Abstract
Coccidioidomycosis is an insidious fungal disease, endemic to arid regions of the Americas, which is becoming more frequently recognised worldwide. While most infections resemble a mild respiratory illness, a subset of cases progress to severe pneumonia or systemic dissemination. Here, we describe a case of disseminated coccidioidomycosis in a 54-year-old immunocompetent African-American man with geographic and demographic risk factors for Coccidiodes acquisition who presented with 2 months of fevers, fatigue, weight loss and painful skin lesions. Blood count and serum chemistry studies initially demonstrated leukocytosis, anaemia, hyponatraemia and acute renal failure. Chest imaging revealed numerous small pulmonary nodules and skin biopsy, serological studies and blood cultures eventually confirmed disseminated infection with Coccidioides immitis. This case highlights important features regarding the risk factors, spectrum of clinical findings, evaluation and treatment of coccidioidomycosis relevant to providers in endemic areas and throughout the world.
Background
Coccidioidomycosis is a potentially severe fungal disease that most often presents as a mild respiratory syndrome but has the potential to develop into disseminated infection. Caused by the genus Coccidioides, the incidence of this illness is rising dramatically in endemic arid regions of the Americas and consequently also in non-endemic areas worldwide.1–4 The incidence of coccidioidomycosis in the south-western USA increased 804% between 1998 and 2011 (from a rate of 5.3 to 42.6/100 000) and Mexico has also reported a significant rise in new cases.1 4–8 Coccidioides immitis is responsible for most infections in California while Coccidioides posadasii causes the majority of disease in other endemic locations.1 8 More frequent international travel and trade in endemic regions has allowed Coccidiodes to emerge as a clinically significant global pathogen.1 9
The typical presentation of coccidioidomycosis is a self-limiting illness resembling a mild community-acquired pneumonia. Nonetheless, this disease notoriously masquerades as a variety of clinical syndromes and up to 40% of symptomatic patients develop severe disease requiring hospitalisation.5 8 10 Disseminated coccidioidomycosis occurs in 1% of infections due to haematogeneous spread from the lungs and may occur weeks to years after the initial exposure.1 5 11 Skin is the most common extra-pulmonary site of infection, followed by the skeletal and central nervous systems. Coccidioides fungaemia, while rare, is particularly severe and carries a high mortality rate.12 Disseminated disease is more common in patients with impaired immunity secondary to HIV infection, malignancy, organ transplantation or the use of immune modulating agents. Additional risk factors include male gender, African or Filipino ancestry, pregnancy, comorbid cardiopulmonary disease and type two diabetes mellitus.8 13–15
The case described here illustrates a classic presentation of disseminated coccidioidomycosis in a patient with multiple geographic and demographic risk factors. Notably, however, this individual had no evidence of immune compromise, yet he developed severe extrapulmonary disease and fungaemia. The dramatic rise in incidence, the protean symptoms of early disease and the potential for critical illness highlight the relevance of Coccidiodes as a major human pathogen. Furthermore, the potential for disease acquisition during travel and latent disease reactivation on return to non-endemic areas emphasises that clinicians practising in all regions should consider Coccidioides infection when evaluating patients with compatible symptoms and relevant exposure histories.
Case presentation
A 54-year-old African-American man with a medical history notable only for mild asthma presented with 2 months of intermittent fevers, weakness, 20-pound weight loss and painful skin lesions. He also described progressively worsening nausea and loss of appetite. The skin lesions were tender and had erupted over the course of several weeks on his arms, chest, face and scalp. The patient had not been seen by a general practitioner for many years and took no medications. Three weeks prior to presentation, the man had been evaluated at a walk-in clinic where he was prescribed a course of oral antibiotics and prednisone that he did not complete. The patient had a less than five pack-year history of smoking tobacco and drank two beers a week. He lived near the northern edge of California's San Joaquin Valley and had worked for several years driving trucks throughout the western USA, including to the border of Mexico. The patient had no known sick contacts, was heterosexual, monogamous, had never been tested for HIV and reported no illicit drug use.
The patient's temperature on admission was 39.8°C and his heart rate was 107 bpm with a normal blood pressure. Physical examination revealed a cachectic, ill-appearing African-American man with mild bilateral cervical lymphadenopathy and intermittent diffuse wheezes on pulmonary auscultation. The patient was lethargic but demonstrated no cognitive impairment or focal neurological abnormalities. Cardiac, abdominal and musculoskeletal examinations were essentially normal, however, the patient had mild digital clubbing. The dermatological exam was distinctly notable for multiple tender, darkly pigmented nodules and plaques on the scalp, cheek, chest and wrist, the largest of which (chest) was 4 cm×3 cm in size (figure 1). The lesions varied in appearance from verrucous to crusted, indurated and necrotic and some nodules were surrounded by papular satellite lesions.
Figure 1.
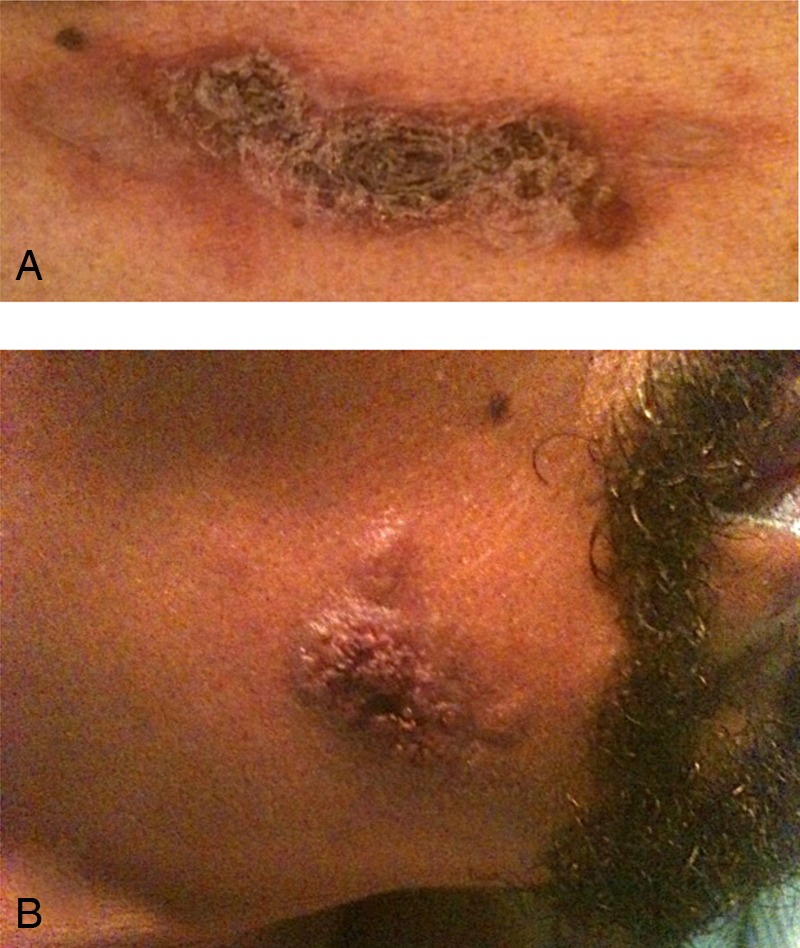
Dermatological findings in a patient with disseminated Coccidioidomycosis. Darkly pigmented, tender, crusting, necrotic, papulonodular skin lesions were present on the patient's upper chest (A), scalp, cheek (B) and wrist. The largest lesion was on the chest and measured 4 cm×3 cm.
Initial laboratory studies revealed a white blood cell count of 11.3 cells/μL with a neutrophilic predominance, haemoglobin of 11.1 mg/dL, platelet count of 29×103/μL, serum sodium of 124 mEq/L, blood urea nitrogen of 33 mmol/L, creatinine of 1.76 mg/dL, calcium 9.2 mg/dL and an erythrocyte sedimentation rate of more than 100 mm/h. Serum total protein was elevated at 9.0 g/dL and serum albumin was low at 2.2 g/dL. Urinalysis showed moderate haematuria and proteinuria. Urine osmolality was 290 mOsm/kg and urine sodium 21 mEq/L. A chest X-ray was unremarkable; however, the presence of digital clubbing raised suspicion for an insidious pulmonary disease and thus CT of the chest was obtained. The CT demonstrated numerous pulmonary nodules distributed in a miliary pattern with extensive hilar and mediastinal lymphadenopathy.
Investigations
Diagnostic laboratory evaluation focused on screening for suspected pathogens and malignancy. Blood cultures for bacteria, acid-fast bacilli (AFB) and fungi were obtained on admission, as were serological studies for Coccidioides, Histoplasma and Blastomyces species. Core skin biopsies were obtained on three of the skin lesions and sent for culture. Lumbar puncture was performed given somnolence on admission and cerebral spinal fluid (CSF) analysis revealed two neutrophils per high powered field with an otherwise normal cell count and differential, and a negative Gram stain for organisms. Bacterial, fungal and AFB cultures of CSF also returned negative, as did the Venereal Disease Research Laboratory (VDRL) test and the Cryptococcus neoformans antigen.
To assess for malignancy, iron studies, peripheral blood smear, and serum and urine protein electrophoresis (SPEP/UPEP) were obtained. The serum iron level was 25 µg/dL, ferritin was 5472 µg/L and transferrin was 82 mg/dL; findings most consistent with anaemia of chronic disease. Peripheral blood smear showed occasional plasma cells, immature granulocytes and rare erythrocytes in rouleaux formation; findings most suggestive of non-specific reactive changes but also potentially compatible with neoplastic disease. A polyclonal gammopathy and normal UPEP were most consistent with the presence of active infection. HIV 1/2 antibody and HIV viral load returned negative as did a rapid influenza and full viral respiratory PCR panel for common viruses, hepatitis B surface antigen, hepatitis C antibody, serum Cryptococcus neoformans antigen, serum galactomannan antigen, urine Histoplasma capsulatum antigen and serum Blastomyces dermatitidis antibody. An interferon-γ release assay returned indeterminate but three AFB smears and cultures of sputum were negative.
Skin biopsy fungal cultures ultimately grew Coccidioides on hospital day 4. Biopsy samples stained positive with Periodic acid-Schiff diastase (PAS-D) and demonstrated endosporulating spherules characteristic of Coccidioides with surrounding suppurative and granulomatous lesions (figure 2). Serum Coccidioides immitis immunodiffusion IgM and IgG antibodies returned positive on hospital day 6, and complement fixation titres later resulted at 1:256. Blood cultures returned positive for C. immitis on hospital day 10. CSF studies including Coccidioides antibody immunodiffusion and complement fixation were negative.
Figure 2.
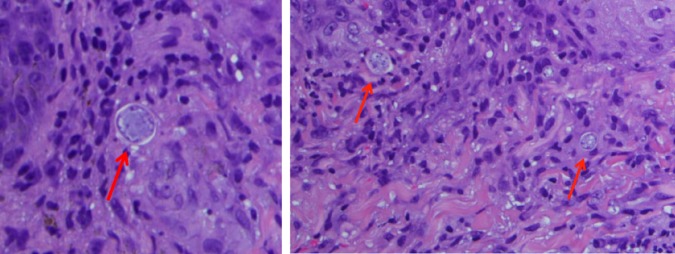
Skin biopsy of right wrist papule. H&E stain of a skin biopsy from a right wrist papule demonstrating endosporulating spherules (red arrows) characteristic of Coccidioides with surrounding suppurative and granulomatous lesions.
Differential diagnosis
The patient's constellation of symptoms including chronic fevers, weight loss and skin lesions, over a subacute to chronic timeframe, was suggestive of a systemic infection. Malignancy was also considered due to the presence of anaemia, an elevated total serum protein to albumin ratio and evidence of renal failure. At the time of admission, HIV status was unknown and thus infection due to pathogens such as Mycobacterium tuberculosis, non-tuberculous mycobacteria, Actinomyces, Nocardia and HHV-8 associated Kaposi's sarcoma were also thought to be possibilities. Fungal diseases considered in the differential diagnosis included histoplasmosis, aspergillosis, blastomycosis, cryptococcosis and coccidioidomycosis. However, the patient's occupational history as a truck driver in the southwestern US and residence near the northern border of California's San Joaquin Valley were distinct risk factors for exposure to Coccidioides.
Treatment
Prior to admission, the patient was empirically started on vancomycin and piperacillin/tazobactam in the hospital's emergency department. Given persistent fevers and a high suspicion for systemic Coccidioides infection, empiric antifungal therapy with oral fluconazole 800 mg daily was added to the regimen on hospital day three. Initiation of antifungal therapy resulted in defervescence and improvement in constitutional symptoms, and thus antibacterial therapy was discontinued. Repeat blood cultures drawn 5 days after starting antifungal therapy returned negative. The patient's acute renal failure improved with rehydration; however, hyponatraemia persisted. The patient's low urine sodium concentration and elevated urine osmolality suggested a syndrome of inappropriate antidiuretic hormone, potentially representing an active pulmonary infection.
Outcome and follow-up
The patient was discharged from the hospital after 8 days with a prescription for oral fluconazole 800 mg daily for a planned 2-month course and subsequent reassessment in the clinic. He lacked medical insurance and did not live in close proximity to the hospital, so a follow-up visit was booked with a general practitioner in his home community. The patient was also scheduled for an infectious disease clinic appointment after 6 weeks, at which time a decrease in fluconazole dosing would be considered. He was scheduled for repeat CT imaging of the chest in 6 months to verify resolution of pulmonary disease.
The patient failed to appear for scheduled follow-up visits with a primary care practitioner and infectious disease specialist following discharge. Further investigation revealed that the patient suffered an unrelated cardiac arrest approximately 1 month after returning home and was rehospitalised at another institution. His subsequent course was further complicated by cardiogenic and septic shock, acute renal failure and a necrotising infection of his lower extremities, which resulted in bilateral below-the-knee limb amputations. No cause was identified for the patient's cardiac arrest and no fungal blood cultures were drawn during the hospitalisation, although repeat fungal blood cultures shortly before this admission had been negative. The patient was eventually discharged to home with wheelchair dependence and is currently being followed by a general practitioner and infectious diseases specialist.
Discussion
Coccidioidomycosis is a fungal infection diagnosed with increasing frequency and characterised by protean clinical findings and outcomes ranging from mild disease to critical illness. A remarkable 804% increase in incidence has occurred over the past 15 years in Arizona and California in the USA, and in some regions Coccidiodes infection is a leading cause of community-acquired pneumonia.1 7 10 16 Geoclimatic and sociodemographic factors, including warmer temperatures, altered rainfall patterns, soil disturbance from land development, and changes in laboratory diagnostic and reporting practices are all considered contributory to this extraordinary increase.1 7
The number of cases reported outside of endemic areas is also on the rise and is becoming an emerging public health concern.1 4 9 Factors contributing to this expansion into non-endemic regions include increased national and international travel and trade through endemic areas and a growing population of individuals with impaired immune system function. Since relatively few aerosolised Coccidiodes arthroconidia are required to establish an infection, travellers to endemic regions with only minimal exposures may become infected and may not have sufficient symptoms to seek medical care until well after their return.1 4 9 Cases have been reported in travellers attending conferences, recreating outdoors and even during a layover while transferring airlines.1 4 9 Clinicians in non-endemic regions may not consider coccidioidomycosis during their initial evaluations, causing a delay in diagnosis and permitting progression to more advanced stages of disease.1
Patients treated with corticosteroids or biological immunomodulating therapies are at risk for both acquiring new infections and reactivating latent disease following medication initiation. Individuals with haematological malignancies and HIV infection face a particularly high risk of disease and are more likely to experience severe outcomes.1 17–19 Organ transplant recipients are also highly susceptible to both primary coccidioidomycosis and latent disease reactivation, particularly within the first year post-transplant when immunosuppression is most intense.1 17–22 Notably, organs transplanted from patients with coccidioidomycosis can serve as a source of infection in recipients with no history of exposure or travel to endemic areas.1 17–22
Most primary cases of coccidioidomycosis are self-limited and characterised by non-specific respiratory symptoms. Consistent with this are reports estimating that more than 25% of community-acquired pneumonias in endemic regions are the result of Coccidioides infection.1 10 16 Nonetheless, an estimated 40% of symptomatic patients do ultimately require hospitalisation and a subset of these develop advanced pulmonary or systemic disease.1 10 16 Disseminated coccidioidomycosis, which affects less than 1% of patients, occurs by haematogeneous spread from the lungs and affects the skin, skeleton and meninges.8 16 In affected immunosuppressed patients, the rate of extrapulmonary disease is much higher at 30–50%.1 2
Cutaneous Coccidioides lesions usually present as indurated nodules or papules; however, almost any kind of lesion can occur including plaques, gummas, pustules, ulcers, abscesses, fistulas and lesions mimicking warts or rosacea. Lesions can occur anywhere on the body, but are seen most frequently on the head and face.8 Though rare, dissemination to almost all organs has been reported and notably has been observed in patients with competent immune function.2 8 23–25
Hypercalcaemia may be a feature of disseminated coccidioidomycosis and is thought to occur via parathyroid hormone-related peptide secretion from granulomas.26–29 Coccidioides fungaemia is a rare entity with high mortality and is most often seen in patients with underlying immunosuppression and although unusual, it can also occur in those with fully functional immune systems as in the case described here.12 30 Diffuse reticulonodular or miliary infiltrates on imaging confer an increased risk of fungaemia and should prompt immediate evaluation for bloodstream infection.2
Screening for Coccidioides infection should be performed in all patients presenting with compatible clinical features, geographic exposure history and classic risk factors. Particular attention should be given to patients with HIV, malignancy, a history of solid-organ transplant or treatment with immunosuppressive medications. Patients with poorly controlled diabetes, cardiopulmonary disease and Filipino or African ethnicity are also at higher risk.2 Severe illness is presumed in patients with weight loss of more than 10%, persistent night sweats, reticulonodular or pulmonary infiltrates on chest imaging, hilar adenopathy, anticoccidiodal complement-fixing antibody concentrations in excess of 1:16, inability to work, symptoms that persist for 12 months or an age greater than 55 years.2
In patients with evidence of severe systemic symptoms, rapid evaluation for extrapulmonary disease is imperative. Coccidioides bloodstream infections carry a mortality in excess of 70%, and any evidence of sepsis in an affected patient warrants immediate treatment. Patients with any evidence of neurological dysfunction should receive a lumbar puncture given the concern for Coccidioides meningitis, however, in patients without focal central nervous system (CNS) symptoms, lumbar puncture is not routinely indicated.31
Laboratory diagnosis of coccidioidomycosis is based on a combination of serological and culture-based assays. Commercially available enzyme-linked immunoassays (EIA) for IgM and IgG are the most sensitive assay and should be performed first when available.32–35 Anti-Coccidioides IgM and IgG immunodiffusion assays are the most specific tests, may be qualitative or quantitative, and typically become positive at 1–3 weeks and 3 weeks after infection, respectively.32–35 Quantitative complement fixation (CF) assays, which detect complement depletion that occurs when antibodies in positive patient sera react against the Coccidioides chitinase, are less sensitive but offer reasonable specificity and are typically utilised for monitoring treatment response and assessing for disease relapse.35 36 When EIA or qualitative immunodiffusion tests return positive, CF or quantitative immunodiffusion assays should be obtained and if either is also positive, the presence of disease is confirmed. A serum complement fixation titre of >1:32 is often associated with extrapulmonary dissemination, as was the case in the patient presentation described here.35
Coccidioides can be cultured using a variety of media from a diversity of sample types including blood, bronchoalveolar lavage fluid, tissue and CSF. Fungal agars including brain–heart, potato-dextrose and Sabouraud-dextrose as well as bacterial medias including blood and chocolate agars support growth of the organism and are utilised for diagnosis.8 35 Culture is typically highly sensitive and returns positive by 1 week unless the patient has been previously exposed to antifungal agents.37 The sensitivity of sputum fungal cultures is unclear.35 38 Finally, histopathological studies using calcofluor white stain, silver stain and PAS stain, while often performed, lack sensitivity and specificity and are thus of limited utility.35 37 Nucleic acid-based detection methods including amplification by real-time PCR are being developed and offer both enhanced sensitivity, specificity and take less processing time.35 37
In general, any patient with disseminated coccidioidomycosis should be treated, and according to the Infectious Diseases Society of America guidelines, first-line therapy for disseminated coccidioidomycosis utilises triazole antifungals.2 In rapidly progressive disease, respiratory failure, sepsis or in pregnancy, amphotericin B is the treatment of choice. Recommended triazole regimens include fluconazole 400–800 mg daily, ketoconazole 400 mg daily or itraconazole 200 mg twice daily, all of which are orally available.2 Itraconazole may be superior to fluconazole in the treatment of non-meningeal coccidioidal infections, especially for patients with skeletal lesions.2 39 In patients with Coccidioides meningitis who fail to respond despite triazole therapy, intrathecal amphotericin B therapy is recommended.2
Owing to a relapse rate for disseminated disease exceeding 20% at 12 months, treatment duration is generally continued for more than a year.39 In the event of CNS involvement, lifetime triazole treatment is indicated with a goal to ultimately transition to a once-daily low dose suppressive regimen. Furthermore, for patients with severe immunodeficiency, oral triazole therapy should be continued as secondary prophylaxis for the patient's lifetime.2 Finally, in patients with obstructive or highly malignant lesions such as large abscesses, pericardial effusions or impinging spinal disease, surgical interventions may be required.2
Coccidioidomycosis is an increasingly common fungal disease in arid regions of the Americas that is emerging as a significant pathogen in both historically endemic areas and now worldwide. While most infections are self-limiting syndromes resembling community-acquired pneumonia, a subset of cases progresses to disseminated disease involving the skin, skeleton or CNS. Impaired immunity is a strong risk factor for extrapulmonary disease although cases of disseminated coccidioidomycosis do occur in patients with intact immune systems. Coccidioides bloodstream infections confer particularly high mortality and thus evaluation for fungaemia should be considered in all patients with systemic symptoms. Reactivation of latent disease may occur several months after initial exposure when affected individuals are no longer residing in areas of known disease incidence, emphasising the need for healthcare providers everywhere to consider Coccidioides infection in their patient populations.
Learning points.
Coccidioidomycosis is a fungal disease endemic to arid regions of North and South America.
The incidence of coccidioidomycosis has increased by 804% in the southwestern USA and is rising globally with cases reported throughout North America and in Europe and Asia.
Primary coccidioidomycosis most commonly presents as a self-limiting syndrome with non-specific respiratory symptoms, however, presentations involving other organ systems are possible.
Disseminated coccidioidomycosis can involve the skin, skeleton and central nervous system and is most frequently seen in immunosuppressed populations and those of African or Filipino ancestry.
Symptoms of coccidioidomycosis may not occur until several months after pathogen exposure and consequently in regions distant from areas of known disease endemicity.
First-line treatment for coccidioidomycosis is a triazole antifungal, and treatment for disseminated disease should be continued for at least 1 year due to a high risk of relapse.
Acknowledgments
The authors would like to acknowledge Faye Chan, MD and Steven Ludwin, MD for their efforts in the clinical management of this patient.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brown J, Benedict K, Park BJ, et al. Coccidioidomycosis: epidemiology. Clin Epidemiol 2013;5:185–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis 2005;41:1217–23 [DOI] [PubMed] [Google Scholar]
- 3.Hector RF, Laniado-Laborin R. Coccidioidomycosis—a fungal disease of the Americas. PLoS Med 2005;2:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the Western hemisphere. Ann N Y Acad Sci 2007;1111:19–34 [DOI] [PubMed] [Google Scholar]
- 5.Ampel NM. New perspectives on coccidioidomycosis. Proc Am Thorac Soc 2010;7:181–5 [DOI] [PubMed] [Google Scholar]
- 6.Ampel NM. What's behind the increasing rates of coccidioidomycosis in Arizona and California? Curr Infect Dis Rep 2010;12:211–16 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease C, Prevention. Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb Mortal Wkly Rep 2013;62:217–21 [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh O, Vera-Cabrera L, Rendon A, et al. Coccidioidomycosis. Clin Dermatol 2012;30:573–91 [DOI] [PubMed] [Google Scholar]
- 9.Panackal AA, Hajjeh RA, Cetron MS, et al. Fungal infections among returning travelers. Clin Infect Dis 2002;35:1088–95 [DOI] [PubMed] [Google Scholar]
- 10.Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis 2006;12:958–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang A, Thomas R, Hoffman RS. Disseminated coccidioidomycosis in an immunocompetent person living in New York City. J Urban Health 2005;82:339–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keckich DW, Blair JE, Vikram HR. Coccidioides fungemia in six patients, with a review of the literature. Mycopathologia 2010;170:107–15 [DOI] [PubMed] [Google Scholar]
- 13.Adam RD, Elliott SP, Taljanovic MS. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med 2009;122:770–7 [DOI] [PubMed] [Google Scholar]
- 14.Peterson CM, Schuppert K, Kelly PC, et al. Coccidioidomycosis and pregnancy. Obstet Gynecol Surv 1993;48:149–56 [DOI] [PubMed] [Google Scholar]
- 15.Seitz AE, Prevots DR, Holland SM. Hospitalizations associated with disseminated coccidioidomycosis, Arizona and California, USA. Emerg Infect Dis 2012;18:1476–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis 2008;14:1053–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair JE, Kusne S, Carey EJ, et al. The prevention of recrudescent coccidioidomycosis after solid organ transplantation. Transplantation 2007;83:1182–7 [DOI] [PubMed] [Google Scholar]
- 18.Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation. Clin Infect Dis 2001;33:1536–44 [DOI] [PubMed] [Google Scholar]
- 19.Blair JE, Smilack JD, Caples SM. Coccidioidomycosis in patients with hematologic malignancies. Arch Intern Med 2005;165:113–17 [DOI] [PubMed] [Google Scholar]
- 20.Logan JL, Blair JE, Galgiani JN. Coccidioidomycosis complicating solid organ transplantation. Semin Respir Infect 2001;16:251–6 [DOI] [PubMed] [Google Scholar]
- 21.Vikram HR, Dosanjh A, Blair JE. Coccidioidomycosis and lung transplantation. Transplantation 2011;92:717–21 [DOI] [PubMed] [Google Scholar]
- 22.Wright PW, Pappagianis D, Wilson M, et al. Donor-related coccidioidomycosis in organ transplant recipients. Clin InfectDis 2003;37:1265–9 [DOI] [PubMed] [Google Scholar]
- 23.Cunningham ET, Jr, Seiff SR, Berger TG, et al. Intraocular coccidioidomycosis diagnosed by skin biopsy. Arch Ophthalmol 1998;116:674–7 [DOI] [PubMed] [Google Scholar]
- 24.Erly WK, Labadie E, Williams PL, et al. Disseminated coccidioidomycosis complicated by vasculitis: a cause of fatal subarachnoid hemorrhage in two cases. AJNR Am J Neuroradiol 1999;20:1605–8 [PMC free article] [PubMed] [Google Scholar]
- 25.Reach P, Paugam A, Kahan A, et al. Coccidioidomycosis of the spine in an immunocompetent patient. Joint Bone Spine 2010;77:611–13 [DOI] [PubMed] [Google Scholar]
- 26.Ali MY, Gopal KV, Llerena LA, et al. Hypercalcemia associated with infection by Cryptococcus neoformans and Coccidioides immitis. Am J Med Sci 1999;318:419–23 [DOI] [PubMed] [Google Scholar]
- 27.Caldwell JW, Arsura EL, Kilgore WB, et al. Hypercalcemia in patients with disseminated coccidioidomycosis. Am J Med Sci 2004;327:15–18 [DOI] [PubMed] [Google Scholar]
- 28.Fierer J, Burton DW, Haghighi P, et al. Hypercalcemia in disseminated coccidioidomycosis: expression of parathyroid hormone-related peptide is characteristic of granulomatous inflammation. Clin Infect Dis 2012;55:e61–6 [DOI] [PubMed] [Google Scholar]
- 29.Lee JC, Catanzaro A, Parthemore JG, et al. Hypercalcemia in disseminated coccidioidomycosis. N Engl J Med 1977;297:431–3 [DOI] [PubMed] [Google Scholar]
- 30.Rempe S, Sachdev MS, Bhakta R, et al. Coccidioides immitis fungemia: clinical features and survival in 33 adult patients. Heart Lung 2007;36:64–71 [DOI] [PubMed] [Google Scholar]
- 31.Thompson G, III, Wang S, Bercovitch R, et al. Routine CSF analysis in coccidioidomycosis is not required. PLoS ONE 2013;8:e64249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman L, Sekhon AS, Moledina N, et al. Comparative evaluation of commercial Premier EIA and microimmunodiffusion and complement fixation tests for Coccidioides immitis antibodies. J Clin Microbiol 1995;33:618–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pappagianis D. Coccidioides immitis antigen. J Infect Dis 1999;180:243–4 [DOI] [PubMed] [Google Scholar]
- 34.Pappagianis D. Serologic studies in coccidioidomycosis. Semin Respir Infect 2001;16:242–50 [DOI] [PubMed] [Google Scholar]
- 35.Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol 2007;45:26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair JE, Coakley B, Santelli AC, et al. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 2006;162:317–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binnicker MJ, Buckwalter SP, Eisberner JJ, et al. Detection of Coccidioides species in clinical specimens by real-time PCR. J Clin Microbiol 2007;45:173–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hector RF, Rutherford GW, Tsang CA, et al. The public health impact of coccidioidomycosis in Arizona and California. Int J Environ Res Public Health 2011;8:1150–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galgiani JN, Catanzaro A, Cloud GA, et al. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann Intern Med 2000;133:676–86 [DOI] [PubMed] [Google Scholar]


