Abstract
Drosophila melanogaster has been used as an excellent model organism to study environmental and genetic manipulations that affect behavior. One such behavior is spontaneous locomotor activity. Here we describe our protocol that utilizes Drosophila population monitors and a tracking system that allows continuous monitoring of the spontaneous locomotor activity of flies for several days at a time. This method is simple, reliable, and objective and can be used to examine the effects of aging, sex, changes in caloric content of food, addition of drugs, or genetic manipulations that mimic human diseases.
Keywords: Neuroscience, Issue 86, Investigative Techniques, Life Sciences (General), Behavioral Sciences, Drosophila melanogaster, Fruit flies, Spontaneous physical activity, Mobility, Fly behavior, Locomotor Activity
Introduction
Fruit flies, Drosophila melanogaster, have been used as a valuable model organism to study mechanisms underlying complex behaviors, such as learning and memory, social interaction, aggression, drug abuse, sleep, sensory function, courtship, and mating1,2. One behavior that has been studied through multiple protocols is spontaneous locomotor activity. Negative geotaxis was one of the first methods developed for measuring Drosophila activity, and this protocol involves measuring the percentage of flies that reach a certain height of the vial after flies were shaken to the bottom of the container1,3. This method has advantages of being straightforward, inexpensive, and since it does not require any special equipment it can be performed in any laboratory. It has been used as a valuable screening tool to study effects of different genetic manipulations on fly mobility3. However, it is time and labor intensive and has the possibility of bias due to variable shaking of the vials and human recordings.
The negative geotaxis method was improved upon by development of the Rapid Iterative Negative Geotaxis (RING) method4,5, which takes photographs of the fly vials following shaking of the flies to the bottom. The advantage of this protocol is its sensitivity and the possibility of testing a large number of fly vials at the same time. However, this protocol still has the potential for human error, and only measures negative geotaxis. Other laboratories have used simple observation in culture vials to determine locomotor activity6.
Recently several video recording systems for measuring fly locomotor activity have been developed. One video monitoring protocol provides time for adjustment before recording7. The method described by Slawson et al. also uses an air pulse to stop movement until the start of recording, which could potentially be a stressor to the animals7. This method provides information on average speed, max speed, time spend in motion, etc. Another three-dimensional tracking system measures the maximal velocity of individual flies during ~0.2 seconds of free flight takeoff8. A three-dimensional video monitoring protocol uses flies expressing GFP and multiple cameras fitted with filters allowing for detection of fluorescence to determine fly mobility9. Flies in this protocol tend to exhibit cylindrical flight patterns, which is potentially due to the shape of Drosophila culture vials10. This method was improved by using a dome that allows measuring spontaneous movement of two flies11. A high-throughput method that uses a camera to automatically monitor and quantify the individual and social behavior of Drosophila has been also described12. Zou et al. developed a behavioral monitor system (BMS) that uses two computer-assisted cameras to record lifetime behavior and movements such as resting, moving, flying, eating, drinking, or deaths of individual tephritid fruit flies13. Several other video systems have been developed to monitor fly behavioral activity14,15.
Here we describe a method for quantifying Drosophila activity that utilizes population monitors. These monitors are housed in temperature- and humidity-controlled incubators at 25 °C on a 12 hour day-night light cycle. Each population monitor has infrared beams placed in rings positioned at three different heights. Every time a fly moves across the rings it interrupts the infrared beam, which is recorded by a microprocessor that independently records and counts the activity of flies within the vial. A microprocessor uploads the total activity within the vial to the computer at user-defined intervals that could vary from 1 second to 60 minutes. The method described here provides ample time for flies to adjust to the new environment and allows for simultaneous measuring of the spontaneous locomotor activity of as many as 120 populations of flies. In addition, we describe preparation of the food, fly maintenance, setting up the mobility population monitors in temperature controlled incubators, and potential factors that may affect results. This method can be used to study how different environmental or genetic modifications affect spontaneous locomotor activity of the flies.
Protocol
Note: The Canton-S strain is the standard wild-type background line obtained from the Bloomington Stock Center.
1. Food Preparation and Recipe for 1,000 ml of Food
Note: This section describes the protocol for food preparation. Large metal pots are used to prepare about 18 L of food at a time. The protocol described here is downsized and uses 1,000 ml H2O. Food is autoclaved twice.
Mix 113 g sucrose and 28 g brewers yeast in 643 ml water. Leave ingredients on a hot plate set at 25 °C with a stir bar to mix throughout for 15 minutes.
Autoclave food solution for 20 min.
Mix 49 g cornmeal and 8.1 g agar in 268 ml water and add to the autoclaved food mixture described in step 1.2. Mix well with a large spoon or a whisk.
Autoclave food mixture for another 20 min.
Place the food on a plate and let cool down with constant mixing with a stir bar. If additional solutions should be added to food, such as mifepristone (RU486), keep the food on a hot plate set up at 60 °C and add solution when the food reaches the required temperature.
Dissolve 2.4 g tegosept in 10.7 ml 100% EtOH and keep on a cold plate with a stirrer to completely dissolve and mix for about 15 min.
Add tegosept solution to food when the temperature of food is 60 °C and mix well.
Use a pump or a food dispenser to pour about 10 ml of food into a wide vial. By using a food dispenser one can pour food simultaneously into 100 wide, plastic vials (1 tray) at a time.
Cover the vials with Kimwipes and cheese cloth and leave food at room temperature for 12-24 hr to cool down. Keep the food at 4 °C and use within 3-4 weeks. Warm up the food to room temperature before use for fly work.
2. Preparation of Glass Vials
Prepare food according to the protocol listed in step 1.
Aliquot 5 ml of food into each narrow, glass vial, which is the correct size for the population monitors. This amount of food should be low enough to be below the lowest ring of the population monitor.
After the food cools down to room temperature cover the vials with sponge plugs and keep them at 4 °C for up to 2 weeks. Because the amount of food in a vial is rather low, it is best to use the food within a week or two to prevent any drying.
Warm up the vials to room temperature before use.
3. Maintenance of the Parental Flies
Grow the flies in wide plastic vials with standard laboratory food and keep the vials in a humidified, temperature-controlled environmental chamber at 25 °C on a 12 hr light/dark cycle. The daylight period starts at 6:00 AM in this laboratory.
In the morning clear adult flies from the vials from which parental flies will be collected.
Collect newly eclosed flies and separate them by gender on a CO2 pad within 8 hr after eclosion to make sure that the female flies are virgins. Flies start to mate 8 hr after eclosion.
When the virgin male and female flies are between 5 and 10 days of age, put 10 males and 10 female flies in a vial with standard food and several grains of active yeast on top. Note: Control the density of the larvae by using the same number of flies and keeping them in a vial for two days. Addition of active yeast promotes egg production.
Keep the flies to mate and lay eggs in a temperature-controlled environmental chamber at 25 °C with a 12 hr light/dark cycle for 2 days. Set up 5-10 vials of parental flies.
Pass the flies to a new plastic vial every other day and keep the vials with the eggs in an incubator at 25 °C.
4. Collection of Experimental Flies
After 9 days flies will start to eclose from the vials where the parental flies laid eggs (described in step 3.6.). Clear and discard the flies that eclosed during the first day and return the vials to incubator. Most of the flies eclosed on day 1 are females. A more synchronized population of flies will eclose on day 2.
Within 24 hr place newly eclosed flies on CO2 pads and collect 25 male and 25 female flies per vials with a paintbrush or metal spoon. Keep flies on CO2 pads for a short period of time to minimize any effects of CO2. Write down the day of eclosion on the vial. Assemble at least 5 replicate vials for experimental and for control groups.
Keep the vials in temperature-controlled environmental chambers at 25 °C with a 12 hr light/dark cycle.
Pass the flies to a new plastic vial every other day using a funnel.
Age the flies until the desired age for experimentation is reached.
5. Setting Up the Mobility Monitors
Place the population monitors in a temperature-controlled incubator.
Connect each monitor with a 4-wire telephone cable to the Power Supply Interface Unit (PSIU) via 5-way splitters (multi-line), which can connect up to 5 individual monitors to one opening in the PSIU. See Figures 1A and 2B.
Connect the PSIU to a line power outlet (100-240 V). Plug the power supply output connector into one of the 2 mating PSIU jacks. The adjacent green light illuminates green when connected properly.
Connect the PSIU to the Universal Serial Bus (USB) hardware. Connect the USB cable between the USB hardware with a Macintosh or a Windows PC for data recording. It would be best to have a computer dedicated only for data collection since collection runs for days at a time.
Download the USB software (PSIUdrivers.zip). USB driver software is used by the Power Supply interface and needs to be downloaded only once. It synthesizes a data link between the computer program and the PSIU/activity monitors. For a PC use a COM port and for a Macintosh use a simple serial port.
Download the computer program for Macintosh OSX (Intel) or for Windows PC (XP/Vista/7) programs by following instructions provided by manufacturer Notes 308.pdf.
Start the computer program and set up the program by clicking on the Preferences, Lights or Monitors. The program will run until the user selects “quit” to stop the program. If the computer program or the computer is shut down the monitors will continue to count beam interruptions, but the counts will not be recorded until the program is re-launched. In that case the first reading will include all the counts since the last time the PSIU sent the data to computer.
Select the Preferences tab and choose the Serial Port, PSIU for Macintosh and COM for the PC.
Select the reading interval that ranges from seconds, minutes, or an hour.
Select the monitors: Each monitor has its unique number that is given by the manufacturer. Select the Monitor Range that corresponds to the numbers given to the monitors by the manufacturer.
The Lights box: Make sure that all the monitors are properly connected, which is marked by a green light next to the monitor number on the software. A red light indicates that the connection is lost, and a black box indicates that the system is off or improperly set up.
6. Setting Up the Experiment
Remove glass vials containing food from 4 °C and let them warm to room temperature.
Separate male and female flies of the same age on CO2 pad. For aging studies it is possible to start mobility studies as early as 3 days of age.
Put 10 male or 10 female flies into each glass vial containing food. Use at least three vials for each experimental and control line of flies and for each gender.
Keep the vials on their side until the flies recover from CO2 to ensure the flies do not get stuck in the food. Separate flies at about 8:00 AM and leave them for about 2 hr at room temperature to recover from CO2.
Place the vials inside the population monitors housed in the incubators.
Discard the data collected within the first 24 hr after the flies are put into the incubator to let them adjust to the novel environment.
Pass the flies after 3 or 4 days to new vials to avoid drying of the food. If flies are prone to death or are age 40 days or older, pass the flies after 2 days and use data collected for day 2. Also, use more than three vials per group to ensure adequate replicates. Data from vials with dead flies should be disregarded and not included in analysis.
7. Running the Activity Monitors and Calculating the Total Spontaneous Activity
Select preferences - the interval for data collection. Note: The computer program allows collection of the data at intervals ranging from 1 second to 60 minutes. 10 and 30 minute periods have been found to provide adequate information about mobility without having an overwhelming number of time points. At the selected time period, the program will send the current total count for each monitor to the computer and start counting again from zero. The computer program stores the data in a new folder created by the computer data system. The data collected in each monitor are stored separately, and individual text documents are created for each vial. The data are continuously collected as long as the program operates.
At the end of the experiment, scan the data using the FileScan110X for Macintosh OSX (Intel) or SystemMB108 for Windows PC (XP/Vista/7) program. Note: The Scan program eliminates duplicate readings and makes sure that the recordings are complete.
Save the data collected within a specific time and period of days. Choose an experimental name and copy the files from the computer data folder for analysis. Note: At this time, activity intervals can be changed and converted to different ones. The original data will stay stored in the computer data folder and can be retrieved as long as they are not deleted.
8. Data Analysis
Copy the data collected in the text files into columns of Excel spreadsheets for data analysis. Data collected by this software are in columns, which contain numbers representing total activity in a single monitor over a period of time selected by the investigator. Note: Data collected for each monitor are in separate text files. There are 32 columns for each monitor. The first six columns are empty and contain only 0; next three contain the data collected at the bottom ring, in the middle, and at the top ring. The rest of the channels can be deleted since they do not contain any data. Each ring outputs a single value per time. See screen shot of the raw data in Figure 2.
Calculate the total activity within a desired period of time for each monitor that represents the sum of activity collected at three different heights of infrared beams. Note: The time period can range from several hours, 24 hours or several days.
Determine the average locomotor activity and the standard deviation between the 3 monitors that represent 3 biological replicates. Note: The data can be analyzed for statistical significance by using a number of tests. A two-tailed Students’s t-test, a one-way analysis of variance (ANOVA) and a Tukey HSD post-hoc test could be used to determine the effects of several environmental or genetic manipulations on 24 hours of spontaneous locomotor activity16. There are a number of other programs that can be used and have been previously published17.
Representative Results
The spontaneous locomotor activity in Drosophila depends on fly gender (Figure 3A), calorie content of the food (Figure 3B) and the light/dark cycle. Once the light is switched off fly activity dramatically decreases. Figure 3A illustrates 24 hours of locomotor activity recordings of male and female flies. An asterisk on the x-axis marks the time when the light was switched off and the transition to dark cycle. Figure 3B illustrates the standard deviation between the average spontaneous locomotor activity collected in three population monitors for Male flies age 3 days on corn food. The data collected for the spontaneous physical activity during the 24 hours can be also expressed as the total activity per fly during a 24 hour period, Figure 3C.
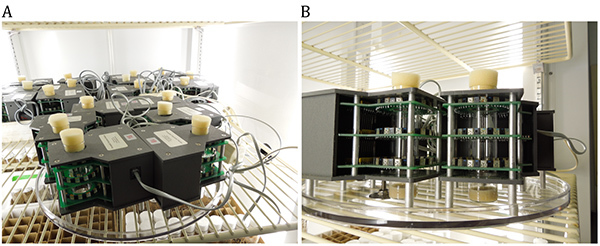 Figure 1: Population monitor setup for monitoring of spontaneous locomotor activity of flies. A) Several population monitors are connected with a 4-wire telephone cable to 5-way splitters and placed in a temperature-controlled incubator. B) Higher magnification of two population monitors, which show placement of the vials within the population monitors and three rings with infrared beams positioned at three different heights. Click here to view larger image.
Figure 1: Population monitor setup for monitoring of spontaneous locomotor activity of flies. A) Several population monitors are connected with a 4-wire telephone cable to 5-way splitters and placed in a temperature-controlled incubator. B) Higher magnification of two population monitors, which show placement of the vials within the population monitors and three rings with infrared beams positioned at three different heights. Click here to view larger image.
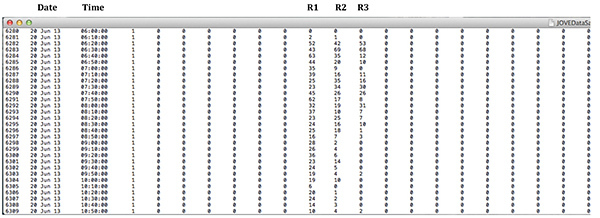 Figure 2: Screen shot of the raw data generated by the software showing Date, Time and data collected in Rings 1, 2, and 3. R stands for Ring. Click here to view larger image.
Figure 2: Screen shot of the raw data generated by the software showing Date, Time and data collected in Rings 1, 2, and 3. R stands for Ring. Click here to view larger image.
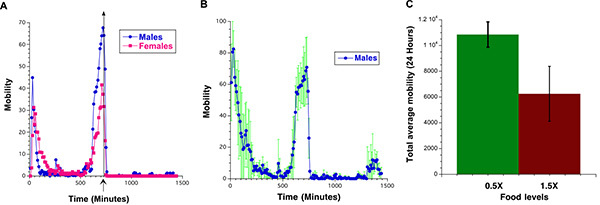 Figure 3:A) Average spontaneous locomotor activity of male (Black) and female (Magenta) flies during 24 hours on standard laboratory diet. The data are collected in 10 minute bins and represent average activity per fly calculated as average activity between three vials each containing 10 flies. B) Average spontaneous locomotor activity of male flies during 24 hours on standard laboratory diet. The data are collected in 10 minute bins and represent average activity per fly calculated as average activity between three vials. Standard deviations are marked in green. C) Total activity of 20 days old male flies on low-calorie (0.5X) (Green) and high-calorie (1.5X) (Brown) food over 24 hours. Click here to view larger image.
Figure 3:A) Average spontaneous locomotor activity of male (Black) and female (Magenta) flies during 24 hours on standard laboratory diet. The data are collected in 10 minute bins and represent average activity per fly calculated as average activity between three vials each containing 10 flies. B) Average spontaneous locomotor activity of male flies during 24 hours on standard laboratory diet. The data are collected in 10 minute bins and represent average activity per fly calculated as average activity between three vials. Standard deviations are marked in green. C) Total activity of 20 days old male flies on low-calorie (0.5X) (Green) and high-calorie (1.5X) (Brown) food over 24 hours. Click here to view larger image.
Discussion
Spontaneous locomotor activity of flies is influenced by many factors such as age, genetic background, and gender2,13,18,19. In addition, environmental factors such as caloric content of the food, temperature of the environment, addition of different drugs, and day/night light cycle can affect fly activity. For instance, male flies of the same age have a higher spontaneous physical activity compared to females (Figure 1). Therefore, flies of the same age and gender should be compared to each other. When examining the effect of genetic manipulations on fly activity, such as overexpression or loss of function of a particular gene, the experimental and control flies must be in the same genetic background to remove any potential effects of different genetic background or second site modifiers. This can be achieved by backcrossing experimental female flies to w1118or yw males for 10 generations. After 10 generations of backcrossing, w1118or yw flies could be used as a genetic control. Another way to control for the genetic background is to use the inducible GAL4 GeneSwitch (GAL4-GS)-UAS binary system, which allows overexpression or down-regulation (RNAi) of the gene of interest in a time and tissue-specific manner in flies fed food with the addition of mifepristone (RU486) 20,21. RU486 is necessary for GAL4 to dimerize and bind to the UAS sequence. Genetic controls are sibling flies kept on food with the addition of EtOH (Ru486 diluent).
Various methods have been used to record Drosophila mobility. The method described here is simple, reliable, more informative, and has less potential of bias compared to other methods used to determine Drosophila mobility, such as negative geotaxis. It has the advantage of objective simultaneous recording of multiple populations of flies for a long period of time in standard culture conditions. Measuring locomotor activity by using population monitors can be useful for studying how different caloric contents of the food affect fly activity or to study genetic mechanisms underlying increased activity of flies on CR16. Similarly, this system has been used to study the effects of different genetic mutations, aging, or the addition of different drugs on fly spontaneous physical activity. Use of individual tubes instead of population monitors allows measuring H2O2 resistance in different genotypes of flies, studying circadian rhythms in vivo, analyzing sleep behavior, and others17,22-24.
Like any method, there are limitations to this monitoring system. When monitoring flies for a long period of time, there is a potential for fly death, especially if using aged flies. Using only healthy flies will help prevent this. We also try to use more than 3 biological replicates per group if the flies are old or prone to dying. One solution is to keep flies only for 2 days in the mobility monitors and use data collected during day 2, after flies have adjusted to the environment. If death occurs we do not use the data collected for the vial in calculations. Although we have been using vials positioned only vertically in the Trikinetics activity monitors, there is a possibility to place the vials horizontally. We choose to place vials vertically because the food is at the bottom of the vial, which is similar to standard incubator culture conditions. This allows flies to have more space to walk up and down the vials, and it is more similar to negative geotaxis experiments. The humidity of the incubator should also be monitored if food desiccation becomes a problem24. This system provides data in terms of average activity, and does not provide specific details about the nature of the activity. In addition, if two flies cross the beam at the same time, it will only be recorded as one interruption. The protocol described here is useful for quantifying total activity, but other protocols could provide useful data if more precise information such as flight trajectory or velocity are desired12,14,25.
Following this experiment, differences in spontaneous locomotor activity due to genetic or environmental manipulations will be known. A future modification of this protocol could be to analyze the different levels of activity of flies at the top, middle, and bottom rings of the population monitors. This would determine whether the fly populations spend most of their time at the bottom of the vial near the food or at the top. The protocol in its current form allows for accurate, simultaneous quantification of spontaneous locomotor activity of Drosophila experimental and control populations.
Disclosures
We have nothing to disclose.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (AG023088 to B.R.).
References
- Ali YO, Escala WE, Ruan K, Zhai RG. Assaying Locomotor, Learning, and Memory Deficits in Drosophila Models of Neurodegeneration. J. Vis. Exp. 2011;49:2504. doi: 10.3791/2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MA, Grotewiel M. Drosophila as a model for age-related impairment in locomotor and behaviors. Exp. Gerontol. 2011;46(5):320–325. doi: 10.1016/j.exger.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel MS, Martin I, Bhandari p, Cook-Wiends E. Functional senescence in Drosophila melanogaster. Aging Res. Rev. 2005;4(3):372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid Iterative Negative Geotaxis (RING): a New Method for Assessing Age-related Locomotor Decline in Drosophila. Exp. Gerontol. 2005;40(5):386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Bechnel J, Pandey UB. Methods to assay Drosophila behavior. J. Vis. Exp. 2012;61 doi: 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TA, Rice WR. Adult locomotor activity mediates Intralocus sexual conflict in a laboratory-adapted population of Drosophila melanogaster. Proc. Biol. Sci. 2007;274(1629):3105–3112. doi: 10.1098/rspb.2007.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson JB, Kim EZ, Griffith LC. High-resolution video tracking of locomotor in adult Drosophila melanogaster. J. Vis. Exp. 2009;24(24) doi: 10.3791/1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JH, Rogina B, Montooth KL, Helfand SL. Conditional tradeoff between aging and organismal performance of Indy long-lived mutant flies. Proc. Natl. Acad. Sci. USA. 2003;100(6):3369–3372. doi: 10.1073/pnas.0634985100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover D, Yang J, Tavaré S, Tower L. Simultaneous tracking of fly movement and gene expression using GFP. BMC Biotechnol. 2008;8:93. doi: 10.1186/1472-6750-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover D, Yang J, Tavaré S, Tower J. Simultaneous tracking of movement and gene expression in multiple Drosophila melanogaster flies using GFP and DsRED fluorescent reporter transgenes. BMC Res Notes. 2009;2(58):1–11. doi: 10.1186/1756-0500-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani R, et al. Three-dimensional tracking and behaviour monitoring of multiple fruit flies. J. R. Soc. Interface. 2013;10(78) doi: 10.1098/rsif.2012.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson KA, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6(6):451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, et al. Recording Lifetime Behavior and Movement in an Invertebrate Model. PLOS One. 2011;6(4) doi: 10.1371/journal.pone.0018151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente D, Golani I, Mitra PP. Analysis of the trajectory of Drosophila melanogaster in a circular open field arena. PLoS One. 2007;2(10):1083. doi: 10.1371/journal.pone.0001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan OT, Marcu O, Sanchez ME, Bhattacharya S, Kovacs KT. A portable system for monitoring the behavioral activity of Drosophila. J Neurosci. Methods. 2011;202(1):45–52. doi: 10.1016/j.jneumeth.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Parashar V, Rogina B. dSir2 mediates the increased spontaneous physical activity in flies on calorie restriction. Aging. 2009;1(6):529–541. doi: 10.18632/aging.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneuchi T, Togawa T, Matsuo T, Fuyama Y, Aigaki T. Efficient measurement of H2O2 resistance in Drosophila using an activity monitor. Biogerontology. 2003;4(3):157–165. doi: 10.1023/a:1024145822785. [DOI] [PubMed] [Google Scholar]
- Carey JR, et al. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp. Gerontol. 2006;41(1):93–97. doi: 10.1016/j.exger.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp. Gerontol. 2008;43(8):739–749. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA. 2001;98(22):12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Chiu JC, Low KH, Pike DH, Yildirim E, Edery I. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J. Vis. Exp. 2010;43:2157. doi: 10.3791/2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harbor Protoc. 2010;11 doi: 10.1101/pdb.prot5518. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Processing circadian data collected from the Drosophila Activity Monitoring (DAM) System. Protoc. Vol. 11. Cold Spring Harbor; 2010. [DOI] [PubMed] [Google Scholar]
- Ardekani R, Tavaré S, Tower J. Assessing senescence in Drosophila using video tracking. Methods Mol. Biol. 2013;965:501–516. doi: 10.1007/978-1-62703-239-1_33. [DOI] [PubMed] [Google Scholar]


