Abstract
Background
The evidence of an association between the Sulfotransferase 1A1 (SULT1A1) Arg213His polymorphism (rs9282861) and bladder cancer risk is still conflicting. We conducted a meta-analysis to assess the association between this polymorphism and bladder cancer risk.
Material/Methods
PubMed, EMBASE, HuGE Navigator, and Web of Science databases were searched for correlative articles. The risk (odds ratio, OR) was used to estimate the association between SULT1A1 Arg213His polymorphism and bladder cancer risk. All of the studies used either fixed-effects or random-effects models. For assessing the credibility of an association, we applied the Venice criteria.
Results
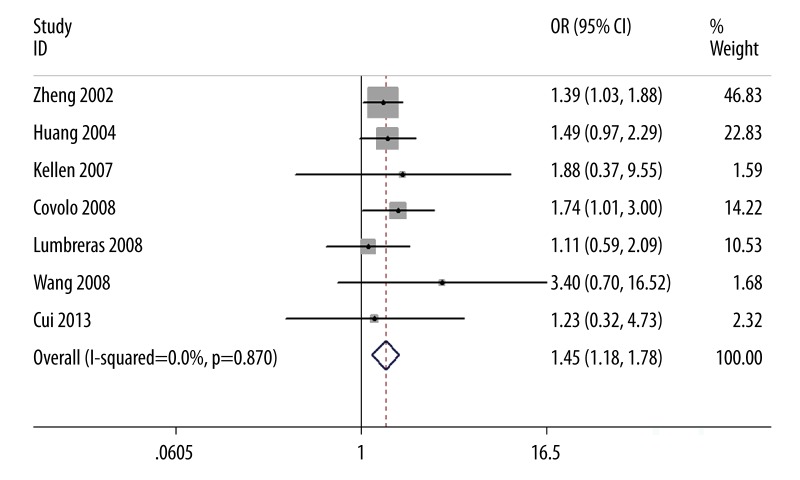
Seven published case-control studies with 1688 cases and 2842 controls were included in this meta-analysis. There were 5 studies of Caucasians and 2 studies of Asians. Four studies reported data on smoking behavior. The percentage of Arg/Arg was higher in Asians and non-smokers than that in Caucasians and smokers, respectively. A significant association of this polymorphism with bladder cancer was found (OR=1.45, 95% CI 1.18–1.78, P=0.0004). In the subgroup analysis by ethnicity, a significant association was found among Caucasians (OR=1.43, 95% CI 1.16–1.77, P=0.0008) but not among Asians (OR=1.89, 95% CI 0.68–5.26, P=0.22). In the subgroup analysis by smoking behavior, increased bladder cancer risk was found in the smokers (OR=1.39, 95% CI 1.01–1.91, P=0.04) and non-smokers (OR=1.74, 95% CI 1.24–2.43, P=0.001).
Conclusions
In conclusion, this meta-analysis indicated that SULT1A1 Arg213His polymorphism is associated with bladder cancer risk.
MeSH Keywords: Genetics, Meta-Analysis, Urinary Bladder Neoplasms, Epidemiology
Background
Bladder cancer is the most common malignancy of the urinary tract, with an estimated incidence of 73 510 cases and 14 880 deaths in the United States for 2012 [1]. Despite recent multidisciplinary advances in its treatment, bladder cancer continues to carry unacceptably high rates of morbidity and mortality [2,3]. Thus, the identification of subjects at high risk of bladder cancer is a major concern for clinicians. Recently, several lines of evidence have indicated that inherited genetic factors influence the development and progression of bladder cancer.
Sulfotransferase 1A1 (SULT1A1) is expressed in the liver, as well as in many extrahepatic tissues (e.g., colonic mucosa), and is a component in the detoxification pathway of numerous xenobiotics [4]. It plays an important role in the metabolism and bioactivation of many dietary and environmental mutagens, including heterocyclic amines implicated in carcinogenesis of bladder cancer [5]. A non-synonymous SNP in the SULT1A1 gene has been identified in the coding region at nucleotide 638 (a G to A transition). This base change leads to an amino-acid substitution at codon 213 (Arg to His). Several studies have investigated the association between SULT1A1 Arg213His polymorphism (rs9282861) and bladder cancer risk. However, the results are contradictory [6–11]. The purpose of this meta-analysis was to access the overall relationship between SULT1A1 Arg213His polymorphism and bladder cancer risk.
Material and Methods
This meta-analysis was performed according to a predetermined protocol described in the following paragraph, using standard systematic review techniques, as outlined in the HuGE Review Handbook [12].
Publication search
Two investigators independently obtained relevant articles through searches of PubMed, EMBASE, HuGE Navigator, and Web of Science databases using the following search terms: “Sulfotransferase 1A1”, “polymorphism”, and “bladder cancer”. The following MeSH terms were used in PubMed: “SULT1A1 protein, human” and “polymorphism, genetic” and “Urinary Bladder Neoplasms”. The search was last updated in May 2014. No language restrictions were applied. A study was included in the current meta-analysis if: (1) it was a case-control study of the SULT1A1 Arg213His polymorphism and bladder cancer, and (2) genotype distributions in both cases and controls were available for estimating an odds ratio (OR) with 95% confidence interval (CI). We excluded studies in which family members were studied. When there were multiple studies from the same population, only the largest study was included.
Data extraction
The following data were collected from each study: first author’s surname, year of publication, ethnicity, age, sex, smoking behavior, and sample size. Authors of the relevant studies were contacted via email if further study data were needed.
Qualitative assessment
The quality of included studies was assessed independently by 2 investigators. The quality scoring system was based on traditional epidemiological considerations and genetic issues [13]. The criteria covered the representativeness of cases and controls, the ascertainment of cases and controls, genotyping examination, Hardy-Weinberg equilibrium (HWE), and association assessment. Scores ranged from 0 to 13.
Statistical analysis
The strength of the associations between the SULT1A1 Arg213His polymorphism and bladder cancer was measured by ORs and 95% CIs. Because most of the studies reported ORs and the corresponding 95% CIs in a recessive model, we chose the recessive genetic model and pooled the results. The between-study heterogeneity was assessed across by the chi-square–based Q statistics and I2 test. Heterogeneity was considered at either a P value of <0.10 or I2>50%. If heterogeneity was observed among the studies, the random-effects model was used to estimate the pooled OR (the DerSimonian and Laird method). Otherwise, the fixed-effects model was adopted (the Mantel-Haenszel method). Departure from HWE in controls was tested by the chi-square test. Subgroup analyses were carried out by ethnicity and smoking. We estimated the difference between the estimates of the subgroups according to tests for interaction. Sensitivity analysis was performed through sequentially excluded individual studies to assess the stability of the results. Potential publication bias was estimated using Egger’s linear regression test by visual inspection of the Funnel plot [14]. All statistical tests were performed using STATA 11.0 software (Stata Corporation, College Station, TX, USA). A P value <0.05 was considered statistically significant.
Estimating the credibility of statistically significant association
Nominally statistically significant results of the meta-analysis were graded on the basis of the Human Genome Epidemiology Network Venice criteria for the assessment of cumulative evidence of genetic associations [15]. These criteria take into account the amount of evidence (sample size measured as the number of minor alleles), consistency of replication (heterogeneity across studies measured as I2), and protection from bias (the bias reason, in particular including sensitivity analysis as outlined above, assessments of the strength of the association, small-study bias, and evidence of an excess of statistically significant results). On the basis of the analysis, the overall epidemiological credibility was graded as strong (grade A), moderate (grade B), or weak (grade C).
Results
Study characteristics
A total of 7 case-control studies with 1688 cases and 2842 controls on the association between SULT1A1 Arg213His polymorphism and bladder cancer risk were included in this meta-analysis [6–11]. There were 5 studies of Caucasians and 2 studies of Asians. Four studies reported information on smoking behavior. The quality scores ranged from 7 to 9, suggesting that the methodological quality was generally acceptable. All studies were in HWE. The characteristics of each case-control study are presented in Table 1. Genotype numbers and HWE examination results are listed in Tables 2 and 3. The percentage of Arg/Arg was higher in Asians than that in Caucasians (79.1% vs. 47.3%, P=0.001). The percentage of Arg/Arg was higher in non-smokers than that in smokers (63.8% vs. 52.3%, P=0.006).
Table 1.
Characteristics of the studies included in this meta-analysis.
| First author/year | Ethnicity | Age | Sex | Smoking habit | Case number | Control number | Score |
|---|---|---|---|---|---|---|---|
| Zheng/2002 | Caucasian | 63 | Reported | Reported | 384 | 386 | 8 |
| Hung/2004 | Caucasian | 65 | Mixed | Reported | 201 | 214 | 7 |
| Kellen/2007 | Caucasian | 70 | Mixed | Mixed | 200 | 385 | 7 |
| Covolo/2008 | Caucasian | 50 | Mixed | Mixed | 197 | 211 | 9 |
| Lumbreras/2008 | Caucasian | NA | Mixed | Mixed | 124 | 1089 | 9 |
| Wang/2008 | Asian | 63 | Mixed | Reported | 300 | 300 | 8 |
| Cui/2013 | Asian | 60 | Mixed | Reported | 282 | 257 | 7 |
HWE – Hardy-Weinberg equilibrium.
Table 2.
Distribution of SULT1A1 Arg213His genotype among patients and controls.
| Studies | Patients | Controls | HWE | ||||
|---|---|---|---|---|---|---|---|
| Arg/Arg | Arg/His | His/His | Arg/Arg | Arg/His | His/His | ||
| Zheng | 196 | 155 | 33 | 164 | 174 | 48 | Yes |
| Hung | 121 | 72 | 8 | 116 | 88 | 10 | Yes |
| Kellen | 111 | 79 | 18 | 191 | 151 | 47 | Yes |
| Covolo | 120 | 77* | 114 | 97* | Yes | ||
| Lumbreras | 57 | 54 | 13 | 513 | 451 | 125 | Yes |
| Wang | 261 | 37 | 2 | 240 | 54 | 6 | Yes |
| Cui | 218 | 59 | 5 | 201 | 52 | 4 | Yes |
Combined numbers of Arg/His and His/His genotypes.
HWE – Hardy-Weinberg equilibrium.
Table 3.
The SULT1A1 Arg213His genotype and bladder cancer risk stratified by smoking behavior.
| Studies | Smoking status | Patients | Controls | ||||
|---|---|---|---|---|---|---|---|
| Arg/Arg | Arg/His | His/His | Arg/Arg | Arg/His | His/His | ||
| Zheng | Smoker | 286 | 282* | 186 | 238* | ||
| Non-smoker | 53 | 47* | 71 | 103* | |||
| Hung | Smoker | 109 | 68 | 7 | 86 | 69 | 6 |
| Non-smoker | 12 | 4 | 1 | 30 | 19 | 1 | |
| Wang | Smoker | 124 | 23* | 88 | 23* | ||
| Non-smoker | 137 | 16* | 152 | 37* | |||
| Cui | Smoker | 155 | 42* | 139 | 37* | ||
| Non-smoker | 63 | 22* | 62 | 19* | |||
Combined numbers of Arg/His and His/His genotypes.
Meta-analysis results
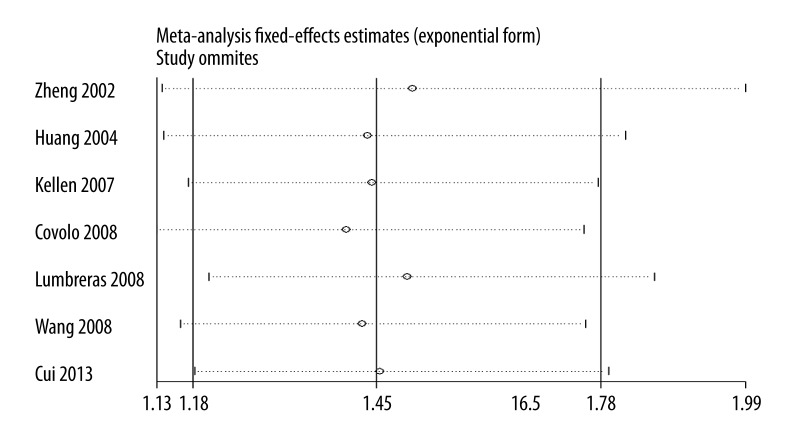
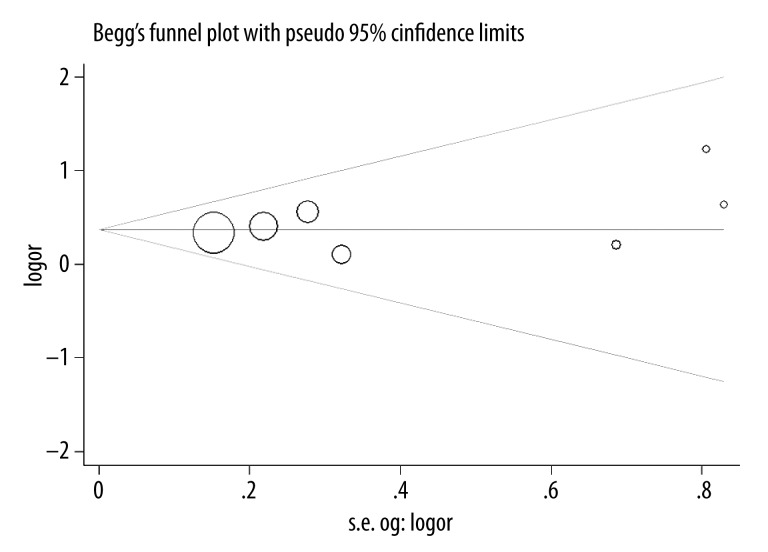
Our results suggest that SULT1A1 Arg213His polymorphism is significantly associated with bladder cancer risk (OR=1.45, 95% CI 1.18–1.78, P=0.0004; Figure 1). In the subgroup analysis by ethnicity, a significant association was found among Caucasians (OR=1.43, 95% CI 1.16–1.77, P=0.0008) but not among Asians (OR=1.89, 95% CI 0.68–5.26, P=0.22). In the subgroup analysis by smoking behavior, increased bladder cancer risk was found in the smokers (OR=1.39, 95% CI 1.01–1.91, P=0.04) and non-smokers (OR=1.74, 95% CI 1.24–2.43, P=0.001), respectively. No significant difference was observed between subgroup analyses of ethnicity and smoking habit by the test of interaction (P=0.61 and 0.34, respectively). Summary results of comparisons are listed in Table 4. The sensitivity analysis did not influence the results excessively by omitting any single study (Figure 2). The shape of the funnel plot was symmetrical (Figure 3). The result of Egger’s linear regression test showed no evidence of publication bias (P=0.388).
Figure 1.
Association between SULT1A1 Arg213His polymorphism and bladder cancer risk.
Table 4.
The genetic effect of SULT1A1 Arg213His polymorphism on bladder cancer.
| Comparison | Characteristics | Model | Test of association | P for interaction | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | I2 (%) | P value | ||||
| Arg/Arg vs. Arg/His + His/His | Overall | F | 1.45 (1.18–1.78) | 0 | 0.0004 | |
| Arg/Arg vs. Arg/His + His/His | Asian | F | 1.89 (0.68–5.26) | 0 | 0.22 | 0.61 |
| Arg/Arg vs. Arg/His + His/His | Caucasian | F | 1.43 (1.16–1.77) | 0 | 0.0008 | |
| Arg/Arg vs. Arg/His + His/His | Smoker | F | 1.39 (1.01–1.91) | 0 | 0.04 | 0.34 |
| Arg/Arg vs. Arg/His + His/His | Non-smoker | F | 1.74 (1.24–2.43) | 0 | 0.001 | |
F – fixed-effects model.
Figure 2.
Sensitivity analysis between SULT1A1 Arg213His polymorphism and bladder cancer risk.
Figure 3.
Funnel plot between SULT1A1 Arg213His polymorphism and bladder cancer risk.
2 Estimating the credibility of association
To assess the credibility of genetic associations, we considered the Venice criteria, which indicated that the association represented a strongly credible association.
Discussion
To our knowledge, this meta-analysis is the first study to systemically assess the association between SULT1A1 Arg213His polymorphism and bladder cancer risk. Results of this meta-analysis showed that SULT1A1 Arg/Arg genotype was significantly associated with increased bladder cancer risk. Although the percentage of Arg/Arg genotype was higher in Asians than in Caucasians, a significant association was found in Caucasians in the subgroup analysis by ethnicity. No significant association was observed in Asians. However, there was no significant interaction in this subgroup analysis. Thus, the positive association between Asians and bladder cancer could not be ruled out, because studies with small sample size may have insufficient statistical power to detect a slight effect. Since only 2 of the studies were done in Asian populations, more studies with Asians should be performed to determine the relationship between SULT1A1 Arg213His polymorphism and bladder cancer risk. The percentage of Arg/Arg genotype was higher in non-smokers than in smokers. Results of a previous study were similar to our results [16]. In the subgroup analysis by smoking behavior, this polymorphism was associated with increased bladder cancer risk in both smokers and non-smokers. Furthermore, there was also no significant interaction in this subgroup analysis. These results suggest that smoking does not change the role of SULT1A1 Arg213His polymorphism in the development of bladder cancer. However, this result should be confirmed by further studies.
Recently, studies have suggested that the SULT1A1 Arg/Arg genotype is associated with an increased risk for development of cancers of the esophagus, breast, and lung [17–19]. The single-nucleotide G/A transition at codon 213 in exon 7 results in an Arg to His amino acid substitution and this polymorphism has been shown to have a functional impact in humans. The allele encoding histidine at position 213 was uniformly associated with lower sulfotransferase activity and lower thermal activity [20]. Thus, it is possible that the SULT1A1 Arg213His polymorphism influences the risk of bladder cancer.
Some limitations should be pointed out. One limitation is the insufficient sample size used in this meta-analysis, especially for the sex subgroup analysis. A second limitation is that lack of original data of each study may prevent more detailed analyses, such as joint effects of SNP-SNP. The third limitation is that in our meta-analysis all the case-control data were used without adjustment by detailed individual information such as age, sex, and lifestyle. The fourth limitation is that we did not include data from genome-wide association studies (GWAS).
Conclusions
This meta-analysis suggests that there is a significant association between SULT1A1 Arg213His polymorphism and bladder cancer risk. Future large-scale, population-based association studies are warranted to verify the risk identified in the current meta-analysis and to investigate the potential gene-gene and gene-environment interactions involving the SULT1A1 Arg213His polymorphism and bladder cancer.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Zhang HH, Qi F, Zu XB, et al. Recurrence of inflammatory myofibroblastic tumor in bladder secondary to prostate treated with laparoscopic radical cystectomy. Med Sci Monit. 2012;18(8):CS63–66. doi: 10.12659/MSM.883255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Zhang J, Liu Y, Ye G. Increased expression of DNA repair gene XPF enhances resistance to hydroxycamptothecin in bladder cancer. Med Sci Monit. 2012;18(4):BR156–62. doi: 10.12659/MSM.882618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris RM, Picton R, Singh S, Waring RH. Activity of phenolsulfotransferases in the human gastrointestinal tract. Life Sci. 2000;67:2051–57. doi: 10.1016/s0024-3205(00)00791-8. [DOI] [PubMed] [Google Scholar]
- 5.Glatt H. Sulfotransferases in the bioactivation of xenobiotics. Chem Biol Interact. 2000;129:141–70. doi: 10.1016/s0009-2797(00)00202-7. [DOI] [PubMed] [Google Scholar]
- 6.Zheng L, Wang Y, Schabath MB, et al. Sulfotransferase 1A1 (SULT1A1) polymorphism and bladder cancer risk: a case-control study. Cancer Lett. 2003;202:61–69. doi: 10.1016/j.canlet.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Hung RJ, Boffetta P, Brennan P, et al. GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int J Cancer. 2004;110:598–604. doi: 10.1002/ijc.20157. [DOI] [PubMed] [Google Scholar]
- 8.Kellen E, Zeegers M, Paulussen A, et al. Does occupational exposure to PAHs, diesel and aromatic amines interact with smoking and metabolic genetic polymorphisms to increase the risk on bladder cancer?; The Belgian case control study on bladder cancer risk. Cancer Lett. 2007;245:51–60. doi: 10.1016/j.canlet.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Lumbreras B, Garte S, Overvad K, et al. Meat intake and bladder cancer in a prospective study: a role for heterocyclic aromatic amines? Cancer Causes Control. 2008;19:649–56. doi: 10.1007/s10552-008-9121-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang YH, Lee YH, Tseng PT, et al. Human NAD(P)H: quinone oxidoreductase 1 (NQO1) and sulfotransferase 1A1 (SULT1A1) polymorphisms and urothelial cancer risk in Taiwan. J Cancer Res Clin Oncol. 2008;134:203–9. doi: 10.1007/s00432-007-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui X, Lu X, Hiura M, et al. Association of genotypes of carcinogen-metabolizing enzymes and smoking status with bladder cancer in a Japanese population. Environ Health Prev Med. 2013;18:136–42. doi: 10.1007/s12199-012-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little J, Higgins JP, editors. The HuGENet™ HuGE Review Handbook, Version 1.0. Ottawa, Ontario, Canada: HuGENet Canada Coordinating Centre; 2006. http://www.hugenet.ca. [Google Scholar]
- 13.Thakkinstian A, McEvoy M, Minelli C, et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol. 2005;162:201–11. doi: 10.1093/aje/kwi184. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannidis JP, Boffetta P, Little J, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2008;37:120–32. doi: 10.1093/ije/dym159. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Spitz MR, Tsou AM, et al. Sulfotransferase (SULT) 1A1 polymorphism as a predisposition factor for lung cancer: a case-control analysis. Lung Cancer. 2002;35:137–42. doi: 10.1016/s0169-5002(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 17.Wu MT, Wang YT, Ho CK, et al. SULT1A1 polymorphism and esophageal cancer in males. Int J Cancer. 2003;103:101–4. doi: 10.1002/ijc.10805. [DOI] [PubMed] [Google Scholar]
- 18.Han DF, Zhou X, Hu MB, et al. Sulfotransferase 1A1 (SULT1A1) polymorphism and breast cancer risk in Chinese women. Toxicol Lett. 2004;150:167–77. doi: 10.1016/j.toxlet.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Liang G, Miao X, Zhou Y, et al. A functional polymorphism in the SULT1A1 gene (G638A) is associated with risk of lung cancer in relation to tobacco smoking. Carcinogenesis. 2004;25:773–78. doi: 10.1093/carcin/bgh053. [DOI] [PubMed] [Google Scholar]
- 20.Raftogianis RB, Wood TC, Otterness DM, et al. Phenol sulfotransferase pharmacogenetics in humans: association of common SULT1A1 alleles with TS PST phenotype. Biochem Biophys Res Commun. 1997;239:298–304. doi: 10.1006/bbrc.1997.7466. [DOI] [PubMed] [Google Scholar]