Abstract
Aim
The objective of the current study was to evaluate the efficacy of aminaphtone to control gum bleeding.
Patients and methods
Fifteen male and 15 female children, aged between 10 and 18 years with a mean age of 13.4 years and with gingival bleeding, were enrolled in this randomized, double-blind, placebo-controlled, Phase IV clinical trial. The inclusion criterion was gingivitis with gingival bleeding. Participants were prescribed either aminaphtone or placebo. Thirty identical boxes containing blister packs of identical pills of either aminaphtone or placebo were produced and coded with unique numbers by the manufacturer (Baldacci Laboratory, Brazil) and donated for this trial. A research assistant administered aminaphtone (Capilarema 75 mg) to fifteen patients or placebo to fifteen patients twice daily for 5 days. Intraoral clinical evaluations of bleeding were made before starting medication/placebo and then at 3 and 5 days after administration.
Results
On comparing the number of bleeding points before and after treatment between the aminaphtone and placebo groups, we found significantly higher reductions with the medication (P<0.0001).
Conclusion
Aminaphtone reduces gum bleeding in gingivitis, and may have a supportive role in the control of bleeding.
Keywords: periodontitis, bleeding, treatment
Introduction
Periodontal disease, or periodontitis, is one of the most underdiagnosed human diseases; it affects about 80% of the adult population.1 The mean percentage of the maximum score of the Community Periodontal Index in adults between the ages of 35 and 44 years indicates that only around 10% of individuals do not seem to have any kind of gingival inflammation.2 In the early stages of the disease, there is an accumulation of bacterial biofilm in the supragingival region that stimulates inflammation of the gingival tissue. When the inflammation is restricted to soft tissue, it is called gingivitis.3,4
The presence of bleeding, edema, and increased crevicular fluid resulting from inflammation mediated by bacteria or endotoxins, and the immunological humoral response of the host with gingival hyperemia are common signs in both gingivitis and periodontitis.5 Edema of the gingival tissue and bleeding in periodontitis can lead to bone resorption; the final stage of this process is the loss of teeth due to total elimination of support tissue.6
Bleeding is unpleasant while brushing the teeth, and often discourages patients from performing oral hygiene. Studies have shown that aminaphtone interferes in bleeding.7–9 Recently, studies have shown that this drug helps to control bleeding in patients on oral warfarin therapy and in cases of ocher dermatitis.7,10,11 Gingival bleeding has proven to be a viable model in the therapeutic assessment of aminaphtone. The objective of the current study was to evaluate the efficacy of aminaphtone to control gum bleeding.
Patients and methods
Patients
Fifteen male and 15 female children aged between 10 and 18 years with a mean age of 13.4 years and with gingival bleeding were enrolled in this randomized, double-blind, placebo-controlled, Phase IV clinical trial. The inclusion criteria were that the participants were students at the Professora Iria Barbieri Vita State School in Mirassol, Brazil and had gingivitis and periodontitis with gingival bleeding.
Medication
Patients with gingivitis were selected and invited to participate in the study after parents gave their informed consent. Participants were prescribed either aminaphtone or placebo. Thirty identical boxes containing blister packs of identical pills of either aminaphtone or placebo were produced and coded with unique numbers by the manufacturer (Baldacci Laboratory, Brazil) and donated for this trial. The significance of each coded number was not revealed until after the practical part of the trial had been completed. A research assistant administered the medication or placebo to patients, with fifteen patients receiving aminaphtone (Capilarema® 75 mg) and the other 15 receiving placebo twice daily for 5 days.
Intraoral clinical examination
All participants were examined by a single dentist in her office located inside the school, where she is employed as a dentist. The clinical histories of the patients were taken, and they were submitted to an intraoral examination to calculate the bleeding or gingival index. The following clinical parameters were evaluated. The gingival index uses a probe to identify the presence or absence of bleeding on the four faces of the teeth (mesial, distal, vestibular, and lingual or palatal). For this, the tip of a periodontal probe (Hu-Friedy 1511BBR PC) was inserted into the periodontal pocket or next to the root extension. It was inserted parallel to the long axis of all teeth with smooth but firm force, and run around the tooth between its surface and the free gingival margin or the inner wall of the periodontal pocket. A check was made up to 10 seconds after delicately opening the gingival sulcus.12 The plaque index was obtained using the visible plaque index, as described by Ainamo and Bay;13 visible plaque was evaluated on teeth after staining both arches with a specific dye. Records of the dental examinations were noted on special sheets together with the patient’s identification and a questionnaire on relevant data. On probing, bleeding of at least 50% of teeth was adopted to characterize periodontal disease. Intraoral clinical evaluations were made before starting medication/placebo and then at 3 and 5 days after administration.
Statistical analysis
The main outcome variable was the reduction of bleeding. The sample size was calculated based on a pilot study (Godoy and Godoy, unpublished data, 2012) on the reduction of light bleeding (nosebleeds). According to this study (α-error =0.05, power =0.80), each group needed to include a minimum of 12 participants. On anticipating a dropout rate of 10%, the total sample size was increased to 15 participants.
Data were analyzed using the StatsDirect Statistical Software, (StatsDirect Ltd, Altrincham, UK). Initially, descriptive analysis was done. Calculations of the mean and standard deviation were made using parametric tests (Student’s t-test) for continuous variables with Gaussian (normal) distribution. An α-error of 5% (P≤0.05) was considered significant. The study was approved by the Ethics Committee of the Medical School in São Jose do Rio Preto (008/2012) and the Brazilian Clinical Trial Register (REQ 1284).
Results
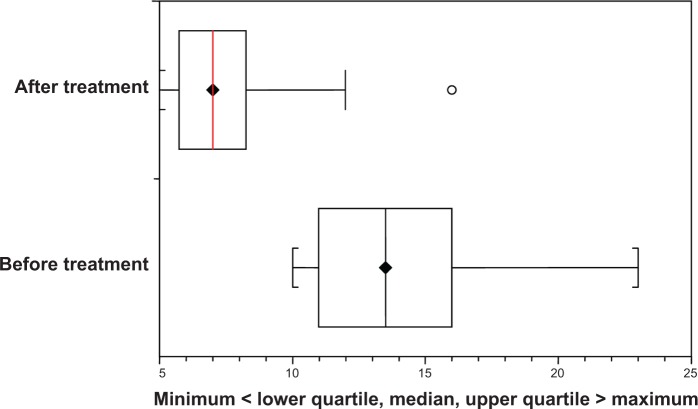
After the final evaluation of bleeding of all patients, the pharmaceutical company revealed which children received aminaphtone and which received a placebo. Table 1 shows that bleeding was reduced after treatment in both groups (paired t-test, P<0.0001 for both groups). Figures 1 and 2 show the variations in bleeding, comparing before and after treatment, for the placebo and aminaphtone groups, respectively (paired t-test, P<0.0001).
Table 1.
Descriptive analysis of the number of bleeding points in both the placebo and aminaphtone groups before and after treatment
| Placebo
|
Aminaphtone
|
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Mean | 14.2 | 7.7 | 15.5 | 1.1 |
| Standard deviation | 3.53 | 2.92 | 3.44 | 1.39 |
Figure 1.
Box-and-whisker plot showing the variations of the number of gingival bleeding points before and after treatment using a placebo.
Figure 2.
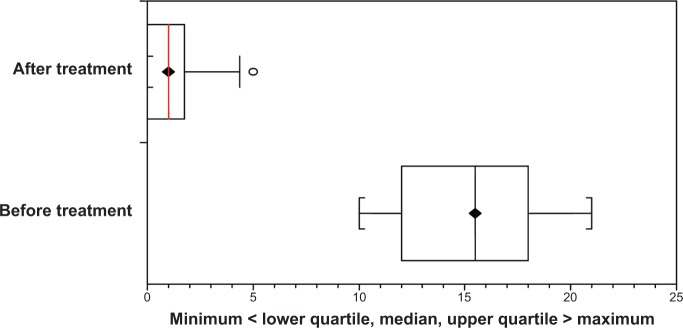
Box-and-whisker plot showing the variations in the number of gingival bleeding points before and after treatment with aminaphtone.
On comparing the number of bleeding points before and after treatment between the aminaphtone and placebo groups, we found significantly higher reduction with the medication (P<0.0001). The mean reduction in the number of points for the placebo group was 6.5, and for the aminaphtone group it was 14.4.
Discussion
The present study evaluated the effect of aminaphtone on the reduction of gingival bleeding in young patients with gingivitis. Both groups in this study – placebo and aminaphtone – experienced reductions in the number of bleeding points. However, the patients treated with aminaphtone had significantly better control of bleeding. There are no publications in the literature on the issue of using aminaphtone or any other drug that may affect capillary fragility.
The hypothesis for this bleeding is that capillary fragility occurs during inflammatory processes, and so drugs that reduce this fragility may improve control of bleeding. There are few studies evaluating the use of aminaphtone in processes that involve bleeding.8,9 Recently however, a series of case reports were published that suggest that this drug reduces capillary fragility. These studies show the control of nosebleeds and report on improvement in cases of ocher dermatitis.7,10,11
Bleeding gums are normally controlled with oral hygiene. The explanation for the reduction in bleeding in the placebo group was better oral hygiene as a result of participating in this study. Therefore, the improved hygiene that was associated with treatment using aminaphtone may have had a synergistic effect in reducing gingival bleeding. Another aspect that catches the attention is that the control in bleeding was 44% of the patients in the aminaphtone group but zero in the placebo group.
The objective of this study was to evaluate the clinical control of bleeding; gingivitis has proven to be an attractive research model to study capillary fragility. Another aspect is the reduction of edema in patients who take aminaph-tone, with a faster improvement in gum color than for those who took the placebo, thereby suggesting control of capillary permeability, which is also expected with the use of aminaphtone.14 Bleeding is unpleasant when using dental floss, and the reduction of edema and gingivitis improves adherence to treatment.
Conclusion
Aminaphtone reduces gum bleeding in gingivitis and may have a supportive role in the control of bleeding, but the main approach to treatment must be to brush the teeth well and use dental floss.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kim JJ, Kim CJ, Camargo PM. Salivary biomarkers in the diagnosis of periodontal diseases. J Calif Dent Assoc. 2013;41(2):119–124. [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen PE1, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83(9):661–669. [PMC free article] [PubMed] [Google Scholar]
- 3.Ngo LH, Darby IB, Veith PD, Locke AG, Reynolds EC. Mass spectrometric analysis of gingival crevicular fluid biomarkers can predict periodontal disease progression. J Periodontal Res. 2013;48(3):331–341. doi: 10.1111/jre.12012. [DOI] [PubMed] [Google Scholar]
- 4.Teles R, Sakellari D, Teles F, et al. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol. 2010;81(1):89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol. 2008;79(8 Suppl):1577–1584. doi: 10.1902/jop.2008.080220. [DOI] [PubMed] [Google Scholar]
- 6.Paizan ML, Vilela JF. Alterações Metabólicas se relacionam à doença Periodontal/Mara Lucia Macedo Paizan São José do Rio Preto [master’s thesis] São José do Rio Preto, Brazil: Faculdade de Medicina de São José do Rio Preto; 2010. [Google Scholar]
- 7.Pereira Godoy JM, Batigália F, Paiva JV, Mendes RN, Oliveira JD. Aminaftona no tratamento da epistaxe [Amnaphtone in the treatment of nosebleed] Rev Bras Hematol Hemoter. 2003;25(1):65–71. Portuguese. [Google Scholar]
- 8.Chimenti S, Faina P. Therapeutic activity of aminaphtone in purpuric disorders. Clin Ter. 1978;84(5):501–508. Italian. [PubMed] [Google Scholar]
- 9.Doni A, Mondani F, Somigli M, Comparini L, Gavazzi M, Ventimiglia V. Modification of primary hemostasis and capillary fragility induced by aminaphtone in chronic renal insufficiency. Clin Ter. 1983;107(1):45–50. Italian. [PubMed] [Google Scholar]
- 10.de Godoy JM. Treatment of stasis dermatitis using aminaphtone: a case series. J Med Case Rep. 2010;4:295. doi: 10.1186/1752-1947-4-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Godoy JM, Batigália F. Aminaphtone in the control of Schamberg’s disease. Thromb J. 2009;7:8. doi: 10.1186/1477-9560-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(6):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 13.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229–35. [PubMed] [Google Scholar]
- 14.Pereira de Godoy JM. Aminaphtone in idiopathic cyclic oedema syndrome. Phlebology. 2008;23(3):118–119. doi: 10.1258/phleb.2007.007054. [DOI] [PubMed] [Google Scholar]