Abstract
Background
Parathyroid reoperations are challenging and achieving a cure requires multidisciplinary treatment team cooperation.
The aims of this study were to summarize our experience in revision surgery for persistent (pHPT) or recurrent primary hyperparathyroidism (rHPT) and to explore factors underlying failure to cure at initial surgery.
Material/Methods
This was a retrospective cohort study of patients who underwent reoperations for pHPT or rHPT at a tertiary referral center. The database of parathyroid surgery was searched for eligible patients (treated in the years 2000–2012). The primary outcome was the cure rate. All the patients were followed-up for at least 12 months postoperatively. Factors underlying failure to cure at initial surgery were reviewed based on hospital records.
Results
The study group comprised 88 patients (69 women, 19 men) operated on for persistent (n=57) or recurrent disease (n=31), who underwent 98 reoperations, including 26 (2.4%) patients first operated on at our institution, and 72 (81.8%) patients operated on elsewhere, but referred for revision surgery. A long-term cure was achieved in 83/88 patients (94.3%). The mean post-reoperation follow-up was 91.7 (12–176) months. Missed hyperfunctioning parathyroid gland was found on reoperation in eutopic position in 49 (55.5%) patients, and in ectopic position in 39 (44.3%) patients, including 20 (22.7%) cases of cervical ectopy and 19 (21.6%) cases of mediastinal ectopy.
Conclusions
Multidisciplinary treatment team cooperation at a tertiary referral center, consisting of an accurate preoperative localization, expertise in parathyroid re-explorations, and correct use of intraoperative adjuncts, contribute to the high success rate of parathyroid reoperations.
MeSH Keywords: Hyperparathyroidism, Primary, Parathyroid Hormone, Reoperation
Background
Despite remarkable progress in parathyroid imaging and improvements of surgical technique, persistent primary hyperparathyroidism (pHPT) and recurrent primary hyperparathyroidism (rHPT) are therapeutically challenging. The most common reasons for failed parathyroid surgery are: low-volume surgeons lacking in experience in parathyroid surgery, unrecognized multiglandular disease, ectopic localization of parathyroid adenoma, inadequate extent of parathyroid tissue resection, parathyroid capsule rupture causing parathyromatosis, or (rarely) parathyroid cancer [1–5]. Inaccurate preoperative imaging may result in failure of unilateral neck exploration (UNE) or minimally invasive parathyroidectomy (MIP) [6]. A thorough knowledge of parathyroid pathology and understanding of embryological glands migration, as well as proficiency in conventional parathyroid exploration supported by a sound clinical use of modern technical adjuncts during parathyroidectomy, are crucial in intraoperative surgical decision-making and minimizing the risk of a failed primary operation [2,3,7,8].
Having reached the decision about reoperation in pHPT or rHPT, a detailed analysis of possible causes of the failed initial operation and positive and accurate hyperfunctioning parathyroid tissue imaging should be determined preoperatively. The surgical re-exploration aims at removing the diseased parathyroid tissue by means of limited surgical dissection in order not to increase the risk of hypoparathyroidism or recurrent laryngeal nerve (RLN) injury. In recent years, the improved outcomes of parathyroid reoperations were reported in a few retrospective cohort studies from academic centers, with success and complication rates approaching those achieved in the unexplored patient [8–10]. However, to date a systematic review and meta-analysis of hitherto published data in the field has not been undertaken and published. To make such an analysis possible we still need more published data from varying healthcare systems.
The objectives of this study were to summarize our experience in revision parathyroid surgery for pHPT and rHPT and to explore factors underlying failure to cure at initial surgery. The reported series of patients represents the largest cohort of parathyroid reoperations in Poland.
Material and Methods
Study design
This was a retrospective cohort study of consecutive patients who underwent reoperations for pHPT or rHPT at the Department of General, Vascular, and Transplantation Surgery, Medical University of Warsaw, Poland. The prospectively collected database of parathyroid surgery was searched for eligible patients (treated 2000–2012). All patients provided written informed consent for the storage and use of their data.
The inclusion criterion was a biochemically confirmed pHPT or rHPT in a clinically symptomatic patient meeting 1 of 3 indications for surgery: hypercalcemia exceeding 0.25 mmol/l above the upper limit of the reference range (2.2–2.6 mmol/l), recurrent renal stones, progressing osteopenia or osteoporosis, and impaired renal function. Asymptomatic patients with persistent or recurrent mildly elevated serum calcium levels underwent surveillance, but were not qualified for reoperation. The exclusion criteria were incomplete clinical data or follow-up information.
The primary outcome was the cure rate from hyperparathyroid state. All the patients were followed for at least 12 months postoperatively to define a cure. The secondary outcomes were: prevalence of missed multiglandular disease at initial surgery, localization of the missed hyperfunctioning parathyroid tissue (eutopic vs. ectopic), diagnostic pre-re-exploration imaging accuracy, surgical approach at re-exploration, number of surgical re-explorations needed to achieve a cure, utility of intraoperative adjuncts during redo-parathyroidectomy and morbidity. Additionally, factors underlying failure to cure at initial surgery were reviewed based on hospital records and compared between patients initially operated on at our institution versus elsewhere. The protocol of this study was approved by the Institutional Review Board.
Preoperative work-up
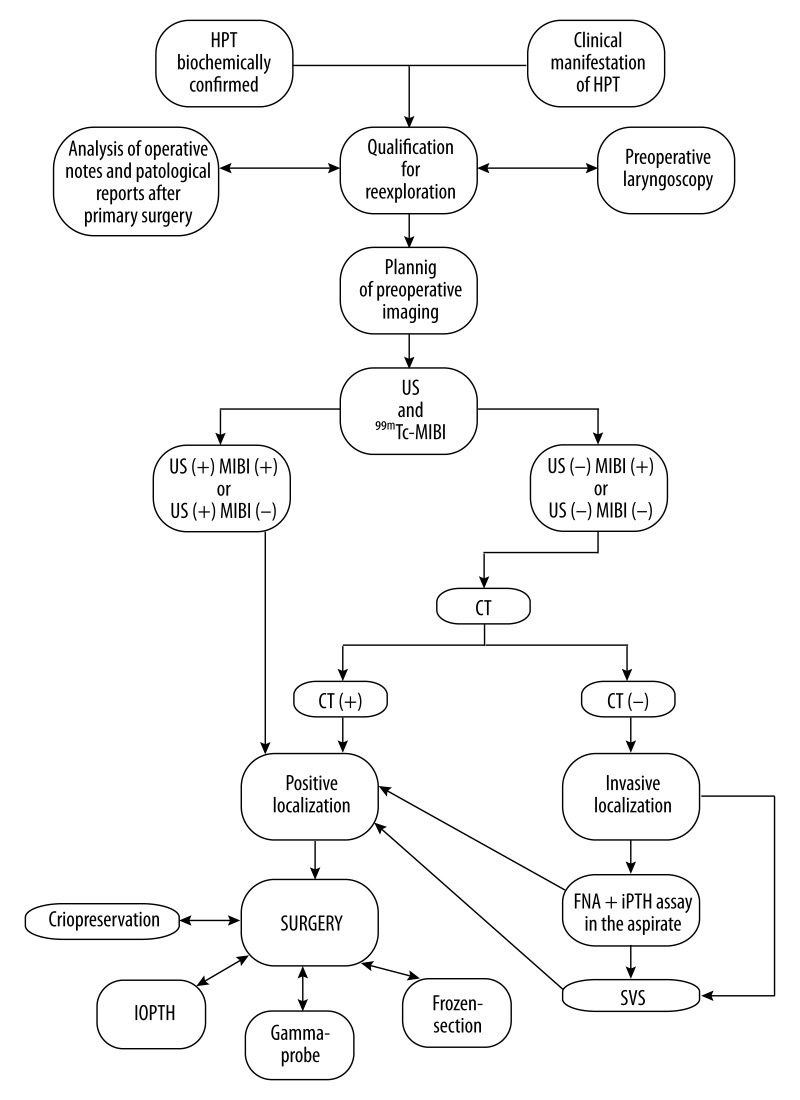
All the patients had biochemically confirmed primary hyperparathyroidism, either persistent or recurrent. Table 1 presents baseline characteristics of the study patients. The current approach to re-exploration for pHPT and rHPT was introduced at our institution in 2000 and gradually modified in the following years based on the growing experience in parathyroid imaging and application of intraoperative adjuncts allowing for intraoperative prognostication of parathyroidectomy outcomes: intraoperative iPTH assay (IOPTH) or gamma-probe (Figure 1). This algorithm is similar to concepts published by other investigators [9–11]. All patients qualified for re-exploration had parathyroid imaging with at least 2 modalities: neck ultrasound and subtraction/dual-phase parathyroid scintigraphy, or SPECT-CT with 99mTc-MIBI. In doubtful cases, CT scans of the neck and chest were done. Neck ultrasonography was performed using a 7.5–15 MHz linear-array transducer by a radiologist experienced in parathyroid imaging. In patients with negative non-invasive parathyroid imaging, the method of selective venous sampling (SVS) with iPTH assay was used for regionalization. Ultrasound-guided fine-needle aspiration (FNA) of suspected lesions with iPTH assay in the aspirate was used in carefully selected cases.
Table 1.
Preoperative demographic, clinical and biochemical characteristics of patients in the study.
| Factor | N=88 patients and 98 reoperations |
|---|---|
| Disease, No. (%) | |
| pHPT | 57 (64.8) |
| rHPT | 31 (35.2) |
|
| |
| Initial parathyroid surgery, No. (%) | |
| At our institution, total | 16 (18.2) |
| pHPT | 6 (6.8)* |
| rHPT | 10 (11.4)* |
| Outside our institution, total | 72 (81.8) |
| pHPT | 51 (60.0)* |
| rHPT | 21 (23.8)* |
|
| |
| Sex (F / M); No. (%) | 69 (78.4)/19 (21.6) |
|
| |
| Age, mean (range); years | |
| Female | 50.3 (24–78) |
| Male | 40.4 (17–81) |
|
| |
| Calcium, mean (range); mmol/l | 2.71 (2.30–4.24) |
|
| |
| Phosphate, mean (range); mmol/l | 0.77 (0.40–1.38) |
|
| |
| Kreatinine, mean (range); μmol/l | 95.26 (35.36–268.74) |
|
| |
| Alkaline phosphatase, mean (range); IU/l | 120.38 (50.0–167.0) |
|
| |
| Urea, mean (range); mmol/l | 5.93 (2.99–20.92) |
|
| |
| iPTH, mean (range); ng/l | 322.75 (70.34–1137.0) |
|
| |
| Symptomatic disease, No (%) | 66 (75.0) |
| Renal stones | 39 (44.3) |
| Osteoporosis | 31 (35.2) |
| Arterial hypertension | 17 (19.3) |
| Risk of hyperkalcemic crisis | 15 (17.0) |
| GI symptoms | 14 (15.9) |
| Impaired renal function | 14 (15.9) |
|
| |
| Asymptomatic disease, No. (%) | 22 (25.0) |
| Calcium, mean (range); mmol/l | 3.04 (2.62–4.24) |
| iPTH, mean (range); ng/l | 285.06 (87.64–509.0) |
|
| |
| Previous surgery before referral, No. (%) | |
| 1 | 80 (90.9) |
| 2 | 8 (9.1) |
|
| |
| Time from initial surgery to reoperation, months (range) | 35.2 (0.13–192.0) |
| pHPT | 17.5 (0.13–84.0) |
| rHPT | 77.3 (15.0–192.0) |
| Solitary disease | 31.0 (0.13–156.0) |
| MEN 1 | 55.0 (12.0–132.0) |
| MEN 2A | 0.13 (0.13–0.13) |
|
| |
| Preoperative unilateral RLN injury, No. (%) | 4 (4.5) |
p=0.011 (χ2-test); pHPT – persistent primary hyperparathyroidism; rHPT – recurrent primary hyperparathyroidism; iPTH – intact parathormone; MEN – multiple endocrine neoplasia; RLN – recurrent laryngeal nerve; reference ranges: total serum calcium (2.15–2.60 mmol/l); total serum phosphate (0.81–1.45 mmol/l); serum creatinine (60.0–130.0 μmol/l); serum alkaline phosphatase (38.0–126.0 IU/l); urea (2.5–6.7 mmol/l); iPTH (15.0–65.0 ng/ml).
Figure 1.
Our institutional algorithm of preoperative work-up before parathyroid reexploration. HPT – primary hyperparathyroidism; US – ultrasound; 99mTc-MIBI – parathyroid scintigraphy; CT – computed tomography; SVS – selective venous sampling with iPTH determination; IOPTH – intraoperative iPTH assay.
Surgical technique
For cervical re-exploration, either unilateral or bilateral, the lateral approach between the sternohyoid and sternothyroid muscles, and medial to the sternocleidomastoid muscle and carotid sheath was used. This approach allows for good exposure of the superior posterior mediastinal/tracheoesophageal groove and avoids the scarred midline field encountered with the standard midline approach [12]. Revision surgery was initially focused on the preoperative image-indexed side of the neck. With negative re-exploration in that area, the dissection was oriented towards the most common parathyroid eutopic locations and on the tract of the embryological parathyroid migration. Should uncertainty arise regarding the number of parathyroid glands left in situ following the initial parathyroid operation, cryopreservation of parathyroid tissue stored in the tissue bank was routinely used with intent of delayed parathyroid autotransplantation, if necessary. Depending on circumstances, the following adjuncts were used intraoperatively to assure a cure: IOPTH, gamma probe, or a frozen section of the removed surgical specimen. STAT-IntraOperative-Intact-PTH Immunoassay (Future Diagnostics, Wijchen, the Netherlands) was used for intraoperative iPTH measurement, with a total turnaround time from blood sample collection to result in 8 min. The Miami criterion was used for cure prognostication [13–15]. A gamma probe was used intraoperatively in cases of discordant results of preoperative imaging following an I.V. injection of 100 MBq of 99mTc-MIBI administered 20 min before surgery. Radioactivity count 20% higher than background was used to identify hyperfunctioning parathyroid tissue. Intraoperative frozen section was used in cases of uncertain preoperative imaging/regionalizing studies and/or an inadequate iPTH level decrease following the removal of the suspected parathyroid lesion.
Follow-up
All data on diagnosis, preoperative imaging, and outcomes were collected on a Microsoft Excel spreadsheet. Successful surgery was defined as normalization of the adjusted serum calcium following revision parathyroidectomy. Patients with elevated adjusted serum calcium within 6 months of surgery were defined as having pHPT, whereas rHPT was defined as the onset of hypercalcemia more than 6 months after parathyroidectomy.
Hypocalcaemia was defined as total serum calcium level below 2.0 mmol/l, irrespective of iPTH level. A serum calcium level below 2.0 mmol/l with a subnormal serum iPTH level (<10 ng/l) was defined as transient hypoparathyroidism if restored to normal within 12 months following withdrawal of oral calcium or calcium plus calcitriol therapy. Persistent hypocalcemia with serum iPTH level below 10 ng/l for more than 12 months postoperatively, requiring substitution with calcium with or without calcitriol, was considered permanent hypoparathyroidism.
Laryngoscopy was mandatory before revision surgery and used to evaluate and follow RLN injury. Vocal cord paresis for more than 12 months postoperatively was considered permanent palsy.
Statistical analysis
Data are presented as mean (standard deviation) or mean (range), unless stated otherwise. The statistical significance of categorical variables was evaluated using the χ2 test, whereas the t-test or Fischer’s exact test was used for the analysis of continuous variables. Receiver operating characteristics (ROC) curve analysis was performed to assess the diagnostic accuracy of preoperative imaging and intraoperative adjuncts. The nerve events incidence was calculated based on the number of nerves at risk. All data were collected prospectively, stored in a computer-based institutional register of parathyroid surgery, and analyzed retrospectively by a statistician, assuming that p<0.05 indicated significance. Statistical analyses were performed with Statistica→ 10 for Windows→ (StatSoft, Cracow, Poland).
Results
Of 657 patients referred for parathyroid surgery during the study interval, 92 had pHPT or rHPT, and thus were potential candidates for the study. Four patients had incomplete histopathology or follow-up data, leaving 88 patients (69 women, 19 men) who were finally enrolled. The study group comprised 57 (64.8%) patients operated on for pHPT and 31 (35.2%) patients operated on for rHPT, who underwent 98 re-explorations. Of this group, 16 (18.2%) patients were initially operated on at our institution, whereas 72 (81.8%) patients were operated on elsewhere and referred for revision parathyroid surgery to our tertiary referral center. Comparing indications for revision parathyroid surgery between patients initially operated on at our institution vs. explored initially elsewhere, the latter group was found to more frequently have pHPT than rHPT (70.8% vs. 37.5%, respectively; p=0.011). Initial parathyroidectomy was undertaken in 56 (77.8%) of 72 patients operated outside our institution by a low-volume parathyroid surgeon (<10 cases per year), whereas the remaining 16 (22.2%) operations were performed by experienced parathyroid surgeons (>30 cases per year). Baseline demographic, clinical, and biochemical characteristics of the studied patients are presented in Table 1. Table 2 shows characteristics of preoperative imaging and diagnostic accuracy of the localization studies.
Table 2.
Diagnostic accuracy of preoperative localization studies in 88 patients in the study.
| Diagnostic study | No. (%) | PPV | Sensitivity |
|---|---|---|---|
| Ultrasound | 88 (100.0) | 72.4 | 60.5 |
| 99mTc-MIBI scintigraphy | 88 (100.0) | 88.2 | 85.1 |
| CT | 27 (30.7) | 53.1 | 49.4 |
| SVS | 3 (3.4) | 100.0 | NC |
| US-guided FNA + iPTH assay in the aspirate | 4 (4.5) | 100.0 | NC |
PPV – positive predictive value; SVS – selective venous sampling with iPTH measurements; CT – computed tomography; US – ultrasound; FNA – fine-needle aspiration; iPTH – intact parathoromone; NC – not calculated.
A cure from hyperparathyroid state was achieved in 83/88 patients (94.3%) (Table 3). Sixty-four (66.3%) patients underwent unilateral neck exploration (UNE), whereas 34 (34.7%) patients underwent bilateral neck exploration (BNE), including 19 (21.6%) mediastinal explorations (16 transcervical approaches and 3 by sternotomy). Ten (11.4%) patients needed more than 1 re-exploration to achieve a cure (3 patients were re-operated on 2 times, 6 patients were re-operated on 3 times, and 1 patient was re-operated 4 times), whereas 5 patients remained hypercalcemic after reoperation/s (characteristics are shown in Table 4). The following intraoperative adjuncts were successfully used: IOPTH in 50/52 patients (96.2%), gamma probe in 25/27 patients (92.6%), and frozen section in 10/11 patients (90.9%). The mean post-reoperation follow-up was 91.7 (12–176) months.
Table 3.
Primary and secondary outcomes of the study.
| Parameter | N = 88 patients and 98 reoperations |
|---|---|
| Reeplorations, mean ±SD (range) | 1.18±0.58 (1–4) |
|
| |
| Surgical approach, No. (%) | |
| Unilateral | 64 (66.3) |
| Bilateral | 34 (34.7) |
|
| |
| Medistinal exploration, No. (%) | |
| Transcervical | 16 (18.2) |
| Sternotomy | 3 (3.4) |
|
| |
| Localization of diseased parathyroid tissue missed at initial exploration, No. (%) | |
| Eutopic | 49 (55.7) |
| Ectopic | |
| In the neck | 20 (22.7) |
| Mediastinal | 19 (21.6) |
|
| |
| Diseased parathyroid glands removed, No. (%) | |
| 1 | 77 (87.5) |
| 2 | 6 (6.8) |
| 2 and a half | 4 (4.5) |
| 3 | 1 (1.1) |
|
| |
| Use of intraoperative adjuncts, No. (%) | |
| IOPTH | 52 (53.1) |
| Gamma-probe | 27 (27.6) |
| Frozen-section | 11 (12.5) |
|
| |
| Operative time, mean (range) | 93.6 (35.0–180.0) |
|
| |
| Pathological report, No. (%) | |
| Solitary parathyroid adenoma | 60 (68.2) |
| Multiple parathyroid disease | 25 (28.4) |
| Double adenoma | 6 (6.8) |
| Non-MEN associated parathyroid hyperplasia | 9 (10.2) |
| MEN 1 | |
| pHPT | 4 (44.4) |
| rHPT | 5 (55.6) |
| MEN 2A | |
| pHPT | 1 (1.1) |
| rHPT | 0 (0) |
| Parathyroid cancer | 3 (3.4) |
|
| |
| Weight of diseased parathyroid/s, g (range) | 1.37 (0.36–30.2) |
|
| |
| Cure rate, No. (%) | |
| Total | 83 (94.3) |
| pHPT | 53 (93.0) |
| rHPT | 30 (96.8) |
|
| |
| Serum calcium on postoperative day 2, mean (range); mmol/l | 2.18 (1.61–3.1)* |
|
| |
| Short-term morbidity, No. (%) | |
| Transient unilateral RLN injury | 10 (11.4) |
| Postoperative hypocalcemia | 72 (81.8) |
| Bleeding | 0 (0.0) |
| Wound infection | 0 (0.0) |
| Others | 0 (0.0) |
|
| |
| Long-term morbidity, No. (%) | |
| Permanent unilateral RLN injury | 6 (6.8) |
| Permanent bilateral RLN injury | 1 (1.1)** |
| Permanent hypoparathyroidism | 12 (13.6) |
|
| |
| Need for delayed parathyroid tissue autotransplantation, No. (%) | 0 (0.0) |
SD – standard deviation; IOPTH – intraoperative iPTH assay; MEN – multiple endocrine neoplasia; pHPT – persistent primary hyperparathyroidism; rHPT – recurrent primary hyperparathyroidism; RLN – recurrent laryngeal nerve; (*) increased serum calcium was found in 5 patients with persistent disease following reexplorations; (**) bilateral RLN injury occurred in 1 patient with pre-reoperative unilateral RLN palsy.
Table 4.
Characteristics of the 5 failed reexplorations.
| Case No. | Age at reoperation/gender | Surgical approach | IOPTH Miami criterion met | Comments |
|---|---|---|---|---|
| 9 | 49/F | BNE | No | Pathological report: 1 normal parathyroid gland was identified in the surgical specimen, whereas the remaining tissues were lymph nodes. Unknown localization of the diseased parathyroid gland |
| 15 | 40/M | BNE | Yes | Pathological report: 6 parathyroid glands with nodular hyperplasia were found in the surgical specimen |
| 22 | 39/F | BNE + sternotomy | No | Pathological report: parathyroid cancer with positive lymph nodes was found in the surgical specimen; persistent hypercalcemia as a result of dissemination |
| 55 | 59/M | BNE | No | Underwent reoperation outside our institution and right-sided parathyroid adenoma was removed from the anterior superior mediastinum (just above the aortic arch) via transcervical approach |
| 75 | 45/F | UNE | Yes | The right inferior parathyroid adenoma was initially removed. Parathyromathosis found on reexploration. En-block excision with the inferior portion of the right thyroid lobe |
IOPTH – intraoperative iPTH assay; BNE – bilateral neck exploration; UNE – unilateral neck exploration; F – female; M – male.
Hyperfunctioning parathyroid gland missed at initial surgery was found on reoperation in eutopic position in 49 (55.7%) patients, and in ectopic position in 39 (44.3%) patients, including 20 (22.7%) cases of cervical ectopy (within the carotid sheath in 9 cases, within the tracheal-esophageal groove in 9 cases, and subcapsular within the thyroid in 2 cases), and 19 (21.6%) cases of mediastinal ectopy (within the thymus in 10 cases, and in the posterior mediastinum in 9 cases). Table 3 shows morbidity following re-explorations.
Discussion
Operations for both pHPT and rHPT are challenging due to the need for dissection of scarified tissues in search of diseased parathyroid gland/s, and common localization of parathyroids in the ectopic sites. Previously, the outcomes of cervical re-explorations in search for hyperfunctioning parathyroid gland/s missed at initial surgery were successful in less than 65–75% of patients [12]. However, in expert hands, the outcomes of revision parathyroid surgery are nowadays comparable to outcomes of initial parathyroidectomy (success rate exceeding 95%), which has been recently reported by others and confirmed in this study [9,15,16]. This remarkable shift was possible due to improved preoperative localization of diseased parathyroid tissue (99mTc-MIBI scintigraphy, high-resolution ultrasound, CT scan of the neck and mediastinum, SVS or US-guided FNA-aspiration of suspected parathyroid lesions with iPTH determination in the aspirate) and development of intraoperative adjuncts allowing for intraoperative quality control (IOPTH, gamma probe) [9,15–22]. In consequence, most parathyroid reoperations can be now performed by unilateral or focused approach (66.3% in this study), leading to a minimized risk of bilateral RLN palsy and decreased prevalence of permanent hypoparathyroidism [9,15,16,23–25].
Importantly, only 16 (2.4%) of 657 patients operated on during the study period underwent initial surgery at our institution. All the remaining 72 (11.0%) patients were operated elsewhere and referred for parathyroid re-exploration. Considering that 56 (77.8%) of 72 patients with pHPT or rHPT were operated on elsewhere by a low-volume parathyroid surgeon (<10 cases per year), and missed parathyroid gland was a solitary parathyroid adenoma in eutopic position (n=39), followed by ectopic location of the diseased gland (n=25), or rarely unrecognized (n=4), or inadequately resected multiglandular disease (n=4), clearly most of those re-explorations could have been avoided if initial surgery was undertaken by an experienced parathyroid surgeon. This observation is also supported by other reports showing prevalence of pHPT or rHPT in as many as 30% of patients operated on by a casual parathyroid surgeon [2,3,9,15]. Chen et al. analyzed 159 patients with persistent/recurrent hyperparathyroidism subsequently cured with additional surgery. Despite a higher incidence of multiglandular disease (which increased the likelihood of operative failure) in patients initially operated on in a high-volume hospital, patients who underwent surgery in a low-volume hospital were more likely to have a missed parathyroid gland in a normal anatomic location (89% vs. 13%, p<0.001), hence a higher proportion of preventable operative failures [2]. Similar data were reported by Mitchell et al., who analyzed parathyroid reoperations at a tertiary-care hospital, noting that 77% of failures occurring in low-volume centers were avoidable compared to 22% from high-volume centers (p<0.001) [3]. Bagul et al. analyzed outcomes of 541 patients who underwent first-time surgery for primary hyperparathyroidism in 1 of the 2 surgical units with a high volume of parathyroid operations to determine factors underlying failure to cure [26]. Persistent disease in the entire cohort was diagnosed in 25 (5%) patients. In patients who had undergone dual imaging with an ultrasound scan and 99mTc-sestamibi scintigraphy, failure rates with “lateralized and concordant” imaging, “non-concordant” imaging, and “dual-negative” imaging were 2%, 9%, and 11%, respectively (p=0.01). Of 25 patients with persistent disease, multiglandular disease was present in 13/25 patients (52%) and ectopic adenoma in 6/25 patients (24%) [26]. Thus, a significant proportion of failures were due to the inability to recognize the presence and/or extent of multiglandular disease in patients with negative or discordant dual imaging.
Recently, IOPTH was recognized as the most reliable adjunct during parathyroid reoperations [27]. On the one hand, it allows for limiting neck exploration in case of an adequate decrease of iPTH levels after resection of a culprit parathyroid gland. On the other hand, it is helpful in recognition of persistent hyperfunctioning parathyroid tissue in case of an inadequate decrease of serum iPTH values. In the current study, IOPTH directly influenced the surgery course in 23 (92.0%) of 25 patients with multiglandular disease, since the lack of PTH decrease after excision of 1 or 2 unilateral pathological parathyroid glands indicated the need to explore the contralateral neck side, which turned out to be harboring additional diseased parathyroid tissue. Irvin et al. reported that with IOPTH used to facilitate localization and confirm excision of all hyperfunctioning tissue, the success rate of reoperative parathyroidectomy improved from 76% to 94% [28]. Many other surgeons reported similar results [9,10,15,16,29].
Despite progress in the operative management of pHPT and rHPT, morbidity following cervical re-explorations is still high. Karakas et al. reported permanent RLN palsy in 9% and permanent hypoparathyroidism in 6% of patients undergoing parathyroid reoperations, with a success rate of 95.2% [9]. Intraoperative neural monitoring was not used in this study, but this technique was reported to decrease the prevalence of RLN injury in thyroid reoperations, which could also be expected after revision parathyroidectomy monitored in this way [30]. Moreover, in patients with uncertain status of remaining normal parathyroid tissue, cryopreservation with autotransplantation was recommended to correct permanent hypoparathyroidism [1,11,12,31]. Indications for cryopreservation in our practice were found in only 8/88 patients (9.1%). None of the stored tissue was used for autotransplantation, because conservative treatment with calcium and vitamin D analogues was sufficient to maintain asymptomaticity in all mildly hypocalcemic patients, consistent with other reports [12,31].
Conclusions
Multidisciplinary treatment team cooperation at a tertiary referral center consisting of accurate preoperative localization, expertise in parathyroid re-explorations, and correct use of intraoperative adjuncts contribute to a high success rate of parathyroid reoperations, which is comparable to outcomes of primary neck exploration for hyperparathyroidism.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Henry JF. Reoperation for primary hyperparathyroidism: tips and tricks. Langenbecks Arch Surg. 2010;395:103–10. doi: 10.1007/s00423-009-0560-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Wang T, Yen T, et al. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg. 2010;252:691–95. doi: 10.1097/SLA.0b013e3181f698df. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell J, Milas M, Barbosa G, et al. Avoidable reoperations for thyroid and parathyroid surgery: effect of hospital volume. Surgery. 2008;144:899–906. doi: 10.1016/j.surg.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Wells SA, Jr, Debenedetti MK, Doherty GM. Recurrent or persistent hyperparathyroidism. J Bone Miner Res. 2002;17(Suppl 2):N158–62. [PubMed] [Google Scholar]
- 5.Salmeron MD, Gonzales JM, Sancho-Insenser J, et al. Causes and treatment of recurrent hypoparathyroidism after subtotal parathyroidectomy in the presence of multiple endocrine neoplasia 1. World J Surg. 2010;34:1325–31. doi: 10.1007/s00268-010-0605-2. [DOI] [PubMed] [Google Scholar]
- 6.Gough I. Reoperative parathyroid surgery: The importance of ectopic location and multigland disease. ANZ J Surg. 2006;76:1048–50. doi: 10.1111/j.1445-2197.2006.03931.x. [DOI] [PubMed] [Google Scholar]
- 7.McGill J, Sturgeon C, Kaplan SP, et al. How does the operative strategy for primary hyperparathyroidism impact the findings and cure rate? A comparison of 800 parathyroidectomies. J Am Coll Surg. 2008;207:246–49. doi: 10.1016/j.jamcollsurg.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 8.Liew V, Gough IR, Nolan G, Fryar B. Reoperation for hyperparathyroidism. ANZ J Surg. 2004;74:732–40. doi: 10.1111/j.1445-1433.2004.03142.x. [DOI] [PubMed] [Google Scholar]
- 9.Karakas E, Müller HH, Schlosshauer T, et al. Reoperations for primary hyperparathyroidism – improvement of outcome over two decades. Langenbecks Arch Surg. 2013;398:99–106. doi: 10.1007/s00423-012-1004-y. [DOI] [PubMed] [Google Scholar]
- 10.Powell A, Alexander H, Chang R, et al. Reoperation for parathyroid adenoma: a contemporary experience. Surgery. 2009;146:1144–55. doi: 10.1016/j.surg.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Udelsman R. Approach to the patient with persistent or recurrent primary hyperparathyroidism. J Clin Endocrinol Metab. 2011;96:2950–58. doi: 10.1210/jc.2011-1010. [DOI] [PubMed] [Google Scholar]
- 12.Brennan MF, Doppman JL, Marx SJ, et al. Reoperative parathyroid surgery for persistent hyperparathyroidism. Surgery. 1978;83:669–76. [PubMed] [Google Scholar]
- 13.Richards ML, Thompson GB, Farley DR, et al. An optimal algorithm for intraoperative parathyroid hormone monitoring. Arch Surg. 2011;146:280–85. doi: 10.1001/archsurg.2011.5. [DOI] [PubMed] [Google Scholar]
- 14.Barczyński M, Konturek A, Hubalewska-Dydejczyk A, et al. Evaluation of Halle, Miami, Rome, and Vienna intraoperative iPTH assay criteria in guiding minimally invasive parathyroidectomy. Langenbecks Arch Surg. 2009;394:843–49. doi: 10.1007/s00423-009-0510-z. [DOI] [PubMed] [Google Scholar]
- 15.Yen TW, Wang TS, Doffek KM, et al. Reoperative parathyroidectomy: An algorithm for imaging and monitoring of intraoperative parathyroid hormone levels that results in a successful focused approach. Surgery. 2008;144:619–21. doi: 10.1016/j.surg.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Richards ML, Thompson GB, Farley DR, et al. Reoperative parathyroidectomy in 228 patients during the era of minimal-access surgery and intraoperative parathyroid hormone monitoring. Am J Surg. 2008;196:937–43. doi: 10.1016/j.amjsurg.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Witteveen JE, Kievit J, Stokkel MP, et al. Limitations of Tc99m-MIBI-SPECT imaging scans in persistent primary hyperparathyroidism. World J Surg. 2011;35:128–39. doi: 10.1007/s00268-010-0818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones JJ, Brunaud L, Dowd CF, et al. Accuracy of selective venous sampling for intact parathyroid hormone in difficult patients with recurrent or persistent hyperparathyroidism. Surgery. 2002;132:944–50. doi: 10.1067/msy.2002.128477. [DOI] [PubMed] [Google Scholar]
- 19.Witteveen JE, Kievit J, van Erkel AR, et al. The role of selective venous sampling in the management of persistent hyperparathyroidism revisited. Eur J Endocrinol. 2010;163:945–52. doi: 10.1530/EJE-10-0654. [DOI] [PubMed] [Google Scholar]
- 20.Pitt S, Panneerselvan R, Sippel R, et al. Radioguided parathyroidectomy for hyperparathyroidism in the reoperative neck. Surgery. 2009;146:592–98. doi: 10.1016/j.surg.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Sippel R, Schaefer S. The effectiveness of radioguided parathyroidectomy in patients with negative technetium tc 99m-sestamibi scans. Arch Surg. 2009;144:643–48. doi: 10.1001/archsurg.2009.104. [DOI] [PubMed] [Google Scholar]
- 22.Barczyński M, Gołkowski F, Konturek A, et al. Technetium-99m-sestamibi subtraction scintigraphy vs. ultrasonography combined with a rapid parathyroid hormone assay in parathyroid aspirates in preoperative localization of parathyroid adenomas and in directing surgical approach. Clin Endocrinol (Oxf) 2006;65:106–13. doi: 10.1111/j.1365-2265.2006.02556.x. [DOI] [PubMed] [Google Scholar]
- 23.Greene AB, Butler RS, McIntyre S, et al. National trends in parathyroid surgery from 1998 to 2008: a decade of change. J Am Coll Surg. 2009;209:332–43. doi: 10.1016/j.jamcollsurg.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Shen W, Düren M, Morita E, et al. Reoperation for persistent or recurrent primary hyperparathyroidism. Arch Surg. 1996;131:861–67. doi: 10.1001/archsurg.1996.01430200071013. [DOI] [PubMed] [Google Scholar]
- 25.Hessman O, Stålberg P, Sundin A, et al. High success rate of parathyroid reoperation may be achieved with improved localization diagnosis. World J Surg. 2008;32:774–81. doi: 10.1007/s00268-008-9537-5. [DOI] [PubMed] [Google Scholar]
- 26.Bagul A, Patel HP, Chadwick D, et al. Primary hyperparathyroidism: an analysis of failure of parathyroidectomy. World J Surg. 2014;38:534–41. doi: 10.1007/s00268-013-2434-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Mack E, Starling J, et al. A comprehensive evaluation of perioperative adjuncts during minimally invasive parathyroidectomy: which is most reliable? Ann Surg. 2005;242:375–80. doi: 10.1097/01.sla.0000179622.37270.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irvin GL, III, Molinari AS, Figueroa C, et al. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. 1999;229:874–78. doi: 10.1097/00000658-199906000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udelsman R, Donovan P. Remedial parathyroid surgery: changing trends in 130 consecutive cases. Ann Surg. 2006;244:471–79. doi: 10.1097/01.sla.0000234899.93328.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barczyński M, Konturek A, Pragacz K, et al. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg. 2014;38:599–606. doi: 10.1007/s00268-013-2260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caccitolo JA, Farley DR, vanHeerden JA, et al. The current role of parathyroid cyropreservation andautotransplantation in parathyroid surgery: an institutional experience. Surgery. 1997;122:1062–67. doi: 10.1016/s0039-6060(97)90209-9. [DOI] [PubMed] [Google Scholar]