Abstract
Purpose
Within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), we assessed the long-term disease-specific functioning among prostate cancer (PCa) survivors versus noncancer controls, the impact of trial arm (screening/usual care) on functioning, and the effect of treatment modality on functioning.
Patients and Methods
PCa survivors (n = 529), 5 to 10 years postdiagnosis, were frequency-matched to noncancer controls (n = 514) for race, screening center, year of enrollment, and trial arm. Participants completed a telephone interview regarding PCa-specific symptomatology. Weights accounted for patient selection from the five PLCO screening centers. Propensity-score methods were used to balance groups of interest with respect to demographic and medical characteristics.
Results
Weighted linear regression analyses revealed poorer sexual and urinary function among PCa survivors compared with noncancer controls (P < .001). Trial arm was not significantly related to any outcome (P > .31). Compared with radical prostatectomy patients (n = 201), radiation-therapy patients (n = 110) reported better sexual (P < .05) and urinary (P < .001) functioning but poorer bowel outcomes (P < .05). Survivors who received treatment combinations including androgen deprivation (n = 207) reported significantly poorer hormone-related symptoms compared with radical prostatectomy patients (P < .05).
Conclusion
This study demonstrated the persistence of clinically significant, long-term PCa treatment-related sexual and urinary adverse effects up to 10 years postdiagnosis. To our knowledge, this was the first comparison of prostate-related dysfunction among screened survivors versus screened noncancer controls and indicated that these long-term problems were attributable to PCa treatment and not to aging or comorbidities. Finally, differences in long-term adverse effects between treatment modalities are particularly relevant for patients and clinicians when making treatment decisions.
INTRODUCTION
The debate regarding whether prostate cancer (PCa) screening saves lives1 was heightened recently when the European Randomized Study of Screening for PCa2,3 reported a 20% decrease in PCa-related mortality, whereas the US-based Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) reported no impact of screening on mortality.4,5 In addition, PCa-treatment trials have not established whether treatment of early-stage disease results in survival differences for a screened population.6,7 On the basis of these and other results,8,9 PCa screening was rated “not recommended” by the United States Preventive Services Task Force.10 When the survival benefit is uncertain, patient-reported outcomes such as disease-specific function are critical for making informed treatment decisions11–14 and for understanding whether the quality of the years living with PCa justifies screening.
The PLCO provides an exceptional opportunity to evaluate the impact of PCa treatment on disease-specific function, while controlling for aging, comorbidities, and screening. We addressed three aims among men diagnosed within the PLCO. First, because aging and age-related comorbidities can cause symptoms that are associated with PCa treatment, the determination of the cause of these symptoms among PCa survivors is difficult. Because the cancer-free comparison group of the PLCO is similarly aged and regularly screened, this matching provided an opportunity to address this concern that had not been possible in previous large-scale studies.15–18 We compared survivors with noncancer controls to determine whether the sexual, urinary, and bowel symptoms among survivors were due to PCa treatment or aging and comorbidities.
Second, although much is known about the adverse effects of PCa treatment, little is known about disease-specific function among men diagnosed with PCa after regular screening. Because regular screening results in earlier detection and treatment of smaller tumors, it is possible that treatment-related symptoms may be less intrusive after screen-detected cancers19–21 than cancers discovered by symptom presentation or intermittent screening.19–21 Alternatively, earlier detection after regular screening may simply result in a lengthening of the time men must cope with their treatment-related adverse effects but without a reduction of disease-related mortality.5
Third, although several studies have documented disease-specific function up to five years post-treatment,17,22–26 few studies have assessed the impact of treatment modalities on longer-term function.17,18,25,27,28 Such information is particularly important for informed treatment decision making given the younger ages at which screening is recommended and the increasing length of time men are living with the consequences of the disease and treatment. In addition, age-related comorbidities may interact with the adverse effects of different treatment modalities. We have examined these three aims within this PLCO substudy.
PATIENTS AND METHODS
Overview of PLCO
From 1993 to 2001, the PLCO4,29,30 enrolled 76,705 men, aged 55 to 74 years, at 10 screening sites nationwide. PLCO exclusion criteria for men were as follows: a history of prostate, lung, or colorectal cancer; current cancer treatment; previous surgical removal of the prostate, lung, colon, or rectum; current finasteride use; colon cancer screening ≤ 3 years before enrollment; enrollment onto another cancer screening/prevention study; unwilling or unable to provide consent; and 8) one or more prostate-specific antigen (PSA) test ≤ 3 years before the study.
All participants completed a baseline questionnaire (BQ) regarding demographics and screening histories. Participants in the usual care (UC) arm were instructed to follow their normal health care routine. Participants in the screening arm received an annual digital rectal examination in years 0 to 3 and a PSA test during years 0 to 5. Participants received the screening results, recommendations for diagnostic examinations with their own physician, and completed an annual assessment of health status and new cancer diagnoses.
Health-Related Quality-of-Life Substudy
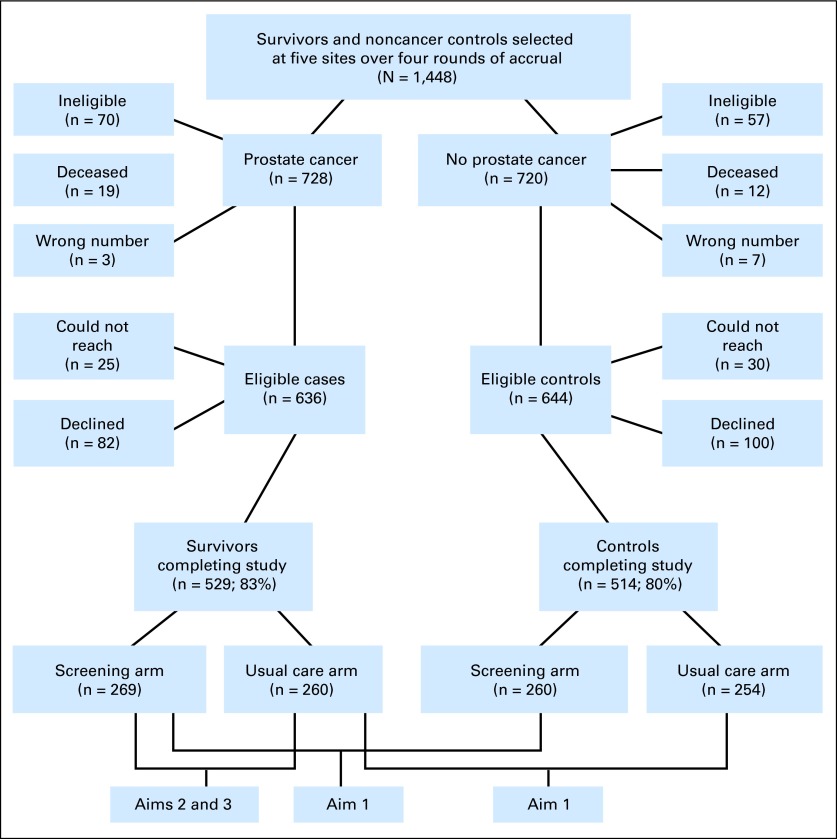
The study design, participation rates, and aims are described in Figure 1. The study aims were as follows: within each trial arm (screening/UC), we compared survivors to noncancer controls on disease-specific functioning (aim 1); among survivors, we assessed the impact of trial arm on disease-specific functioning (aim 2); and among survivors, we assessed the impact of treatment modality on disease-specific functioning (aim 3).
Fig 1.
CONSORT diagram showing study design and participation rates.
Patients.
Inclusion criteria were as follows: completion of the BQ, English speaking, attendance at participating PLCO screening centers (SCs; Table 1), white or African American (other races were excluded as a result of small samples at SCs), and active PLCO participants. Exclusion criteria included having dementia/cognitive difficulties or hearing impairment.
Table 1.
Demographic and Trial-Related Variables Among Screening-Arm Participants Stratified by Cancer Status (unadjusted weighted results)
| Variable | Screening Arm |
P | |
|---|---|---|---|
| PCa Survivors(%; n = 269) | Noncancer Controls(%; n = 260) | ||
| Age at interview, years | .129* | ||
| Mean | 73.95 | 74.71 | |
| SD | 5.59 | 6.20 | |
| Years since trial enrollment | < .001* | ||
| Mean | 11.11 | 11.62 | |
| SD | 1.60 | 1.62 | |
| Race | .231† | ||
| African American | 6.0 | 3.8 | |
| White | 94.0 | 96.2 | |
| Marital status | .150† | ||
| Not married | 15.6 | 20.4‡ | |
| Married | 84.4 | 79.6 | |
| Education level | .702† | ||
| HS or less | 25.7 | 28.6‡ | |
| Some college/trade | 29.1 | 29.1 | |
| BA/BS or higher | 45.2 | 42.2 | |
| Income level | .515† | ||
| < $50,000 | 49.2‡ | 46.3‡ | |
| ≥ $50,000 | 50.76 | 53.7 | |
| Employment status | .149† | ||
| Retired/not employed | 75.6 | 80.7‡ | |
| FT/PT | 24.4 | 19.3 | |
| Comorbidities | .019† | ||
| ≤ 1 | 50.4 | 40.4 | |
| ≥ 2 | 49.7 | 59.6 | |
| Accrual round | .359† | ||
| 1: July to December 2007 | 25.7 | 22.3 | |
| 2: January to June 2008 | 26.2 | 27.4 | |
| 3: July to December 2008 | 19.9 | 25.5 | |
| 4: January to June 2009 | 28.2 | 24.8 | |
| Number of PSAs during 3 years before PLCO enrollment | .466† | ||
| 0 | 43.3 | 44.1 | |
| 1 | 37.8 | 41.2 | |
| 2 | 9.0 | 8.5 | |
| 3+ | 9.8 | 6.3 | |
| Screening center | .286† | ||
| Georgetown University | 11.2 | 8.4 | |
| Henry Ford Health System | 15.3 | 20.4 | |
| Marshfield Clinic | 16.0 | 19.7 | |
| University of Minnesota | 41.3 | 37.3 | |
| Washington University | 16.3 | 14.3 | |
| Urination frequency at night before PLCO enrollment, ≥ 1 time | 28.1 | 33.4 | .176† |
Abbreviations: FT/PT, full time/part time; HS, high school; PCa, prostate cancer; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; PSA, prostate-specific antigen; SD, standard deviation.
t test.
χ2 test.
Columns do not add to 100% because of < 1% missing data.
Additional eligibility criteria included a diagnosis ≥ 5 to less than 10 years before the Health-Related Quality-of-Life (HRQL) interview (diagnosis dates: July 1998 to July 2003) and no other cancer diagnosis, except nonmelanoma skin cancer. We included all disease stages and both screen-detected and interval cancers.
Procedures.
We randomly selected survivors and noncancer controls in four rounds (in May 2007, December 2007, May 2008, and December 2008) to capitalize on the regularly updated participant information of the PLCO. Noncancer controls were frequency matched to survivors for year of randomization (1993 to 2001), trial arm, race, and screening center.
At each round, 180 PCa survivors (90 per arm) and 180 noncancer controls (90 per arm) were selected (Fig 1). All eligible African American survivors were selected.
Each SC mailed personalized invitation letters with a toll-free number to decline or learn more about the study. Each SC made up to 10 calls to nonresponders. Telephone interviews required 15 to 20 minutes for which participants received a $10 gift card. Institutional review board approval was covered under the overall PLCO consent form.
Quality assurance.
Experienced telephone interviewers (N = 8) received training to conduct interviews. During each accrual round, two investigators listened to live interviews and provided feedback to each interviewer. By using a secure tracking database, the project coordinator maintained regular contact with SCs to assess accrual and adherence to procedures.
Measures
Demographic and medical information.
We assessed education, marital status, employment status, income, health insurance, and having a regular physician. Participants indicated whether they had been diagnosed with any of 16 conditions (Appendix Table A1, online only). Conditions were summed and then categorized into one or fewer versus two or more conditions. Approximately 50% of participants had zero to one comorbidy and 50% of participants had two or more comorbidies.
Prostate cancer–specific HRQL.
The Expanded Prostate Cancer Index Composite Short Form31 evaluates function in the past 4 weeks of urinary, bowel, sexual, and hormonal functioning. We included the sexual (six items; α reliability = .89) and urinary (nine items; α = .83) function scores and bowel (six items; α = .78) and hormonal (five items; α = .61) bother scores. Higher scores indicated better functioning and less bother (range, 0 to 100).
Treatment for urinary, bowel, or sexual dysfunction.
We assessed medical, surgical, and other treatments received for urinary, bowel, and sexual functioning. Because of the low rate of treatments, we collapsed the treatment for each adverse effect into a yes or no response.
Cancer treatments and recurrence.
Because the PLCO collected treatment data from medical records only during the first year postdiagnosis, we obtained self-reports of survivors of dates and types of all PCa treatments (radical prostatectomy [RP], external-beam radiation, brachytherapy, hormones, watchful waiting, and cryotherapy) and PCa recurrence (yes or no). Treatments were categorized into the following three groups: surgery only (RP; n = 201), radiation only (radiation therapy [RT]; n = 110 [brachytherapy, n = 65 and external beam therapy, n = 45]), and treatment combinations (TCs;n = 207), which included men who had received hormone therapy (HT) only, as well as any combination of RP/RT/HT. Overall, 135 patients had ever had HT, and 32 patients were receiving HT at the HRQL interview. Men who had received only watchful waiting (n = 9) or only cryotherapy (n = 2) were excluded from treatment-modality analyses. For treatments received within 12 months postdiagnosis, agreement between self-report and medical records was 89.6%; an additional 8.3% indicated partial agreement, which suggested high reliability of the subsequent self-reported treatments.
Information Obtained From PLCO Records
BQ.
We obtained date of birth, race, frequency of nighttime urination (0, none; 1, once per night, and 2, two or more times per night), and the number of PCa screens during the previous 3 years (zero, one, two, or threescreens).
Medical record review.
PLCO staff abstracted treatment records to obtain the disease stage, PSA level, and Gleason score at diagnosis and PCa treatments received during the first year postdiagnosis.
Statistical Analysis
Descriptive analyses.
We compared participants to decliners on demographic, medical, and trial-related variables by using χ2 tests, t tests, and analysis of variance(two-sided tests).
Weights.
Weights to account for the selection of the study sample from the five SCs and to adjust for nonresponse were based on the inverse probability weighting method.32 The weight vector was included in all evaluations of covariate balance and all outcome models (Appendix, online only).
Propensity scores.
To adjust for potential demographic and medical premorbid differences between survivors and noncancer controls, we calculated propensity scores (PSs) to obtain the probability of having received a PCa diagnosis.33,34 We included the PS as a covariate in all analyses in which survivors and noncancer controls were compared.
Similarly, among survivors, we calculated the PS for treatment modality as follows: RP only, RT only, and TCs (including hormone treatment but excluding watchful waiting and cryotherapy). PSs were included as covariates in all analyses that compared treatment modalities (Appendix, online only).
Outcome models.
To determine the impact of the PCa diagnosis on disease-related function among screened participants (aim 1), we conducted weighted linear regression analyses, including the PS and all significant covariates from bivariate analyses. For each analysis, our conclusions were not altered when we adjusted only for the PS and also when we did not include weights.
To determine the impact of trial arm on disease-related functioning among survivors (aim 2), we conducted weighted linear regression analyses, including all significant covariates from bivariate analyses. We assessed whether treatment modality had a differential impact on disease-related functioning among survivors (aim 3) with a series of weighted linear regression models, including the treatment-modality PS, trial arm, and all significant covariates. We used SAS software (version 9.2; SAS Institute, Cary, NC) to conduct all analyses.
Power calculations.
For the comparison of survivors versus noncancer controls among screened participants (269 v 260 patients, respectively) and screening versus UC groups among survivors (269 v 260 patients, respectively), we had 80% power at α = .05 to detect a mean difference ≥ 0.24 SDs. For pairwise comparisons of the three treatment modalities (202 v 110 v 207), we had 80% power to detect mean differences ≥ 0.33 SDs.
RESULTS
Participation Rates
Of the 1,448 patients selected, 31 patients were deceased, and 10 patients had outdated contact information (Fig 1).35 Of the remaining 1,407 patients, 127 patients were ineligible. Of the 1,280 eligible participants, 1,043 patients (81.5%) participated (529 survivors and 514 noncancer controls).
Of the 237 nonparticipants (18.5%), 55 patients were unreachable, and 182 patients declined (eg, because of a lack interest or being too busy). Compared with participants, nonparticipants were less likely to be married, employed, white, or highly educated and more likely to be from the Henry Ford site (P < .05). There were no significant differences in age, cancer status, trial arm, comorbidities, years since trial enrollment, or accrual round (P > .10). Among survivors, participation was unrelated to treatment modality, cancer stage, or time since diagnosis (all P > .10).
Impact of PCa Disease and Treatment on Disease-Specific Functioning (aim 1)
There were few demographic differences between survivors versus noncancer controls in the screening arm (Table 1). The mean age was approximately 74 years, and men were predominantly white, educated, and insured. To assess the impact of PCa versus the impact of aging and comorbidities on disease-specific functioning, we conducted weighted linear regression analyses among screening-arm participants (Table 2). Survivors had significantly worse sexual and urinary function (P < .001) than noncancer controls. Neither the hormonal (P = .78) nor bowel (P = .82) subscales differed significantly in cancer status. Similar relationships were found in the UC arm, which suggested that differences between regular screening and intermittent screening did not contribute to survivors being worse off than noncancer controls (data not shown).
Table 2.
Weighted Linear Regression Analyses for Disease-Specific Function in Screening Arm: PCa Survivors v Noncancer Controls
| EPIC Outcome | Noncancer Controls(0; n = 260) |
Survivors (1; n = 269) |
LS Mean Difference(PCa survivors v controls) |
P | |||
|---|---|---|---|---|---|---|---|
| LS Mean | SE | LS Mean | SE | Estimate | 95% CI | ||
| Sexual function | 55.09 | 3.83 | 32.18 | 3.65 | −22.91 | −28.69 to −17.14 | < .001 |
| Urinary function | 90.43 | 1.62 | 76.13 | 1.63 | −14.31 | −17.93 to −10.68 | < .001 |
| Bowel bother | 87.89 | 1.44 | 88.16 | 1.45 | 0.27 | −2.10 to 2.64 | .82 |
| Hormone bother | 90.52 | 1.04 | 90.83 | 1.01 | 0.31 | −1.86 to 2.48 | .78 |
NOTE. All analyses included the cancer-status propensity score and all significant covariates.
Abbreviations: EPIC, Expanded Prostate Cancer Index Composite Short Form; LS, least squares; PCa, prostate cancer.
Impact of Regular Screening Versus UC on Disease-Specific Functioning Among Survivors (aim 2)
Analyses were conducted that compared the screening arm versus the UC arm among survivors. UC-arm participants were older, less likely to have had a RP, and more likely to have had a higher Gleason score and PSA value (Table 3).
Table 3.
Demographic, Trial-, and Cancer-Related Variables Among PCa Survivors Stratified by Trial Arm (unadjusted weighted results)
| Variable | Usual Care(%; n = 260) | Screening(%; n = 269) | P |
|---|---|---|---|
| Age at interview, years | .003* | ||
| Mean | 75.36 | 73.95 | |
| SD | 5.31 | 5.59 | |
| Years since trial enrollment | .100* | ||
| Mean | 11.34 | 11.11 | |
| SD | 1.62 | 1.60 | |
| Years since diagnosis | .063* | ||
| Mean | 7.30 | 7.52 | |
| SD | 1.30 | 1.35 | |
| Race | .638† | ||
| African American | 7.1 | 6.0 | |
| White | 92.9 | 94.0 | |
| Marital status | .170† | ||
| Not married | 20.2 | 15.6‡ | |
| Married | 79.8 | 84.4 | |
| Education level | .100† | ||
| HS or less | 30.7 | 25.7‡ | |
| Some college/trade | 33.3 | 29.1 | |
| BA/BS or higher | 36.0 | 45.2 | |
| Income level | .484† | ||
| < $50,000 | 49.5‡ | 46.3‡ | |
| ≥ $50,000 | 50.5 | 53.7 | |
| Employment status | .488† | ||
| Retired/not employed | 78.1‡ | 75.6‡ | |
| FT/PT | 21.9 | 24.4 | |
| Comorbidities | .722† | ||
| ≤ 1 | 48.8 | 50.4 | |
| ≥ 2 | 51.2 | 49.7 | |
| Accrual round | .281† | ||
| 1: July to December 2007 | 20.8 | 25.7 | |
| 2: January to June 2008 | 24.0 | 26.2 | |
| 3: July to December 2008 | 26.1 | 19.9 | |
| 4: January to June 2009 | 29.2 | 28.2 | |
| Number of PSAs during 3 years before PLCO enrollment | .187† | ||
| 0 | 42.4 | 44.1 | |
| 1 | 35.7 | 41.2 | |
| 2 | 12.8 | 8.5 | |
| 3+ | 9.1 | 6.3 | |
| Screening center | .581† | ||
| Georgetown University | 10.7 | 11.2 | |
| Henry Ford Health System | 14.6 | 15.3 | |
| Marshfield Clinic | 19.4 | 16.0 | |
| University of Minnesota | 43.4 | 41.3 | |
| Washington University | 11.9 | 16.3 | |
| Urination frequency at night before PLCO enrollment, ≥ 1 time | 29.5 | 28.1 | .722† |
| Years since diagnosis | .409† | ||
| 5-5.99 | 22.9 | 17.4 | |
| 6-6.99 | 22.7 | 20.2 | |
| 7-7.99 | 19.2 | 22.4 | |
| 8-8.99 | 20.6 | 24.3 | |
| 9-9.99 | 14.6 | 15.7 | |
| Stage at diagnosis | .965† | ||
| II | 87.5 | 87.6 | |
| III and IV | 12.5 | 12.4 | |
| Treatment modality | .011† | ||
| Surgery only | 33.0‡ | 45.9‡ | |
| Radiation only | 22.4 | 18.8 | |
| Treatment combination, including ADT | 44.6 | 35.2 | |
| PCa recurrence, yes | 12.1 | 10.5 | .556† |
| Gleason score‡ | < .001† | ||
| 3-6 | 61.2 | 77.2 | |
| 7+ | 38.8 | 22.8 | |
| PSA level at diagnosis | < .001† | ||
| ≤ 4.0 | 8.9 | 22.7 | |
| 4.01-10.0 | 67.8 | 64.8 | |
| ≥ 10.01 | 23.3 | 12.5 |
Abbreviations: ADT, androgen-deprivation therapy; FT/PT, full time/part time; HS, high school; PCa, prostate cancer; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; PSA, prostate-specific antigen; SD, standard deviation.
t test.
χ2 test.
Columns do not add to 100% because of < 1% missing data.
Weighted linear regression analyses, including age, comorbidities, and other significant covariates, revealed no significant relationships between trial arm and disease-specific functioning (all P values > .31; Appendix Table A2, online only), which suggested that screening practices did not play a role in these outcomes.
Impact of Treatment Modality on Long-Term Disease-Specific Functioning (aim 3)
Compared with RT and TC groups, the RP group was significantly younger and more likely to be married, employed, and in the screening arm (Table 4). RT participants were more likely to have had stage II disease, whereas TC participants had higher Gleason and PSA values and were more likely to have had a recurrence. These variables were included in the PS model (Appendix, online only).
Table 4.
Demographic, Trial Arm–, and Cancer-Related Variables by Treatment Modality in PCa Survivors (unadjusted weighted results)
| Variable | RP, Surgery Only (%; n = 201) | RT, Radiation Only (%; n = 110) | TC (%; n = 207)* | P |
|---|---|---|---|---|
| Age at interview, years | < .001† | |||
| Mean | 72.72 | 75.91 | 75.56 | |
| SD | 4.80 | 5.37 | 5.71 | |
| Years since trial enrollment | .171† | |||
| Mean | 11.06 | 11.42 | 11.21 | |
| SD | 1.64 | 1.48 | 1.64 | |
| Years since diagnosis | .187† | |||
| Mean | 7.47 | 7.21 | 7.48 | |
| SD | 1.33 | 1.25 | 1.36 | |
| Race | ||||
| African American | 6.3 | 6.6 | 6.8 | .982‡ |
| White | 93.7 | 93.4 | 93.2 | |
| Marital status | .013‡ | |||
| Not married | 12.5 | 17.9 | 23.6 | |
| Married | 87.5 | 82.1 | 76.4 | |
| Education level | .440‡ | |||
| HS or less | 25.5 | 25.4 | 30.8 | |
| Some college/trade | 29.7 | 31.8 | 33.3 | |
| BA/BS or higher | 44.8 | 42.8 | 36.0 | |
| Income level | .226‡ | |||
| < $50,000 | 43.4 | 50.3 | 51.7 | |
| ≥ $50,000 | 56.6 | 49.7 | 48.3 | |
| Employment status | .029‡ | |||
| Retired/not employed | 70.2 | 80.1 | 80.5 | |
| FT/PT | 29.8 | 19.9 | 19.5 | |
| Comorbidities | .407‡ | |||
| ≤ 1 | 53.2 | 49.2 | 46.6 | |
| ≥ 2 | 46.8 | 50.8 | 53.4 | |
| Accrual round | .172‡ | |||
| 1: July to December 2007 | 24.7 | 19.0 | 24.1 | |
| 2: January to June 2008 | 26.5 | 25.5 | 23.6 | |
| 3: July to December 2008 | 17.1 | 23.7 | 27.9 | |
| 4: January to June 2009 | 31.7 | 31.9 | 24.4 | |
| Site | .003‡ | |||
| Georgetown | 9.3 | 19.7 | 8.7 | |
| Henry Ford | 16.3 | 16.2 | 13.8 | |
| Marshfield | 16.0 | 22.6 | 15.6 | |
| Minnesota | 45.8 | 23.9 | 14.1 | |
| Washington University | 12.7 | 17.5 | 13.3 | |
| PCa recurrence, yes | 2.3 | 1.5 | 25.6 | < .001‡ |
| Urination frequency at night before PLCO enrollment, ≥ 1 time | 26.1 | 28.2 | 31.5 | .477‡ |
| Years since diagnosis | .491‡ | |||
| 5-5.99 | 17.6 | 21.5 | 21.7 | |
| 6-6.99 | 20.7 | 27.0 | 18.3 | |
| 7-7.99 | 23.2 | 21.7 | 18.3 | |
| 8-8.99 | 23.1 | 18.4 | 24.6 | |
| 9-9.99 | 15.3 | 11.4 | 17.1 | |
| Stage at diagnosis | .001‡ | |||
| II | 86.2 | 98.5 | 82.7 | |
| III and IV | 13.8 | 1.6 | 17.3 | |
| Trial arm | < .001‡ | |||
| UC | 38.0 | 50.4 | 51.9 | |
| Screen | 62.0 | 49.6 | 48.1 | |
| Gleason score | < .001‡ | |||
| 3-6 | 76.7 | 85.6 | 53.7 | |
| 7+ | 23.3 | 14.4 | 46.3 | |
| PSA level at diagnosis | < .001‡ | |||
| ≤ 4.0 | 24.2 | 16.5 | 7.8 | |
| 4.01-10.0 | 67.0 | 76.1 | 61.0 | |
| ≥ 10.01 | 8.8 | 7.4 | 31.2 |
Abbreviations: FT/PT, full time/part time; HS, high school; PCa, prostate cancer; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiation therapy; SD, standard deviation; TC, treatment combination; UC, usual care.
TC group included all participants who received androgen-deprivation therapy.
F test.
χ2 test.
We conducted weighted linear regression analyses for the four disease-specific outcomes, which included covariates associated with each outcome. Time since diagnosis and trial arm were not significantly associated with outcomes. The treatment modality was associated with sexual (P < .05) and urinary (P < .001) function (Table 5), and RT participants reported better outcomes than RP participants. RP participants reported better outcomes than RT participants for bowel bother (P < .05). TC patients reported significantly more hormone bother than RP patients (P = .05). When PCa survivors from the screening arm only were included, the results were identical, except that there was no significant difference between RP and RT participants on the bowel subscale (data not shown).
Table 5.
Weighted Linear Regression Analyses for Disease-Specific Function Stratified by Treatment Modality for Prostate Cancer Survivors
| EPIC | RP |
RT |
TC |
LS Mean Difference Between Pairs of Treatment Modalities |
P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LS Mean | SE | LS Mean | SE | LS Mean | SE | Treatment-Modality Pair | Estimate | 95% CI | ||
| Sexual function | 16.90 | 5.43 | 27.96 | 5.83 | 19.11 | 5.46 | RP and RT | −11.06 | −20.69 to −1.43 | |
| RP and TC | −2.21 | −10.75 to 6.33 | ||||||||
| RT and TC | 8.85 | −1.08 to 18.78 | .02 | |||||||
| Urinary function | 71.48 | 2.08 | 84.04 | 2.66 | 77.13 | 2.05 | RP and RT | −12.55 | −20.00 to −5.10 | |
| RP and TC | −5.65 | −12.25 to 0.96 | ||||||||
| RT and TC | 6.91 | −0.75 to 14.56 | .01 | |||||||
| Bowel bother | 88.19 | 1.71 | 84.06 | 1.96 | 85.37 | 1.77 | RP and RT | 4.14 | 0.01 to 8.26 | |
| RP and TC | 2.82 | −0.85 to 6.49 | ||||||||
| RT and TC | −1.32 | −5.57 to 2.94 | .04 | |||||||
| Hormone bother | 94.89 | 2.84 | 93.11 | 2.97 | 91.38 | 32.81 | RP and RT | 1.78 | −2.06 to 5.62 | |
| RP and TC | 3.52 | 0.10 to 6.93 | ||||||||
| RT and TC | 1.74 | −2.22 to 5.70 | .05 | |||||||
NOTE. All analyses included treatment-modality propensity scores and all significant covariates.
Abbreviations: EPIC, Expanded Prostate Cancer Index Composite Short Form; LS, least squares; RP, radical prostatectomy; RT, radiation therapy; TC, treatment combination.
DISCUSSION
We have demonstrated the persistence of clinically significant, long-term PCa treatment-related sexual and urinary adverse effects between 5 and 10 years postdiagnosis, which showed the enduring nature of commonly experienced adverse effects and the need for providers to manage them on a long-term basis.36
To our knowledge, this is the first study to compare a sample of screened PCa survivors to a sample of screened noncancer controls. Survivors reported greater sexual and urinary dysfunction compared with noncancer controls, which suggested that these long-term problems could not be attributed solely to aging or comorbidities but were due to PCa and its treatment (aim 1). These results corroborate those of previous studies that have included noncancer controls and revealed greater symptomatology among PCa survivors.17,18,37 However, this study also controlled for the potential impact of screening/early detection, which was important because the majority of PCas are now screen detected. Screening/early detection did not impact the symptomatology differences between PCa survivors versus noncancer controls.
Among survivors, the route to diagnosis (regular screening v UC) was not associated with disease-specific functioning. This result suggested that survivors with screen-detected PCa reported a similar rate of long-term adverse effects as survivors with PCa detected through UC (aim 2). A previous study that included a nonrandomly assigned sample of survivors with clinically detected disease38,39 also did not find that regular screening impacted disease-specific functioning. In addition, Carlsson et al19 assessed the impact of screening versus UC within a randomized trial. By limiting the analysis to RP patients who had been potent and continent presurgery, Carlsson et al19 concluded that regular screening resulted in a minimal increase in impotence and incontinence.19 However, screening may still impact disease-specific functioning because we were unable to take into account the overdiagnosis that occurred in the screened arm,4 which was likely to have resulted in more sexual and urinary adverse effects compared with noncancer controls. Additional research is needed to fully evaluate the adverse effects that result from regular versus intermittent screening.
The greater long-term urinary and sexual dysfunctions among survivors treated by surgery compared with radiation are particularly important data when making treatment decisions. The differences in adverse effects that exist between treatment modalities up to 5 years postdiagnosis11,17,22,25,38 persisted at 5 to 10 years postdiagnosis. However, both surgery and radiation resulted in poor sexual functioning, which suggested that providers need to be prepared to manage these chronic, long-term adverse effects regardless of the treatment modality (aim 3). More than 95% of men in each treatment group reported having at least some sexual dysfunction, whereas approximately 50% of men in each treatment group reported having at least some urinary and bowel dysfunction (data not shown). We found no significant impact of time since diagnosis (5 to 10 years) on any outcomes or within any treatment group, which suggested that HRQL functioning remained relatively stable over this period.
Several study limitations should be noted. First, we did not have a pretreatment HRQL assessment. Although we used state-of-the-art statistical methods that achieved a balance between groups, groups could have differed on unmeasured characteristics. Second, an association between the time since diagnosis and outcomes may have been obscured by the cross-sectional design. Third, the opportunistic screening that occurred in the UC group4 may have limited our ability to detect differences between trial arms. On the basis of sampling surveys, 40% of UC-arm participants were screened in the first year, and 52% of UC-arm participants were screened in the sixth year.4 As a result, the majority of PCa survivors in both the UC and screening arms were diagnosed with early-stage disease (Table 3), which may have resulted in the absence of differences between trial arms. Fourth, we did not have a large enough sample to assess the impact of brachytherapy versus external-beam RT, which some studies have reported.40–42 Fifth, we did not use a validated index for the assessment of comorbidities, which limited the ability to compare the rate of comorbidities in this sample to previous samples (Appendix Table A1, online only). Finally, generalizability may be limited because randomly assigned trial participants differ from the general population on socioeconomic characteristics and health status.43 However, generalizability was increased because treatments occurred in a wide range of hospitals and not exclusively in tertiary-care cancer centers. Our results corroborated previous results, and the sample characteristics were similar to previous PCa studies,17,44 which suggested that our findings apply to non-PLCO participants.
In conclusion, this study made several important contributions. First, few studies of disease-specific functioning have included a comparable noncancer control group, and none of the studies have included regularly screened controls. Within each arm, survivors reported significantly poorer long-term sexual and urinary function compared with noncancer controls, which suggested that these persistent symptoms were due to PCa treatment rather than comorbidities or aging. Second, to our knowledge, this is the only evaluation of the long-term sequelae of PCa screening in the PLCO. Because of the few differences between study participants versus nonparticipants, these results are generalizable to the five other PLCO screening sites. Third, to our knowledge, this was the first observational study of PCa-related functioning to use PS methods to adjust for potential baseline differences between survivors and noncancer controls and only the third study to use PS to adjust for differences between treatment modalities.25,45,46 Adjustment, such as afforded by PS, is crucial to address bias associated with a cancer diagnosis and treatment selection in observational studies.34,47 Finally, this study provided essential information for patients and clinicians who make screening and treatment decisions by documenting the differences between long-term urinary and sexual dysfunction after RP and RT.
Acknowledgment
We thank the study participants, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial principal investigators Christine Berg, MD, and Phillip Prorok, PhD, and staff members at each of the following five participating screening centers—Georgetown University: Edward Gelmann, MD (currently at Columbia University), Claudine Isaacs, MD, Colleen McGuire, RN, MSN, and Tiffanie Hammond, BS; Henry Ford Health Systems: Paul Kvale, MD, Karen Broski, BS, and Tara Andrews; Marshfield Clinic: Karen Lappe, BSN, and Deb Multerer; Washington University: Gerald Andriole, MD, Robert Grubb, MD, Heidi Lowery, RN, MS, and Elaine Freesmeier; and University of Minnesota: Deb Engelhard, MA, Gavin Watt, Jill Cordes, RN, and Lea Matous. At Georgetown University, telephone interviewers included Elisabeth Kassan, BA, and Caroline Dorfman, BA. At the University of Minnesota, telephone interviewers included Sue Peterson, Janet Manuel, Mary Lynn Steele, and Phyllis Olsson.
Appendix
Weights
Stratum weights were based on trial data collected through October 2009. The few eligibility discrepancies that occurred between the health-related quality of life (HRQL) interview data and the medical record data of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial were resolved by using the more recently collected HRQL data.
Sampling fractions were calculated separately for survivors and controls as total completed/total eligible. These calculations were done overall and separately within each stratum. For survivors, strata were the combination of race, arm, and year of diagnosis, which were the same cells from which subjects were randomly selected for interview. For controls, strata were the combination of race, arm, screening center, and year of randomization, which were the same variables used for frequency matching to survivors. The year of randomization and screening center were collapsed further so that there were no cells without any subjects. The denominator of the sampling weight for a particular stratum was the count of men in that stratum who were ever eligible during the HRQL accrual period (June 2007 to June 2009). The numerator was the count of eligible subjects in that stratum who completed the interview.
Weights for each participant with a completed HRQL interview were calculated separately for each stratum. Each weight was calculated as
The sum of weights for all participants equaled the total number of participants.
Propensity Score Models
Propensity scores for cancer/noncancer.
Variables included in the propensity score (PS) logistic regression model were age, race, education, marital status, employment status, comorbidities, years since Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) enrollment, screening center, trial arm, accrual round, number of prostate-specific antigen (PSA) screens before PLCO enrollment, and nighttime urination frequency (baseline questionnaire of the PLCO). After adjustment for PS, a covariate balance was achieved for each covariate, which was defined as nonsignificant differences on χ2 and t tests (all P > .96) when survivors and controls were compared.
PSs for treatment modality.
With the use of a multinomial logistic regression model, we calculated the three PSs (one PS for each treatment modality) by including age at the time of the interview, race, education level, marital status, employment status, comorbidities, years since diagnosis, screening center, trial arm, accrual round, number of PSA screens prior to enrollment, nighttime urination frequency (baseline questionnaire of the PLCO), PSA at diagnosis, Gleason score, disease stage, and prostate cancer recurrence. After adjustment for two of the PSs (because they all added up to 1 for each observation), a covariate balance was achieved for each covariate, which was defined as nonsignificant differences on χ2 and analysis of variance tests (all P > .41) when the three treatment modalities were compared. We did not use PS methods for comparison of screening versus UC groups among prostate cancer survivors because the two groups of interest were balanced with respect to characteristics measured before PLCO randomization.
Table A1.
Medical Comorbidities for Noncancer Controls and Prostate Cancer Survivors
| Comorbidity | Noncancer Controls(n = 514) | Prostate Cancer Survivors(n = 529) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Diabetes | ||||
| No | 411 | 80.0 | 442 | 83.7 |
| Yes | 103 | 20.0 | 86 | 16.3 |
| Missing | 0 | 1 | ||
| Ulcer disease | ||||
| No | 474 | 92.2 | 506 | 95.7 |
| Yes | 40 | 7.8 | 23 | 4.3 |
| Chronic pulmonary disease | ||||
| No | 468 | 91.0 | 495 | 93.6 |
| Yes | 46 | 9.0 | 34 | 6.4 |
| Myocardial infarction | ||||
| No | 443 | 86.2 | 466 | 88.1 |
| Yes | 71 | 13.8 | 63 | 11.9 |
| Congestive heart failure | ||||
| No | 483 | 94.0 | 501 | 94.7 |
| Yes | 31 | 6.0 | 28 | 5.3 |
| Peripheral vascular disease | ||||
| No | 468 | 91.0 | 485 | 91.7 |
| Yes | 46 | 9.0 | 44 | 8.3 |
| Cerebrovascular disease | ||||
| No | 476 | 92.6 | 487 | 92.1 |
| Yes | 38 | 7.4 | 42 | 7.9 |
| Hypertension | ||||
| No | 246 | 47.9 | 262 | 49.5 |
| Yes | 268 | 52.1 | 267 | 50.5 |
| Liver disease | ||||
| No | 506 | 98.4 | 519 | 98.1 |
| Yes | 8 | 1.6 | 10 | 1.9 |
| Depression or anxiety | ||||
| No | 476 | 92.6 | 495 | 93.6 |
| Yes | 38 | 7.4 | 34 | 6.4 |
| Dementia | ||||
| No | 507 | 98.6 | 523 | 98.9 |
| Yes | 7 | 1.4 | 6 | 1.1 |
| Connective tissue disease (includes arthritis) | ||||
| No | 278 | 54.1 | 307 | 58.0 |
| Yes | 236 | 45.9 | 222 | 42.0 |
| Hemiplegia | ||||
| No | 510 | 99.2 | 528 | 99.8 |
| Yes | 4 | 0.8 | 1 | 0.2 |
| Kidney disease | ||||
| No | 499 | 97.1 | 523 | 98.9 |
| Yes | 15 | 2.9 | 6 | 1.1 |
| HIV or AIDS | ||||
| No | 514 | 100.0 | 528 | 99.8 |
| Yes | 0 | 0.0 | 1 | 0.2 |
| Other illness* | ||||
| No | 445 | 86.6 | 475 | 89.8 |
| Yes | 69 | 13.4 | 54 | 10.2 |
NOTE. Comorbidities assessed had substantial overlap with the Charlson Index (we included 14 of the 19 comorbidities included by Charlson ME, et al: J Chronic Dis 40:373-383, 1987). However, we removed the four cancer-related comorbidities (because men with non–prostate cancer diseases were ineligible for the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial substudy, which made these items irrelevant), and we collapsed mild liver disease with moderate/severe liver disease. We included two additional comorbidities that we expected would be related to our disease-related quality of life outcomes (hypertension and depression/anxiety), and we included an Other illness category to capture additional illnesses that were not included by Charlson et al because of our interest in quality-of-life outcomes rather than mortality outcomes. Participants indicated if they had been diagnosed by a physician with any of the 16 conditions listed in the table. Because of the modifications noted, we did not use a weighted comorbidity index, but instead, conditions were summed to form a total score and then categorized into < 1 v > 2. Approximately 50% of patients had 0-1 comorbidities and 50% had 2+ comorbidities When categorized into four levels (0, 1, 2, and 3+), the associations with cancer status, trial arm, and treatment modality were the same as with the two-level variable. We elected to use the two-level variable for the sake of simplicity and ease of interpretation.
For example, Parkinson's disease, herniated disc, or macular degeneration.
Table A2.
Weighted Linear Regression Analyses for Disease-Specific Function Stratified by Trial Arm Among PCa Survivors
| EPIC | UC (0) | Screen (1) | LS Mean Difference Between UC and Screen | P | |||
|---|---|---|---|---|---|---|---|
| LS Mean | SE | LS Mean | SE | Estimate | 95% CI | ||
| Sexual function | 26.67 | 4.04 | 23.43 | 3.91 | 3.24 | −2.99 to 9.47 | .31 |
| Urinary function | 77.43 | 1.86 | 77.67 | 1.94 | −0.24 | −4.69 to 4.20 | .92 |
| Bowel bother | 84.01 | 1.85 | 82.92 | 1.83 | 1.08 | −1.33 to 3.50 | .38 |
| Hormone bother | 92.63 | 2.58 | 92.58 | 2.60 | 0.06 | −2.05 to 2.16 | .96 |
Abbreviations: EPIC, Expanded Prostate Cancer Index Composite Short Form; LS, least squares; UC, usual care.
Footnotes
Supported by the Midwest Division of the American Cancer Society (Grant No. 113173-RSGPB-07-099-01-CPPB; K.L.T.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kathryn L. Taylor, Anthony B. Miller, Timothy R. Church, Larry R. Muenz, Kimberly M. Davis, Douglas Reding
Provision of study materials or patients: Timothy R. Church, Douglas Reding
Collection and assembly of data: Kathryn L. Taylor, Scott P. Kelly, David L. Dawson, Sara Edmond, Jerome E. Mabie, Thomas L. Riley
Data analysis and interpretation: Kathryn L. Taylor, George Luta, Anthony B. Miller, Timothy R. Church, Scott P. Kelly, Larry R. Muenz, Kimberly M. Davis
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Brawley OW, Ankerst DP, Thompson IM. Screening for prostate cancer. CA Cancer J Clin. 2009;59:264–273. doi: 10.3322/caac.20026. [DOI] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriole GL, Crawford ED, Grubb RL, III, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 7.Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): Design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81–87. doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;341:c4543. doi: 10.1136/bmj.c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilic D, O'Connor D, Green S, et al. Screening for prostate cancer: An updated Cochrane systematic review. BJU Int. 2011;107:882–891. doi: 10.1111/j.1464-410X.2010.10032.x. [DOI] [PubMed] [Google Scholar]
- 10.US Preventive Services Task Force. Screening for prostate cancer, topic page. http://www.uspreventive servicestaskforce.org/uspstf/uspsprca.htm.
- 11.Barry MJ, Gallagher PM, Skinner JS, et al. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J Clin Oncol. 2012;30:513–518. doi: 10.1200/JCO.2011.36.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin GA, Aaronson DS, Knight SJ, et al. Patient decision aids for prostate cancer treatment: A systematic review of the literature. CA Cancer J Clin. 2009;59:379–390. doi: 10.3322/caac.20039. [DOI] [PubMed] [Google Scholar]
- 13.Zafar SY, Alexander SC, Weinfurt KP, et al. Decision making and quality of life in the treatment of cancer: A review. Support Care Cancer. 2009;17:117–127. doi: 10.1007/s00520-008-0505-2. [DOI] [PubMed] [Google Scholar]
- 14.Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another? A review of patient decision making for localized prostate cancer. Cancer. 2006;106:1865–1874. doi: 10.1002/cncr.21822. [DOI] [PubMed] [Google Scholar]
- 15.Bacon CG, Giovannucci E, Testa M, et al. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol. 2001;166:1804–1810. [PubMed] [Google Scholar]
- 16.Hoffman RM, Gilliland FD, Penson DF, et al. Cross-sectional and longitudinal comparisons of health-related quality of life between patients with prostate carcinoma and matched controls. Cancer. 2004;101:2011–2019. doi: 10.1002/cncr.20608. [DOI] [PubMed] [Google Scholar]
- 17.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 18.Mols F, Korfage IJ, Vingerhoets AJ, et al. Bowel, urinary, and sexual problems among long-term prostate cancer survivors: A population-based study. Int J Radiat Oncol Biol Phys. 2009;73:30–38. doi: 10.1016/j.ijrobp.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson S, Aus G, Bergdahl S, et al. The excess burden of side-effects from treatment in men allocated to screening for prostate cancer. The Göteborg randomised population-based prostate cancer screening trial. Eur J Cancer. 2011;47:545–553. doi: 10.1016/j.ejca.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Essink-Bot ML, Korfage IJ, De Koning HJ. Including the quality-of-life effects in the evaluation of prostate cancer screening: Expert opinions revisited? BJU Int. 2003;92(suppl 2):101–105. doi: 10.1111/j.1464-410x.2003.04409.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman RM, Stone SN, Espey D, et al. Differences between men with screening-detected versus clinically diagnosed prostate cancers in the USA. BMC Cancer. 2005;5:27. doi: 10.1186/1471-2407-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korfage IJ, Essink-Bot ML, Borsboom GJ, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int J Cancer. 2005;116:291–296. doi: 10.1002/ijc.21043. [DOI] [PubMed] [Google Scholar]
- 23.Litwin MS, Sadetsky N, Pasta DJ, et al. Bowel function and bother after treatment for early stage prostate cancer: A longitudinal quality of life analysis from CaPSURE. J Urol. 2004;172:515–519. doi: 10.1097/01.ju.0000129236.56712.e7. [DOI] [PubMed] [Google Scholar]
- 24.Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: Results from the prostate cancer outcomes study. J Urol. 2005;173:1701–1705. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 25.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: The prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 26.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 27.Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term followup. J Urol. 2010;183:2206–2212. doi: 10.1016/j.juro.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mols F, van de Poll-Franse LV, Vingerhoets AJ, et al. Long-term quality of life among Dutch prostate cancer survivors: Results of a population-based study. Cancer. 2006;107:2186–2196. doi: 10.1002/cncr.22231. [DOI] [PubMed] [Google Scholar]
- 29.Andriole GL, Levin DL, Crawford ED, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: Findings from the initial screening round of a randomized trial. J Natl Cancer Inst. 2005;97:433–438. doi: 10.1093/jnci/dji065. [DOI] [PubMed] [Google Scholar]
- 30.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 31.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 32.Horvitz DG, Thompson DJ. A generalization of sampling without replacement from a finite universe. J Am Stat Assoc. 1952;47:663–685. [Google Scholar]
- 33.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29:661–677. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 34.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 36.Committee on Cancer Survivorship. Washington, DC: National Academies Press; 2005. Improving Care and Quality of Life, Institute of Medicine and National Research Council: From Cancer Patient to Cancer Survivor: Lost in Transition; pp. 187–191. [Google Scholar]
- 37.Bacon CG, Giovannucci E, Testa M, et al. The association of treatment-related symptoms with quality-of-life outcomes for localized prostate carcinoma patients. Cancer. 2002;94:862–871. doi: 10.1002/cncr.10248. [DOI] [PubMed] [Google Scholar]
- 38.Madalinska JB, Essink-Bot ML, de Koning HJ, et al. Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J Clin Oncol. 2001;19:1619–1628. doi: 10.1200/JCO.2001.19.6.1619. [DOI] [PubMed] [Google Scholar]
- 39.Madalinska JB, Essink-Bot ML, de Koning HJ, et al. Health-related quality of life in patients with screen-detected versus clinically diagnosed prostate cancer preceding primary treatment. Prostate. 2001;46:87–97. doi: 10.1002/1097-0045(20010201)46:2<87::aid-pros1012>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Lips I, Dehnad H, Kruger AB, et al. Health-related quality of life in patients with locally advanced prostate cancer after 76 Gy intensity-modulated radiotherapy vs. 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys. 2007;69:656–661. doi: 10.1016/j.ijrobp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Namiki S, Ishidoya S, Tochigi T, et al. Health-related quality of life after intensity modulated radiation therapy for localized prostate cancer: Comparison with conventional and conformal radiotherapy. Jpn J Clin Oncol. 2006;36:224–230. doi: 10.1093/jjco/hyl002. [DOI] [PubMed] [Google Scholar]
- 42.Namiki S, Ishidoya S, Ito A, et al. Five-year follow-up of health-related quality of life after intensity-modulated radiation therapy for prostate cancer. Jpn J Clin Oncol. 2009;39:732–738. doi: 10.1093/jjco/hyp086. [DOI] [PubMed] [Google Scholar]
- 43.Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165:874–881. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 44.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 45.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92:1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 46.Shen X, Keith SW, Mishra MV, et al. The impact of brachytherapy on prostate cancer-specific mortality for definitive radiation therapy of high-grade prostate cancer: A population-based analysis. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2011.09.055. 10.1016/j.ijrobp.2011.09.055 [epub ahead of print on January 21, 2012] [DOI] [PubMed] [Google Scholar]
- 47.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]