Abstract
A 36-year-old woman who had received long-term treatment with chloroquine for systemic lupus erythematosus developed a third degree atrioventricular block and required a permanent pacemaker. Notably, left ventricular thickening and mild systolic dysfunction were noticed on echocardiography as well as on cardiac MRI. As there was no clear explanation for myocardial findings, the patient underwent an endomyocardial biopsy that demonstrated vacuolar degeneration of myocytes on light microscopy and curvilinear bodies on electron microscopy, both findings consistent with chloroquine toxicity. The drug was withheld and treatment with candesartan and carvedilol was prescribed. At 2-year follow-up, the patient remained asymptomatic and left ventricular systolic function had improved. Physicians who prescribe antimalarial drugs for rheumatic diseases should be aware of the potentially life-threatening effects of chloroquine on the heart.
Background
Chloroquine and hydroxychloroquine are antimalarial drugs (AMD) frequently prescribed in patients with rheumatic diseases because of their immunomodulatory effects.1 These drugs have shown to be useful for preventing acute exacerbations and improve survival in patients with systemic lupus erythematosus (SLE).2 Besides, due to the low risk/benefit ratio, AMD are considered among the safest antirheumatic medications3 and severe side effects are very rare. Although long-term treatment may result in retinopathy,4 neuromyopathy5 and myopathy,6 cardiac toxicity has rarely been described.
Case presentation
A 36-year-old woman with SLE was sent to our hospital for further cardiac work up of the left ventricular (LV) systolic dysfunction. She denied dyspnoea or fatigue with daily activities and had no leg oedema or orthopnoea. Her latest ophthalmological examination was normal. Three months before admission, she had syncope secondary to a complete atrioventricular block of unknown origin and required a permanent pacemaker implant at another hospital. Before pacemaker implantation, an echocardiography revealed mild LV systolic dysfunction (ejection fraction 50%) and biventricular hypertrophy. Besides, LV thickening was confirmed by MRI (CMR); however, there was no evidence of hypertrophic cardiomyopathy or late gadolinium enhancement of LV.
The patient's history was negative for hypertension and chronic renal disease. Relevant medication history on admission was prednisolone 5 mg/day and chloroquine 250 mg/day for 14 years. She denied alcohol ingestion or drug abuse and had no family history of sudden death or cardiac disease. Besides, on arrival, she was found in no distress; her blood pressure was 112/78 mm Hg and heart rate was 83 bpm. The physical examination was otherwise normal with no evidence of volume overload.
Investigations
An ECG revealed an atrial sensed and ventricular paced rhythm. All laboratory test results were normal and included white cell count 8600/μL (normal range, 4000–11 000), haemoglobin 14.2 g/dL (normal range, 12–15), platelet count 261 000 (normal range, 150 000–450 000), creatinine 0.77 mg/dL (normal range, 0.6–1.1), C reactive protein 0.6 mg/dL (normal range, 0.01–0.82), C3 129 mg/dL (normal range, 83–193), C4 16.5 (normal range, 15–57) and urine analysis. As the patient was asymptomatic, neither troponin nor NT-pro BNP levels were considered relevant.
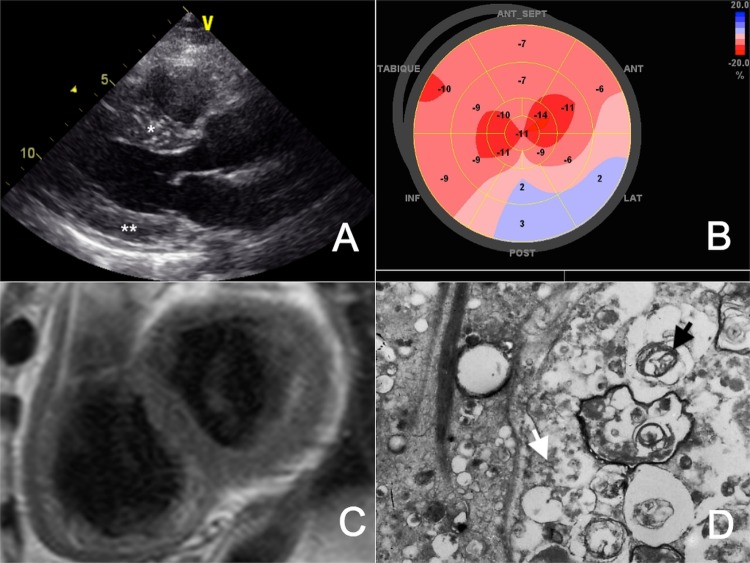
A new transthoracic echocardiography demonstrated symmetrical LV thickening and mild systolic dysfunction (ejection fraction 48%; figure 1A), decreased global longitudinal strain (−10%, normal −18±2%; figure 1B), restrictive filling pattern, right ventricular dysfunction (tricuspid annular plane systolic excursion 12 mm, normal >16 mm) and a systolic pulmonary artery pressure of 25 mm Hg. No pericardial effusion was seen. LV thickening had already been confirmed by prior CMR (figure 1C). Since the clinical picture suggested an infiltrative cardiomyopathy, the patient underwent further cardiac work up. A coronary angiogram did not show obstructive coronary artery disease, but light microscopy examination of an endomyocardial biopsy disclosed diffuse vacuolar degeneration of myocytes and transmission electron microscopy (TEM) revealed curvilinear bodies (figure 1D). These findings led to the diagnosis of cardiac toxicity induced by chloroquine.
Figure 1.
(A) Transthoracic echocardiography demonstrating a normal left ventricular cavity with increased septal (*) and posterior wall (**) thickness. (B) Bull's eye plot of the left ventricle obtained by two-dimensional speckle tracking echocardiography displaying reduced myocardial deformation in all segments (normal −18±2%). (C) Cardiac MRI after gadolinium injection without evidence of late contrast enhancement. (D) Lamellar bodies (black arrow) and curvilinear bodies (white arrow) viewed on transmission electron microscopy.
Differential diagnosis
Most commonly, valvular heart disease and hypertension are the cause of LV dysfunction and hypertrophy. However, this patient did not have these conditions so the differential diagnosis includes hypertrophic cardiomyopathy or restrictive and infiltrative cardiomyopathies such as amyloidosis and Fabry disease. The findings of CMR and light microscopy ruled out all these diagnoses except for Fabry disease, which is a vacuolar cardiomyopathy very similar to chloroquine-induced cardiomyopathy, and which can be distinguished by TEM findings as discussed below.
Treatment
Chloroquine was stopped and the patient was started on candesartan and carvedilol, which were further titrated up to the highest dose recommended.
Outcome and follow-up
At 2-year follow-up, the patient remains free of heart failure symptoms, and though LV thickening has not changed (figure 2A), systolic function returned to normal and global longitudinal strain also improved (figure 2B).
Figure 2.
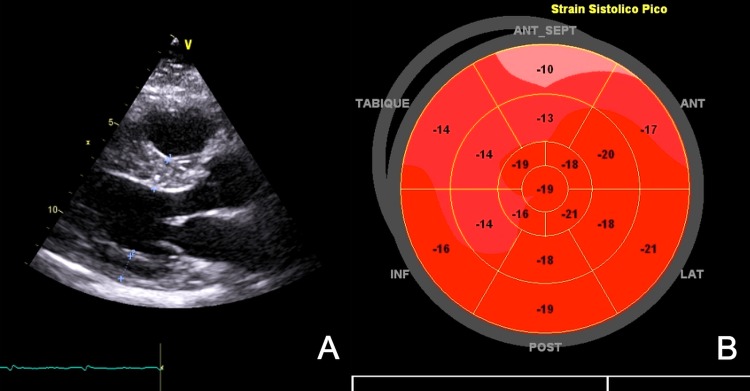
(A) Transthoracic echocardiography at 2 years’ follow-up demonstrating no change in cardiac thickening (dotted lines). (B) Bull's eye plot of the left ventricle shows a significant improvement of myocardial deformation.
Discussion
A recent review of the literature found 77 reports of AMD cardiotoxicity: 47 patients with cardiomyopathy and 30 patients with third-degree AV block (14 of them had cardiomyopathy and complete AV block).7 Complete AV block usually precedes the onset of heart failure symptoms by several years. Our patient was inside this spectrum as she had already needed a pacemaker because of complete AV block, yet she did not have symptoms of heart failure despite the presence of mild LV systolic dysfunction and thickening.
The mechanism of chloroquine cardiotoxicity is not fully disclosed. The AV conduction abnormalities seem to be caused by a direct injury to the infra-Hisian conduction system,8 usually heralded by a complete right branch block9 and left anterior fascicular block before developing a third-degree AV block.7 On the other hand, cardiomyopathy is essentially an intracellular deposition disorder caused by the inhibition of lysosomal hydrolases and, thereby, intralysosomal accumulation of glycosphingolipids.10 This leads to myocyte vacuolation and thickening of the cardiac walls. The predisposing factors to cardiotoxity are not completely understood. Large cumulative doses and long duration of treatment are frequently reported in affected patients, but low-dose AMD11 and latency from months to several years have also been described,9 suggesting that host factors might play a significant role.
A high index of suspicion is required for diagnosing AMD-induced cardiomyopathy, particularly when AV conduction disorders appear, as these may present without symptoms of heart failure. There are no recommendations available for ECG screening or follow-up of patients treated with AMD. So, the available knowledge concerning the diagnostic work up comes from case reports of symptomatic patients. Echocardiography is useful for detecting biventricular thickening,7 usually misnamed hypertrophy, which may be indistinguishable from other infiltrative conditions or hypertrophic cardiomyopathy.12 Besides, it may also demonstrate systolic or diastolic dysfunction, signs of dilated or restrictive cardiomyopathy and valve thickening.7 13 Tissue Doppler and strain imaging have shown impairment of longitudinal systolic LV function in asymptomatic patients with SLE,14 and newer speckle tracking techniques sound promising for detecting early abnormalities in systolic function when the traditional echocardiographic measurements are normal.15 In this case, two-dimensional speckle tracking imaging revealed a diffuse pattern of myocardial involvement with improvement at follow-up. To the best of our knowledge, this is the first report showing the value of speckle tracking imaging in this condition. On the other hand, CMR may assist in the differential diagnosis since late contrast gadolinium enhancement (LGE) in the septal region and/or lateral wall has been noted in patients with AMD cardiomyopathy.9 13 However, such findings are also described in Fabry's cardiomyopathy and hypertrophic cardiomyopathy,16 limiting the role of CMR in definitive diagnosis. As our patient had no LGE, further work up was mandatory.
Given the limitations of non-invasive diagnostic methods, the histological examination of an endomyocardial biopsy specimen is the cornerstone of diagnosis. The findings on light microscopy are those of a lysosomal storage disorder (cytoplasmic vacuolisation with granular material within the vacuoles) and similar to those of Fabry's cardiomyopathy or side effects of amiodarone.17 Thus, the presence of two different kinds of inclusion bodies: lamellar lysosomal structures and, particularly, curvilinear bodies on TEM is essential to prove the diagnosis.10 12 However, medical history of chloroquine exposure must be provided to the pathologist as curvilinear bodies may be overlooked and mislead to the diagnosis of Fabry's cardiomyopathy.12
There is no definite therapy for AMD cardiotoxicity and drug withdrawal is the mainstay of treatment. Regardless of the aetiology, the use of ACE inhibitors or ARB and β blockers has been recommended to prevent symptoms and cardiac remodelling in patients with stage B heart failure.18 Our patient had a partial improvement of the left ventricular systolic function and remodelling, but the prognosis is unpredictable since partial to complete recovery,11 13 cardiac transplantation and death have all been reported.7 12 13 Contrariwise, the resolution of conduction disorders seems to be a rare event.7
Learning points.
Cardiomyopathy is a rare but life-threatening complication of chloroquine and hydroxycloroquine therapy.
Annual ECG examination, although not recommended by any scientific association, should be considered in patients receiving chronic therapy with chloroquine or hydroxychloroquine.
The finding of heart conduction disorders on ECG or left ventricle wall enlargement on echocardiography in these patients warrants further diagnostic work up.
Acknowledgments
Dr Carolina Echeverri (Hospital Pablo Tobon) and Dr Rafael Andrade (Fundacion Santafe de Bogota) for their valuable support with the transmission electron microscopy photographs.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wallace DJ, Gudsoorkar VS, Weisman MH, et al. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 2012;8:522–33 [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8 [DOI] [PubMed] [Google Scholar]
- 3.Choy EHS, Smith C, Doré CJ, et al. A meta-analysis of the efficacy and toxicity of combining disease-modifying anti-rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology (Oxford) 2005;44:1414–21 [DOI] [PubMed] [Google Scholar]
- 4.Marmor MF, Carr RE, Easterbrook M, et al. ; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology 2002;109:1377–82 [DOI] [PubMed] [Google Scholar]
- 5.Aviña-Zubieta JA, Galindo-Rodriguez G, Newman S, et al. Long-term effectiveness of antimalarial drugs in rheumatic diseases. Ann Rheum Dis 1998;57:582–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis 2006;65:385–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol 2013;35:434–42 [DOI] [PubMed] [Google Scholar]
- 8.Teixeira RA, Martinelli Filho M, Benvenuti LA, et al. Cardiac damage from chronic use of chloroquine: a case report and review of the literature. Arq Bras Cardiol 2002;79:85–8 [DOI] [PubMed] [Google Scholar]
- 9.Reffelmann T, Naami A, Spuentrup E, et al. Images in cardiovascular medicine. Contrast-enhanced magnetic resonance imaging of a patient with chloroquine-induced cardiomyopathy confirmed by endomyocardial biopsy. Circulation 2006;114:e357–8 [DOI] [PubMed] [Google Scholar]
- 10.August C, Holzhausen HJ, Schmoldt A, et al. Histological and ultrastructural findings in chloroquine-induced cardiomyopathy. J Mol Med (Berl) 1995;73:73–7 [DOI] [PubMed] [Google Scholar]
- 11.Fragasso G, Sanvito F, Baratto F, et al. Cardiotoxicity after low-dose chloroquine antimalarial therapy. Heart Vessels 2009;24:385–7 [DOI] [PubMed] [Google Scholar]
- 12.Roos JM, Aubry M-C, Edwards WD. Chloroquine cardiotoxicity: clinicopathologic features in three patients and comparison with three patients with Fabry disease. Cardiovasc Pathol 2002;11:277–83 [DOI] [PubMed] [Google Scholar]
- 13.Naqvi TZ, Luthringer D, Marchevsky A, et al. Chloroquine-induced cardiomyopathy-echocardiographic features. J Am Soc Echocardiogr 2005;18:383–7 [DOI] [PubMed] [Google Scholar]
- 14.Buss SJ, Wolf D, Korosoglou G, et al. Myocardial left ventricular dysfunction in patients with systemic lupus erythematosus: new insights from tissue Doppler and strain imaging. J Rheumatol 2010;37:79–86 [DOI] [PubMed] [Google Scholar]
- 15.Huang B-T, Yao H-M, Huang H. Left ventricular remodeling and dysfunction in systemic lupus erythematosus: a three-dimensional speckle tracking study. Echocardiography 2014. 10.1111/echo12515 [DOI] [PubMed] [Google Scholar]
- 16.Pieroni M, Chimenti C, De Cobelli F, et al. Fabry's disease cardiomyopathy: echocardiographic detection of endomyocardial glycosphingolipid compartmentalization. J Am Coll Cardiol 2006;47:1663–71 [DOI] [PubMed] [Google Scholar]
- 17.Stone JR. Diagnostic biopsies of the native heart. Surg Pathol Clin 2012;5:401–16 [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–327 [DOI] [PubMed] [Google Scholar]