuge tube.
Centrifuge the detached cells at 225 x g for 5 min at room temperature. Aspirate the supernatant, and then resuspend the cells in 2-3 ml of complete growth medium. Determine the cell concentration and viability using a hemacytometer and Trypan Blue.
To utilize the harvested cells for study, re-seed additional 75 cm2 flasks with cells at a density of 10,000 cells/cm2 and proceed to step 2.2. Alternatively, cells can be frozen at this point for future studies. For cell freezing, pellet the cells by centrifugation at 225 x g for 5 min at room temperature. Aspirate off the supernatant and resuspend the cell pellet in FBS containing 10% DMSO at a concentration of 1 x 106 cells/ml. The cell suspension is then transferred to cryovials and stored at -80 °C after freezing of the cells in a cell-freezing container.
2. HUVEC Layer Preparation
- Use of frozen 2nd passage HUVECs: revival and culture. If using actively growing cells, proceed to step 2.2.
- Thaw the cryovial containing 2nd passage HUVECs from step 1.8 quickly in a 37 °C water bath. Transfer cells from the cryovial to a 15 ml sterile centrifuge tube and add 10 ml growth medium.
- Centrifuge the cells at 200 x g for 5 min at room temperature and aspirate the supernatant to remove the residual DMSO.
- Resuspend the cell pellet with complete growth medium and transfer the cells into a 75 cm2 flask. Add growth medium to a total volume about 20-25 ml and incubate at 37 °C/5% CO2.
Visually examine the cell culture for confluence each day with media changes every two days. Usually within 2-4 days, cells will reach 80-90% confluence at which time they will be ready for use in the flow chamber assay.
To prepare the culture dish that will be used in the flow chamber (see Section 5), add 1 ml of 10 µg/ml fibronectin and 0.05% (w/v) gelatin to a 35 mm tissue culture dish and pipette several times to make sure the whole plate surface is coated. Remove the excessive fibronectin and gelatin solution and air dry the plate for at least 30 min to optimize the protein matrix formation. The fibronectin and gelatin solution may be reused up to 10x.
Harvest the HUVEC cells from step 2.2 using trypsin-EDTA as described in steps 1.5-1.7. Seed 500,000 cells into each coated tissue culture dish. Add 2 ml growth medium in each dish and incubate at 37 °C/5% CO2.
Allow cells to grow to confluence as in step 2.2. Cells should be visually inspected daily with medium changes every two days. Prior to the flow chamber assay, prime the HUVEC with 20 ng/ml human TNF-α for 4-6 hr to upregulate and stimulate adhesion molecule expression.
3. Purified Ligand Coating
Draw a circle of 0.5 cm diameter with a marker or pen at the center of a 35 mm tissue culture dish.
Plate 20 µl of 20 µg/ml protein A in the marked area. Use the pipette tip to spread the protein A solution to cover the whole area within the 0.5 cm diameter circle. It is important to not touch or scratch the surface of the dish. Incubate at 37 °C for 1 hr.
Wash the protein-A coated plate 3x with 1 ml of PBS (pH 8.0).
Plate 50 µl 1% BSA in the marked area to block non-specific binding on the plate. Incubate for 2 hr at 4 °C.
Wash the blocked plate 3x with 1 ml of PBS as in step 3.3.
Prepare the Fc-adhesion receptor ligand chimeric protein solutions for coating. In this experiment, an ICAM-1/Fc chimera solution at 25 µg/ml and a P-Selectin/Fc chimera at 0.5 µg/ml in PBS (pH 8.0) was used.
Coat the marked area with 50 µl of substrate. Incubate overnight at 4 °C. The dish should be used within two days and the coated area should not be allowed to dry out. Add PBS if necessary to maintain the 50 µl solution on the plate.
4. Neutrophil Separation (All Steps Performed at Room Temperature)
After obtaining informed consent, collect participant blood by phlebotomy into an anticoagulant blood collection tube or vacutainer (EDTA or heparin). After blood collection, dilute the blood 1:1 with PBS before separation.
Prepare a two layer Ficoll for separating PBMC and neutrophils in 50 ml centrifuge tubes. First add 15 ml heavy Ficoll (see List of Materials, ρ=1.118-1.120), and then carefully layer 10 ml light Ficoll (see List of Materials, ρ=1.077-1.080) on top of the heavy Ficoll. There should be a sharp border between the light Ficoll and heavy Ficoll layers. Finally, carefully layer 25 ml of the diluted blood sample on top of the light Ficoll without disturbing the Ficoll layer.
Centrifuge the tube at 250 x g for 30 min at room temperature. Note: The centrifuge rotor brake should be off for these spins to minimize potential disruption of the cell separation during rotor deceleration at the end of the centrifugation. After centrifugation, the following layers (from top to bottom) are present: the top layer is the plasma followed by the PBMC layer on top of the light Ficoll layer, the neutrophil layer with few red blood cells (RBC) is between the light and heavy Ficoll followed by the heavy Ficoll layer and RBCs at the bottom of the tube.
- Harvest and transfer the neutrophil layer into a new 50 ml tube with a transfer pipette, add PBS to a final volume of 50 ml and centrifuge at 225 x g for 10 min at room temperature.
- After centrifugation, there may still be RBCs mixed with the neutrophils. Aspirate the supernatant down to the 10 ml mark. Resuspend the neutrophil-RBC pellet by gentle agitation of the tube (or a brief low speed vortexing) and then wash again with 50 ml of PBS.
- After the second wash, aspirate the supernatant. To remove the contaminating RBCs, resuspend the pelleted cells in the residual PBS by agitation (or short vortexing), add 25 ml H2O and gently vortex for 10 sec to lyse the RBC.
- Add 25 ml of 1.8% NaCl and immediately mix by centrifugation at 225 x g for 10 min at room temperature. The contaminating RBC should now be lysed leaving a white neutrophil cell pellet.
Wash the neutrophil cell pellet with PBS.
Resuspend the isolated neutrophils in RPMI medium with 10% FBS and determine the cell concentration under light microscope with a hemocytometer.
Adjust the cell density to 500,000 cells/ml with complete RPMI-10% FBS medium.
5. Flow Chamber Adhesion Assay
Assemble the flow chamber. Place the 35 mm dish containing the confluent HUVECs or purified adhesion receptor ligands on the microscope table. Connect the parallel plate flow chamber with syringe pump, vacuum system and leave one line open for the neutrophil input. Insert the flow chamber on top of the plate and fasten the flow chamber assembly13. (See Figure 1)
Start the video recording program on the computer connected to the microscope. Adjust the field and focus of the microscope until a clear field with fully grown HUVEC cells or a field within the ligand coating region is visible.
Using the syringe pump, rinse the flow chamber with RPMI medium. Make sure there are no air bubbles within the chamber or the neutrophil input line.
If desired, the neutrophils may be primed with low dose (10-8 M) fMLP for 10 min. This will allow for a matching of the basal level of neutrophil activation between different donors14.
Use the syringe pump to inject the neutrophils into the flow chamber at defined speeds (a speed of 350 µl/min, which equals a shear stress of 1.5 dynes/cm2 is used in this study). Record the video. Because neutrophil adhesion can occur rapidly, a video with a length of 4-5 min is usually sufficient to quantitate adhesion events for analysis.
An adherent cell is defined as a cell that moves less than one cell diameter within 5 sec on the HUVEC or ligand coated surface15,16. Count the total number of adherent cells in the field present in the recorded video using this rule. By recording similar length videos with different donors’ neutrophil, one can calculate the adherent cells/min to compare the adhesion properties between different donors.
Representative Results
Examples of neutrophil binding to a purified ligand (ICAM-1/P-Selectin) coated flow chamber (Figure 2) or of neutrophil binding to a HUVEC coated flow chamber assay (Figure 3) are shown. As shown in the figures, neutrophils continue to accumulate/adhere to the coated surface or HUVEC under conditions of continuous flow. Under our typical experiment conditions, we can observe 50-70 human neutrophils firmly adhering to the coated surface or HUVEC during a four-minute recording period. However, allelic variants of neutrophil adhesion molecules, or allelic variants in the substrate (purified ligand or HUVEC), could substantially alter quantitative neutrophil adhesion14.
We have also assessed the time dependence of the neutrophil adhesion events observed in our studies. While we are maintaining constant flow conditions, it is possible that the adhesive properties of the cells change over time. However, over the relatively short time courses in our studies, we do not observe any consistent differences in the rate of adhesion over time. For example, assessing adhesion at the 1-2 minute time points compared to adhesion observed between the 3-4 min time points are not consistently different. Of course, if cells are being stimulated during the adhesion experiment, then changes in adhesive properties over time could be expected.
In our studies, we controlled for isolation induced neutrophil activation by intentionally priming the cells with low dose fMLP (10-8 M) for 10 min prior to study. Endothelial cells (HUVEC in our studies) also require prior activation to upregulate adhesion molecule expression for optimal leucocyte firm adhesion. In the absence of pre-treatment, endothelial cells (HUVECs) will support very little neutrophil adhesion. In our studies, we used 10 ng/ml TNFα treatment for 4-6 hr prior to use. IL-1β (10 ng/ml) and LPS (0.5-1 µg/ml) can also be used to pre-activate the endothelial cells. Importantly, untreated HUVEC can be used as negative control to ensure that cell adhesion is caused by specific (receptor-mediated) neutrophil-endothelial cell interactions. Alternatively, anti-receptor antibodies can be used to block specific receptors to assess specificity of adhesion.
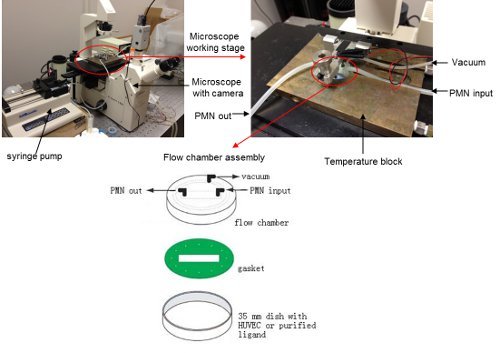 Figure 1. Flow chamber configuration on the microscope stage.
Please click here to view a larger version of this figure.
Figure 1. Flow chamber configuration on the microscope stage.
Please click here to view a larger version of this figure.
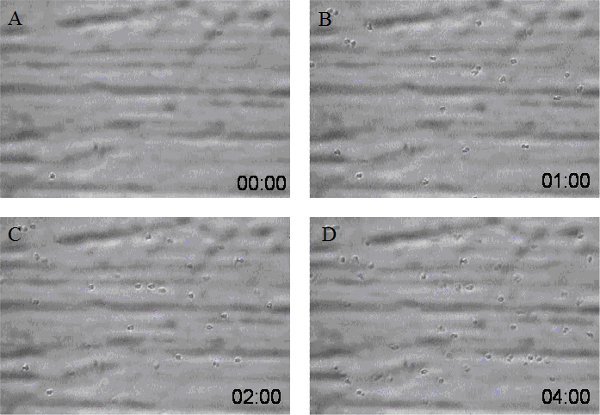 Figure 2. Screen shots from a sample video of neutrophils adhering to ICAM-1/P-Selectin coated surface at different time points (A: 0 time point, B: 1 min time point, C: 2 min time point, and D: 4 min time point). The concentration of ICAM-1 is 25 µg/ml and P-Selectin is 0.5 µg/ml. Neutrophil flow speed is 350 µl/min with neutrophil density at 500,000 cells/ml.
Figure 2. Screen shots from a sample video of neutrophils adhering to ICAM-1/P-Selectin coated surface at different time points (A: 0 time point, B: 1 min time point, C: 2 min time point, and D: 4 min time point). The concentration of ICAM-1 is 25 µg/ml and P-Selectin is 0.5 µg/ml. Neutrophil flow speed is 350 µl/min with neutrophil density at 500,000 cells/ml.
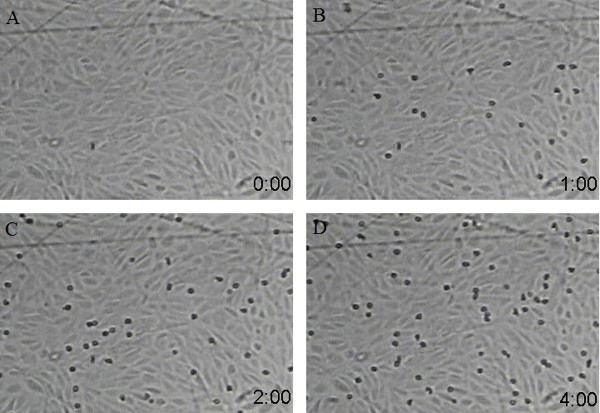 Figure 3. Screen shots from a sample video of neutrophils adhering to HUVEC coated surface at different time points (A: 0 time point, B: 1 min time point, C: 2 min time point, and D: 4 min time point). Neutrophil flow speed is 350 µl/ml with neutrophil density at 500,000 cells/ml.
Figure 3. Screen shots from a sample video of neutrophils adhering to HUVEC coated surface at different time points (A: 0 time point, B: 1 min time point, C: 2 min time point, and D: 4 min time point). Neutrophil flow speed is 350 µl/ml with neutrophil density at 500,000 cells/ml.
| Species | Location | Shear stress (dynes/cm2) |
| Human | Common caroid artery | 11.6 |
| Human | Branchial artery | 6.5 |
| Human | Common femoral artery | 4.3 |
| Human | Suprarenal aorta | 7.3 |
| Human | Supraceliac aorta | 4.2 |
| Human | Superficial fermoral artery | 4.4 |
| Human | Venules | 0.5-5.0 |
| Human | Retinal first arterioles | 40.2 |
| Human | Retinal second arterioles | 0.001 |
| Human | Retinal first venules | 23.2 |
| Human | Retinal second venules | 0.43 |
| Dog | Common caroid artery | 15.8 |
| Rabbit | Common caroid artery | 23.3 |
| Rat | Common caroid artery | 46.6 |
| Mouse | Common caroid artery | 64.8 |
| Dog | Common femoral artery | 9.8 |
| Rabbit | Common femoral artery | 156.8 |
| Rat | Common femoral artery | 65.9 |
Table 1. Sample shear stress in different organs and different species.
* summarized from references 13, 16, 19, and 20.
Discussion
This protocol guides the separation and isolation of minimally activated neutrophils for the careful quantification of neutrophil adhesion under sheer stress conditions. Neutrophil adhesion is a critical process in inflammation. Because genetic variants in multiple molecules in this process have been demonstrated to predispose to the development of autoimmune disease1,2, an assay system capable of quantitatively assessing human neutrophil firm adhesion is required. The method described in this protocol allows for the careful and quantitative determination of the firm adhesive potential of neutrophils in a controlled in vitro environment under sheer stress. This method thus allows the direct comparison of quantitative neutrophil adhesion between genotyped individuals to determine the importance of genetic variation in adhesion molecules14.
Several steps in this method merit careful consideration to achieve highly quantitative and reproducible results. In the HUVEC preparation, it is critical to attain 100% cell confluence before using them in the flow chamber. For using surfaces coated with purified ligand, the substrate coating area should never be allowed to dry out to avoid denaturing the ligand. In addition, the preparation of human neutrophils is critical to the success of the experiment. Key issues in isolating neutrophils from blood include gently handling by minimizing vortexing to avoid activation, keeping the cells at room temperature (i.e. the blood should not be stored on ice and centrifugation steps should be performed at room temperature) and completing the isolation and the experiment in the least amount of time as possible. There are additional neutrophil isolation methods that can also be utilized to prepare cells for these assays17,18.
From a practical perspective, adhesion assays using freshly isolated human neutrophils should be initiated within 3-6 hr after participant phlebotomy. As neutrophils are extremely sensitive to handling, prolonged times between the blood draw and usage could affect assay results. Careful determination of neutrophil cell concentration prior to the flow chamber assay is also necessary to achieve accurate and reproducible results.
During the flow chamber assay, it is important to monitor the video recording carefully to ensure that the flow speed is consistent and there is no turbulence throughout the length of the experiment. Changes in flow speeds or the presence of turbulence would necessitate that the experiment be repeated. After the experiment, it is also important to assess the remaining neutrophils microscopically to ensure that the neutrophils are not clumping. Clumping at this point would indicate that the neutrophils have become activated which could significantly alter neutrophil density during the experiment.
The flow chamber creates a near homogeneous sheer stress within the chamber (τ=6Qμ/(wh2), where Q=flow rate, μ=dynamic viscosity, and w=width of the flow chamber, h=height of the flow chamber15). In our studies, we used a flow rate of 350 µl/min for neutrophil adhesion, which creates a sheer stress of 1.5 dynes/cm2 (w=0.25 cm, h=0.01 in., the viscosity of water at 37 °C (0.007 poise) was used as an approximation of the viscosity of RMPI media). For a specific flow chamber, one can change the flow rate to achieve different levels of sheer stress to mimic different physiological conditions. Typical physiologic shear stress in human blood vessels can ranges between 0.5-5.0 dynes/cm 13,16. Shear stress in other blood vessels and other animals were listed in Table 1113,16,19 20.
While our method has focused on the study of the adhesion of human neutrophils, this method is not limited to neutrophils and can easily be applied to adhesion or rolling studies of other cell types with simple modifications. Also, the substrates in this method can be changed for different purposes.
Although this protocol is easily adaptable to different studies, there are some limitations. The protocol as implemented here requires large number of primary cells for analysis. This may preclude analysis of primary cells from small animals. Additionally, the need to immobilize purified adhesion ligands in an active/available conformation may limit the range of ligands. The use of Fc-fusion proteins greatly enhances the chances of achieving proper ligand conformation on the plate surface. Nonetheless, our method has significant flexibility to allow the quantitative analysis of adhesion events. These studies will greatly enhance our understanding of adhesion receptor-ligand pairs, and the potential function importance of genetic variants in these proteins, in the pathogenesis of human diseases.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is sponsored by the Lupus Research Institute (NY, NY), NIH P01-AR49084, NIH R21-DA026956 and NIH UL1-TR00165. We thank Dr. Robert P. Kimberly for his continued support.
References
- Harley JB, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants. in ITGAM, PXK, KIAA1542 and other. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, et al. Variation in the ICAM1-ICAM4-ICAM5 Locus Is Associated with Systemic Lupus Erythematosus Susceptibility in Multiple Ancestry Populations. Ann. Rheum. Diseases. 2012;71(11):1809–1814. doi: 10.1136/annrheumdis-2011-201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. Molecular mechanism of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996;32(4):733–742. [PubMed] [Google Scholar]
- Korthuls RJ, Anderson DC, Granger DN. Role of neutrophil-endothelial cell adhesion in inflammatory disorders. J. Crit. Care. 1994;9(1):47–71. doi: 10.1016/0883-9441(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8(8):504–512. [PubMed] [Google Scholar]
- Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Invest. 1989;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen. Cell. 1987;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell. 1991;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Smith CW. Possible steps involved in the transition to stationary adhesion of rolling neutrophils: A brief review. Microcirculation. 2000;7:385–394. [PubMed] [Google Scholar]
- Usami S, Chen HH, Zhao Y, Chien S, Skalak R. Design and construction of a linear shear stress flow chamber. Ann. Biomed. Eng. 1993;21(1):77–83. doi: 10.1007/BF02368167. [DOI] [PubMed] [Google Scholar]
- van Kooten TG, Schakenraad JM, Vander Mei HC, Busscher HJ. Development and use of a parallel-plate flow chamber for studying cellular adhesion to solid surfaces. J. Biomed. Mater. Res. 1992;26(6):725–738. doi: 10.1002/jbm.820260604. [DOI] [PubMed] [Google Scholar]
- Munn LL, Melder RJ, Jain RK. Analysis of cell flow in the parallel plate flow chamber: Implications for cell capture studies. Biophys. J. 1994;67:889–895. doi: 10.1016/S0006-3495(94)80550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik DF. Measurement of Adhesion Under Flow Conditions. Current Protocols in Cell Biology. 2003. pp. 9.6.1–9.6.10. [DOI] [PubMed]
- Zhou Y, et al. Multiple Lupus Associated ITGAM Variants Alter Mac-1 Function on Neutrophils. Arthritis. Rheum. 2013. [DOI] [PMC free article] [PubMed]
- Bacabac RG, et al. Dynamic shear stress in parallel-plate flow chambers. J. Biomech. 2005;38(1):159–167. doi: 10.1016/j.jbiomech.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Kucik DF, Wu C. Cell-Adhesion Assay. Methods in Molecular Biology. 2005;294:43–54. doi: 10.1385/1-59259-860-9:043. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Laube B, Abu Abed , Goosmann U, C , Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010;36(36) doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Siano B, Diamond S. Neutrophil isolation protocol. J Vis Exp. 2008. p. 745. [DOI] [PMC free article] [PubMed]
- Cheng C, et al. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis. 2007;195(2):225–235. doi: 10.1016/j.atherosclerosis.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Yoshida A. Noninvasive evaluation of wall shear stress on retinal microcirculation in humans. Invest Ophthalmol Vis Sci. 2006;47(3):1113–1119. doi: 10.1167/iovs.05-0218. [DOI] [PubMed] [Google Scholar]


